Introduction
Colon cancer is the second most prevalent malignancy
and the third leading cause of cancer-related mortalities
worldwide, resulting in ~500,000 mortalities every year. Colon
cancer is highly lethal and aggressively malignant due to its
dormant course, difficult early diagnosis, metastasis, strong
invasion and poor prognosis (1–4).
Surgical resection remains the only curative treatment for
colorectal cancer, however, the outcome is not always satisfactory.
Although 70–80% of patients are eligible for curative surgical
resection at the time of diagnosis, 50% of all newly-diagnosed
patients ultimately develop metastatic disease. Numerous patients
should be considered for palliative treatment, including
chemotherapy and radiotherapy. However, the toxicity of these
chemotherapy medicines to normal tissues and cells has been a major
obstacle in successful cancer treatment (5–11).
There is an urgent requirement to identify novel natural compounds
with a low toxicity and high selectivity for killing cancer
cells.
Traditional Chinese medicine has been practiced for
several millennia and includes a large number of recipes that have
not yet been fully explored scientifically. Sophora
flavescens Ait is an example of one of these unexplored
substances. Sophora flavescens Ait is a leguminous plant
that grows in China, Japan and certain European countries. The dry
root of the plant is extensively used in traditional Chinese
medicine for the treatment of viral hepatitis, cancer, cardiac
diseases and skin diseases, including atopic dermatitis and eczema
(12–16).
Matrine is one of the main alkaloid components that
may be extracted from the Sophora root. The compound, which
was first isolated and identified in 1958 from Sophora
flavescens Ait, has a molecular formula of
C15H24N2O. In China, matrine has
been widely used in the treatment of various diseases, since it has
a wide range of pharmacological effects, including
anti-inflammatory, antiviral, immunoinhibitory, antifibrotic,
analgesic, antiarrhythmic and anti-diarrheal effects. Interest has
been generated in the antitumor activity of matrine. It has been
reported that matrine exerts antitumor effects by inhibiting
proliferation and inducing the apoptosis of gastric and cervical
cancer and leukemia and glioma cells. Matrine has also been shown
to induce apoptosis of murine hepatoma cells in vitro and
in vivo, as well as inhibiting tumor growth. Furthermore,
matrine inhibits the adhesion and migration of cervical cancer HeLa
cells, the invasion and metastasis of human malignant melanoma A375
cells and the growth of established gastric tumors in mice
(17–20). However, whether or not matrine is
able to inhibit the proliferation of human colon cancer HT29 cells
and its molecular mechanisms of action are unclear. Therefore, the
present study aimed to investigate the antitumor effect of matrine
in human colon cancer HT29 cells, and to further elucidate its
molecular mechanism involved in antineoplastic activities.
Materials and methods
Reagents and chemicals
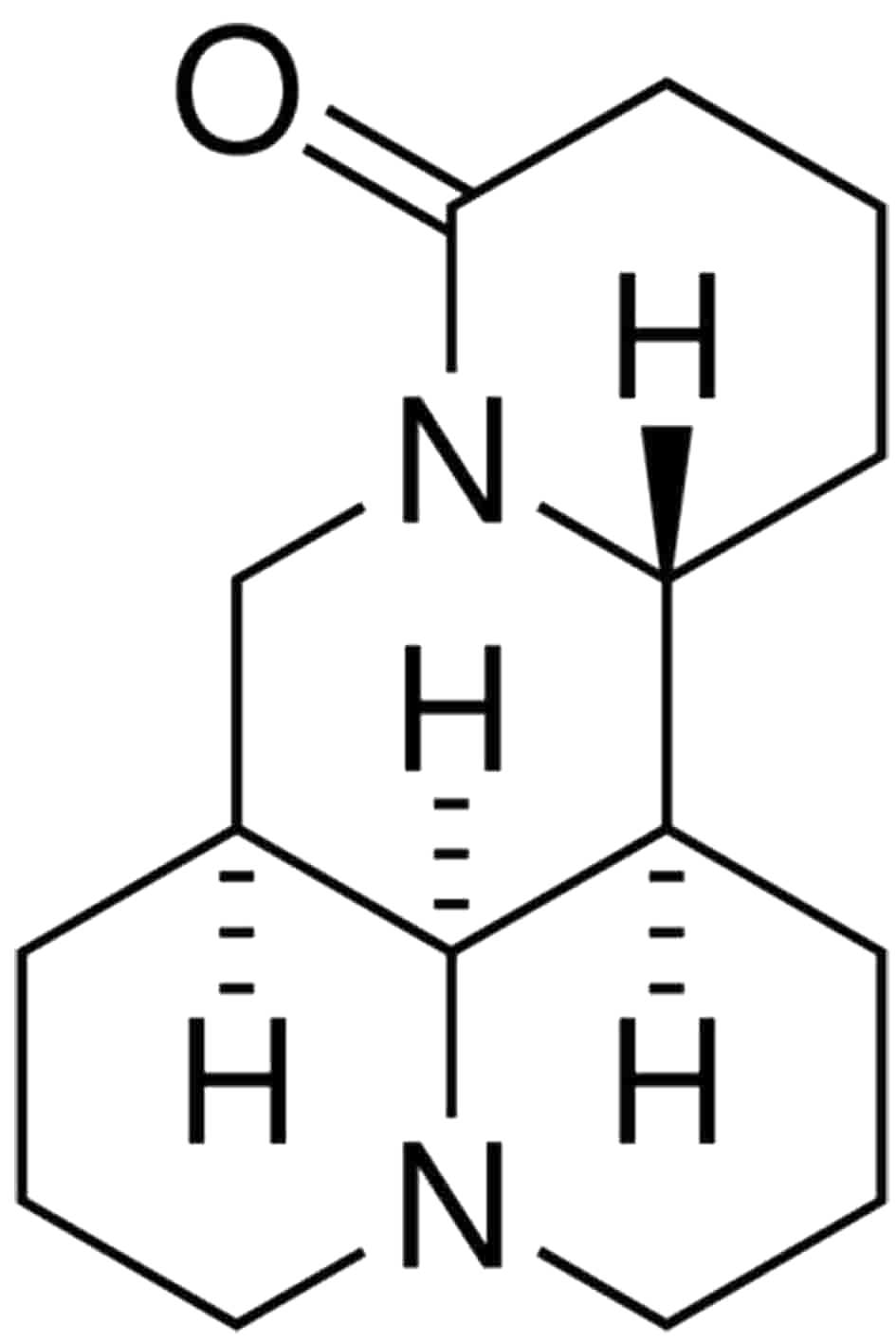
Matrine, the chemical structure of which is shown in
Fig. 1, was purchased from
Sigma-Aldrich (St Louis, MO, USA), with a purity of >98%, as
confirmed by high-performance liquid chromatography (HPLC). The
molecular formula of matrine is
C15H24N2O and its molecular weight
is 248.36. In the present study, matrine was dissolved in cell
culture medium at a stock concentration of 20 mg/ml and stored at
−20°C. The stock solution was freshly diluted in the medium prior
to being used in each experiment. Fetal bovine serum was purchased
from Zhejiang Tianhang Biological Technology Co., Ltd. (Hangzhou,
China). RPMI-1640 medium was bought from Keygen Biotechnology Co.,
Ltd. (Nanjing, China). Sodium dodecyl sulfate (SDS),
3-(4,5-dimethylthiazol2-yl)-2,5-diphenyltetrazolium bromide (MTT),
L-glutamine and Annexin V fluorescein isothiocyanate/propidium
iodide (Annexin V-FITC/PI) apoptosis detection kits were purchased
from Beijing Biosea Biotechnology Co., Ltd. (Beijing, China).
Antibodies specific for Bcl-2, Bax, cytochrome C (Cyto C) and
β-actin were obtained from R&D Systems Inc. (Minneapolis, MN,
USA). Anti-caspase-3 and -9 were purchased from Wuhan Boster
Bio-engineering Co., Ltd., (Wuhan, China) and the JC-1 probe was
from the Beyotime Institute of Biotechnology (Nantong, China).
Cell line and cell culture
The human colon cancer HT29 cell line was obtained
from the Department of Oncology (Zhongnan Hospital of Wuhan
University, Wuhan, China). Cells were grown in RPMI-1640 medium
supplemented with 10% heat inactivated fetal calf serum (FCS), 1%
L-glutamine and 1% penicillin-streptomycin in a humidified
atmosphere containing 5% CO2. The HT29 cells were grown
in a monolayer culture using 25-cm2 tissue culture
flasks and were periodically detached from the flask surface using
1% trypsin-ethylene-diamine tetraacetic acid (trypsin-EDTA)
solution. The cell counts were determined using a CC-108
microcellcounter (Sysmex, Kobe, Japan). Cells in the logarithmic
phase of growth were used for all studies described.
MTT assay
The MTT assay detects the reduction in MTT by
mitochondrial dehydrogenase to form a blue formazan product, which
reflects the normal functioning of mitochondria and hence the cell
viability. Subsequent to being incubated with matrine for 24, 36 or
48 h, in 96-well plates, the cells (104/well) were
washed twice with phosphate-buffered saline (PBS) and MTT (100
μg/0.1 ml PBS ) was added to each well. The cells were incubated at
37°C for 4 h. The formazan crystals were dissolved by adding 100 μl
DMSO and the absorbance was measured at 570 nm using a
spectrophotometer. The cell proliferation inhibition rate was
calculated as 1 - (average OD value of the wells with the
administered drug/average OD value of the control wells) × 100. The
proliferation response of the HT29 cells was determined by the MTT
assay as described previously. The experiments and all the
subsequent assays were repeated three times.
Cell cycle analysis
To analyze the cell cycle, the cells were collected
in the phase of logarithmic growth and the cell concentration was
adjusted to 1×106 cells/ml. The cells were treated with
various concentrations of matrine (4, 8 or 16 mg/ml). Following 24
h of treatment, the floating and attached cells were collected and
centrifuged, washed with cold PBS and fixed in 70% cold ethanol
overnight at 4°C. A fluorochrome solution containing 50 μg/ml PI,
3.4 mmol/l sodium citration, 20 μg/ml RNase A and 1% Triton X-100
was added and the mixture was incubated in the dark at room
temperature for 30 min. The distribution of the cell cycle was
measured using flow cytometry (FCM; Partec, Münster, Germany). FCM
analysis was performed using the Cell Quest software (Beckton
Dickinson and Company, Franklin Lakes, NJ, USA).
Annexin V-FITC/PI double staining for
FCM-assessed apoptosis
The Annexin-V-FITC/PI double staining assay was used
to detect cellular apoptosis. The HT29 cells were equally
distributed into culture flasks and treated with matrine
concentrations of 0, 4, 8 and 16 mg/ml, for 24 h. The cells were
collected, washed with cold PBS and resuspended at 1×106
cells/ml in Annexin-V binding buffer. The supernatant (100 μl/tube)
was incubated with 5 μl Annexin-V-FITC and 5 μl PI for 15 min at
room temperature in the dark. Binding buffer (400 μl) was then
added to each tube and followed by cytometric analysis within 1 h
of staining. All experiments were repeated three times.
Detection of Cyto C release from the
mitochondria to the cytosol
Cyto C determination in cytosolic and mitochondrial
fractions was detected using western blot analysis. The cells were
harvested following the respective treatments and washed once with
ice-cold PBS. In order to isolate the mitochondria and cytosol, the
cells were sonicated in buffer containing 10 mM Tris-HCl pH 7.5, 10
mM NaCl, 175 mM sucrose and 12.5 mM EDTA and the cell extract was
centrifuged at 1,000 × g for 10 min to pellet the nuclei. The
supernatant obtained was centrifuged at 18,000 × g for 30 min to
pellet the mitochondria and purified as previously described. The
resulting supernatant was termed the cytosolic fraction. The pellet
was lysed and the protein content was estimated in the two
fractions using Bradford’s method. Equal amounts of protein were
separated on 15% SDS-PAGE and electrotransferred to a
polyvinylidene fluoride (PVDF) membrane. The membrane was then
incubated in 5% skimmed milk in TBST [Tris-buffered saline (TBS)
composed of 10 mM Tris, 150 mM NaCl (pH 7.6), with 0.1% Tween 20]
for 2 h, followed by overnight incubation with the primary antibody
separately. The incubated membranes were extensively washed with
TBST prior to incubation for 2 h with the secondary antibody.
Subsequent to extensive washing with TBST, the immune complexes
were detected by an enhanced chemiluminescence (ECL) detection kit
(Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Western blot analysis
The effects of matrine on protein expression was
analyzed using western blot analysis. Following treatment with
matrine, the floating and adherent cells were lysed in buffer A [10
mM Tris-HCl (pH 7.6), 1 mM EDTA, 10% glycerol, 1 mg/ml leupeptin, 1
mg/ml pepstatin, 2 μg/ml aprotinin and 3.28 mg/ml PMSF] by three
consecutive 10-sec sonications with a Tekmar sonic disrupter
(Sigma-Aldrich) at power setting 60. Proteins were separated by
SDS-PAGE (5% stacking gel; 10% separating gel) and transferred to
nitrocellulose (Micron Separation Inc., Westborough, MA, USA) using
a semi-dry blotting apparatus (Bio-Rad, Hercules, CA, USA). The
membranes were incubated with 5% skimmed dry milk overnight at room
temperature. The membrane was then washed three times with TBST [10
mM Tris-HCl (pH 8.0), 150 mM NaCl and 0.5% Tween-20] for 5 min
each, at room temperature and then probed with an appropriate titer
of antibody (Cell Signaling, Beverly, MA, USA) that binds to Bcl-2,
Bax, caspase- 3 or -9 or β-actin, for 1 h at room temperature.
Subsequently, the membrane was washed as described previously and
further incubated with horseradish peroxidase-conjugated sheep
anti-mouse IgG (monoclonal primary antibodies) or goat anti-rabbit
IgG (poly-clonal primary antibodies; Amersham Pharmacia Biotech) at
room temperature for 1 h. The membrane was washed three times with
TBST and developed using the ECL kit (Amersham Pharmacia Biotech).
The intensity of the immunoreactive bands was quantified using a
densitometer (Molecular Dynamics, Sunnyvale, CA, USA).
Statistical analysis
The results were expressed as the mean ± standard
deviation (SD). An analysis of variance (ANOVA) and Dunnett t-test
were used to evaluate the statistical significance. P<0.05 was
considered to indicate a statistically significant difference.
Results
Inhibitory effect of matrine on the
growth of HT29 cells
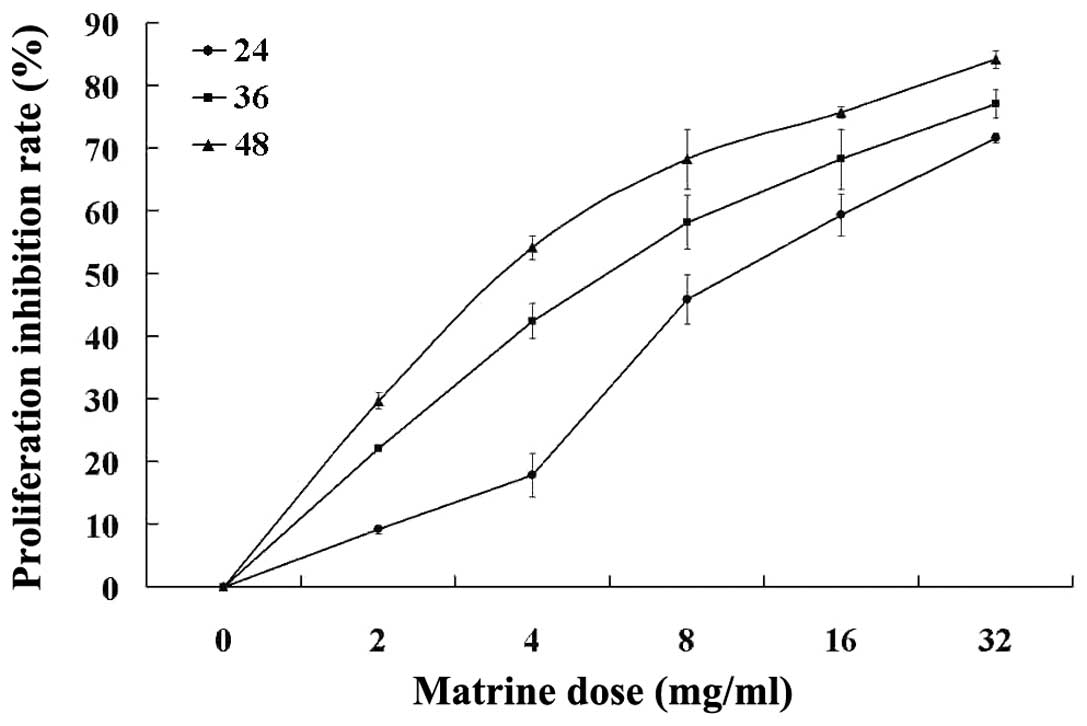
The anti-proliferative effect of matrine on the HT29
cells was detected by MTT assay. The results show that as
concentration increased, the proliferation of the HT29 cells was
markedly inhibited in a dose- and time-dependent manner by matrine
concentrations of 2–32 mg/ml for 24, 36 and 48 h in vitro
(P<0.05; Table I and Fig. 2).
 | Table IVarious concentrations of matrine
inhibit the proliferation of HT29 cells. |
Table I
Various concentrations of matrine
inhibit the proliferation of HT29 cells.
| Concentration
(ml/l) | 24 h | 36 h | 48 h |
|---|
| Control | 0.00±0.07 | 0.00±0.11 | 0.00±0.14 |
| 2 | 9.20±0.65a | 22.06±0.20a | 29.73±1.29a |
| 4 | 17.88±3.51a | 42.48±2.78a | 54.22±1.91a |
| 8 | 45.96±3.95b | 58.20±4.33b | 68.29±4.79b |
| 16 | 59.35±3.36b | 68.29±4.79b | 75.75±0.92b |
| 32 | 71.62±0.76b | 77.14±2.30b | 84.20±1.47b |
Matrine-induced
G0/G1 cell cycle arrest
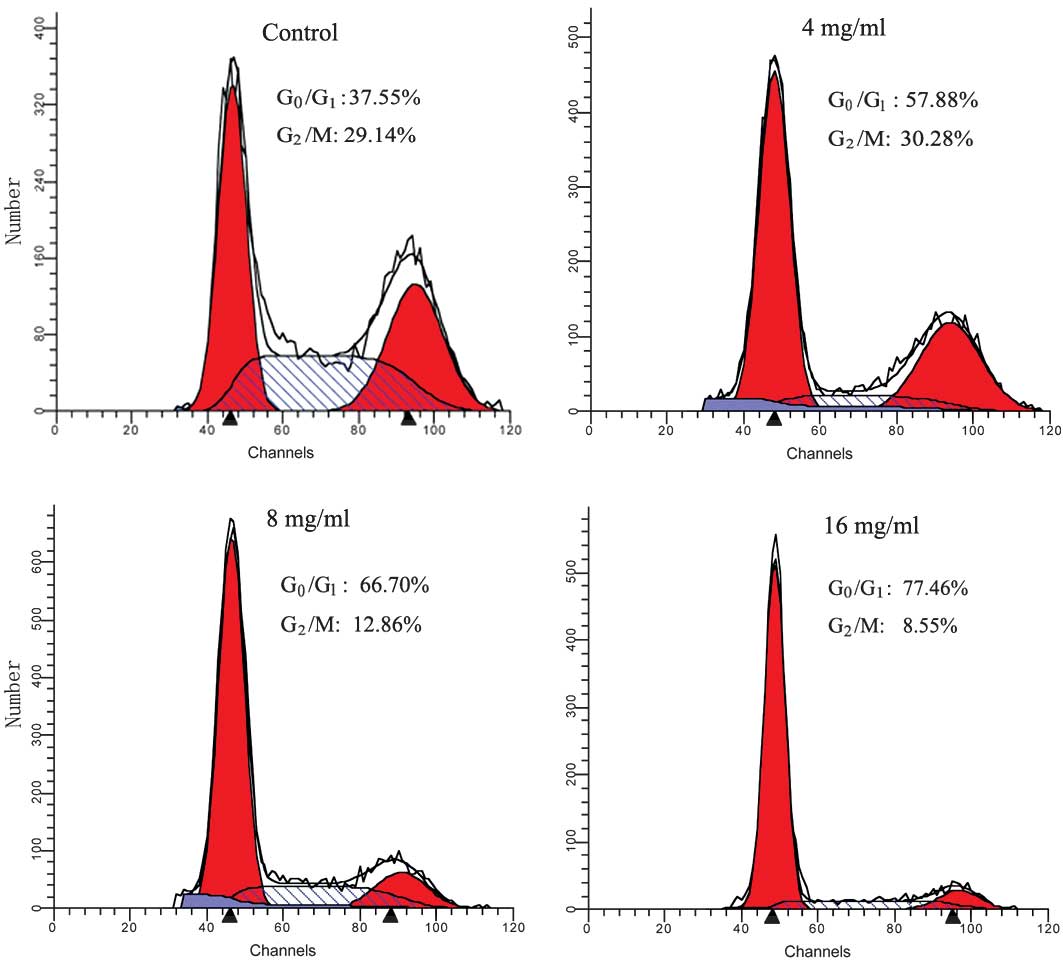
To examine the effect of matrine on cell cycle
progression, the cells that were untreated or treated with matrine
for the indicated concentrations (4, 8 or 16 mg/ml) and the cell
cycle distribution were analyzed using FCM. As shown in Fig. 3, matrine significantly increased the
number of cells in the G0/G1 phase and
decreased the number of cells in the G2/M phase in a
dose-dependent manner, indicating that matrine caused a growth
arrest of HT29 cells in the G0/G1 cell cycle
phase, leading to a depletion of S and G2/M phase
cells.
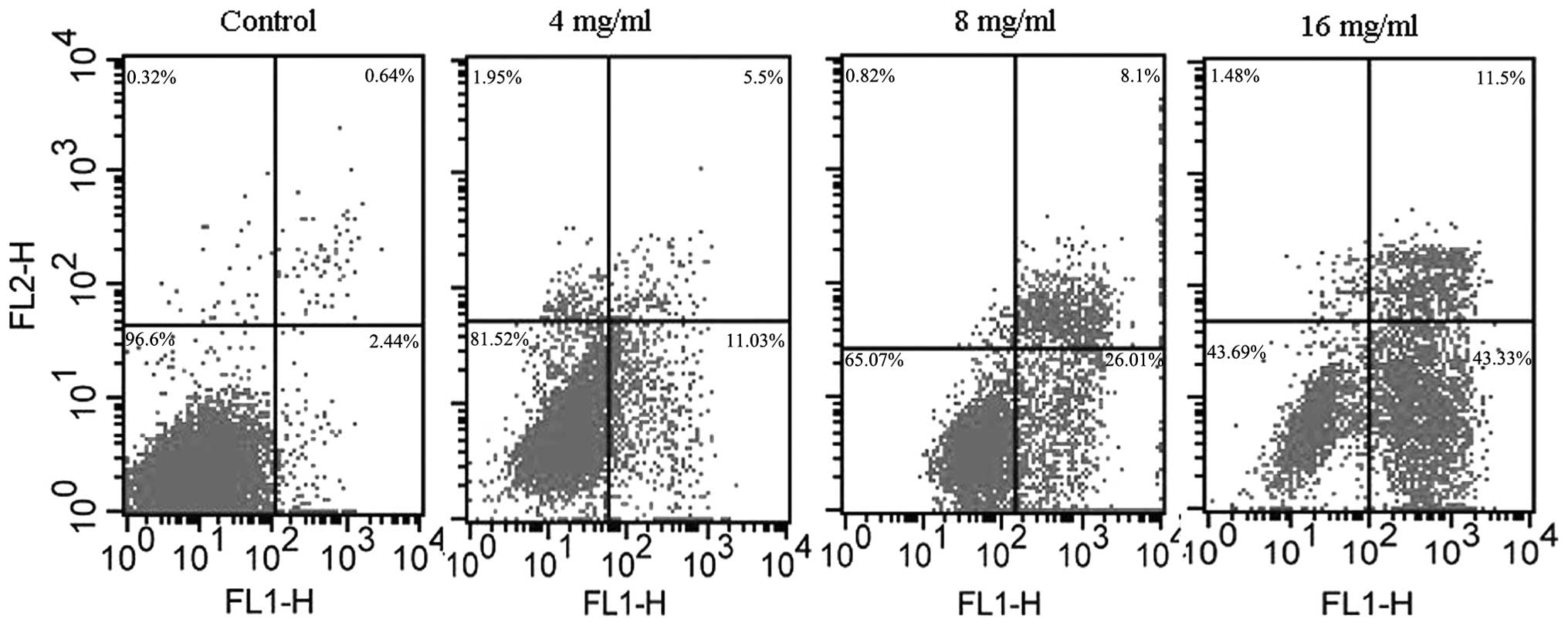
Detection of apoptosis
Perturbations in the cell membrane occur during the
early stages of apoptosis and lead to a redistribution of
phosphatidylserine to the external side of the cell membrane.
Annexin V selectively binds to phosphatidylserine and thus enables
the use of a fluorescein-labeled Annexin V kit to identify the
cells that are undergoing apoptosis (21). An Annexin-V-FITC/PI double staining
assay was performed to detect the apoptosis of the HT29 cells. The
cells were also stained with PI to distinguish early apoptotic
cells from necrotic cells. A FACScan flow cytometer collected
10,000 events. The percentage of live, dead and apoptotic cells was
determined as described in the methods and shown in Fig. 4. The viable cells are located in the
lower left corner (negative in Annexin V-FITC and PI). Early
apoptotic cells are in the lower right corner (Annexin V-FITC
positive). Late apoptotic cells showing signs of progressive
cellular membrane and nuclear damage are in the upper right corner
(double positive).
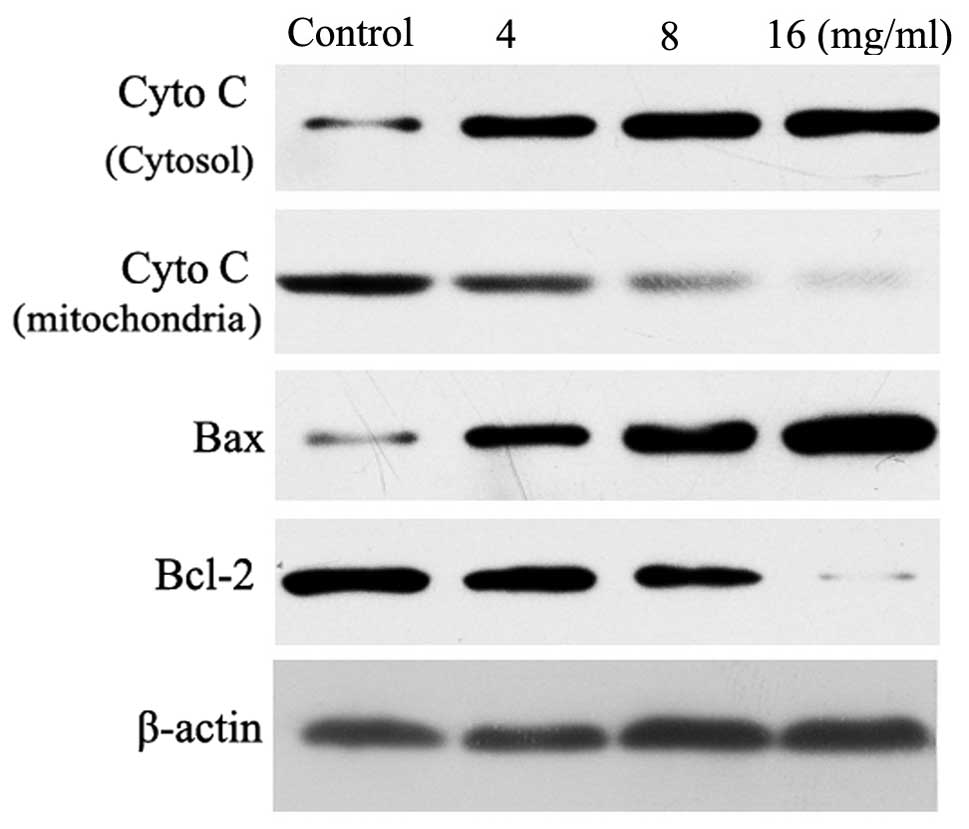
Cyto C release from mitochondria to the
cytosol
In response to certain apoptotic stimuli, Cyto C is
released from the mitochondria to the cytoplasm, where it combines
with Apaf-1 to promote apoptosome assembly and caspase-9
activation. The release of Cyto C from the mitochondria is a
critical step in the apoptotic cascade, since this activates
downstream caspases. Therefore, western blotting was performed in
the cytosolic and mitochondrial fractions to investigate the
release of Cyto C in the matrine-treated HT29 cells. As shown in
Fig. 5, the results demonstrate a
concentration-dependent increase in the cytosolic Cyto C levels
following treatment with matrine. Simultaneously, a decrease in
Cyto C was observed in the mitochondrial fraction.
Expression of Bax and Bcl-2 protein
To understand the anti-proliferative mechanisms of
matrine in human colon cancer HT29 cells, the expression of
apoptotic-related proteins were investigated (Fig. 5). Western blot analysis confirmed
that matrine at concentrations of 4–16 mg/ml dose-dependently
downregulated Bcl-2 protein and upregulated Bax protein, eventually
leading to a reduction in the ratio of Bcl-2/Bax protein. Studies
have shown that Bcl-2 and its dominant inhibitor, Bax, are key
regulators of cell proliferation and apoptosis (22,23).
The overexpression of Bcl-2 enhances cell survival by suppressing
apoptosis, but the overexpression of Bax accelerates cell death.
The induction of apoptosis, cell cycle arrest and a decrease in the
ratios of Bcl-2/Bax protein caused by matrine may be significant
matrine anti-proliferative mechanisms against cancer cells.
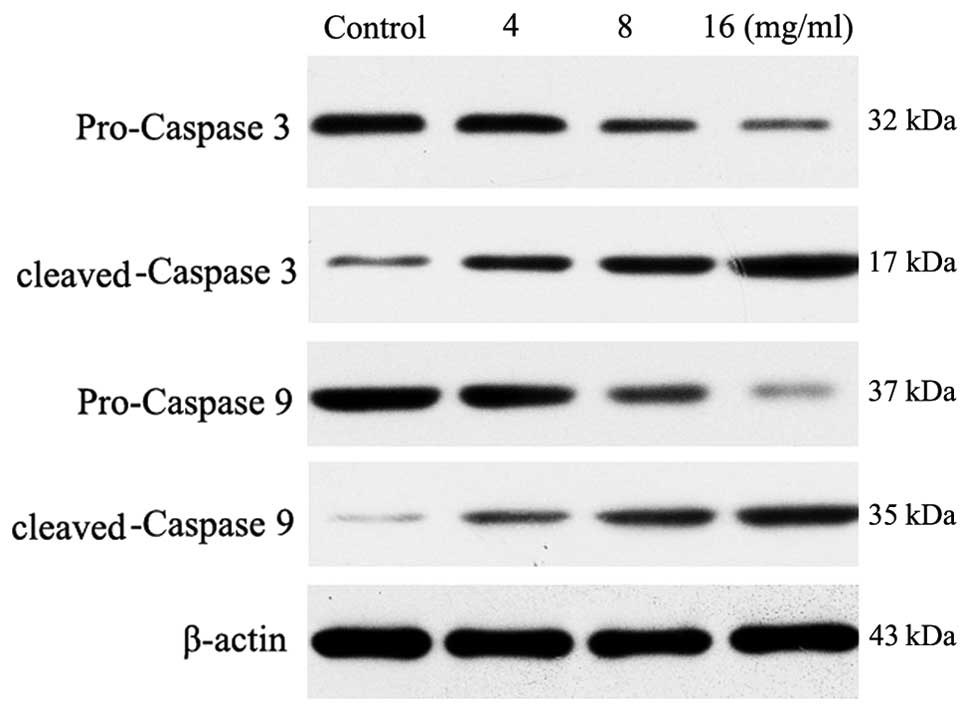
Activation of caspase-9 and -3
Western blot analysis of cleaved and full length
caspase-9 revealed that matrine induced a gradual increase in
caspase-9 processing with various matrine concentrations at 4, 8 or
16 mg/ml for 24 h following treatment (Fig. 6). The amount of cleaved caspase-9
fragments then reached a plateau at the dose of 16 mg/ml. The
appearance of processed fragments was accompanied by a decrease in
the full-length enzyme. No processed fragments were detected in the
control extracts. Processing of caspase-3, an effecter caspase that
is directly activated by caspase-9, was also examined. The 17 kDa
cleaved caspase-3 fragments were detected at 24 h following matrine
treatment and an additional increase in the amount of fragments was
observed.
Discussion
Cancer chemoprevention is defined as inhibiting,
delaying or reversing the carcinogenic process using non-toxic
chemicals, and is considered to be a promising strategy for
controlling cancer progression. A variety of chemical compounds
have been reported to protect against chemical carcinogenesis and
thus, are considered to be cancer chemopreventive agents. Among
these, matrine is a promising phytochemical agent that has
attracted interest due to its cancer chemopreventive activity in
multistage carcinogenesis. Matrine is a naturally occurring
polyphenolic phytoalexin, which has been demonstrated to display
cancer chemopreventive activity in in vivo animal
experiments. It was first isolated and identified in 1958 from
Sophora flavescens Ait and has been widely used in China as
a therapeutic agent against cancer. Matrine has been considered as
a good and convenient Chinese herbal preparation with low toxicity
and few side-effects. It has also been administered to children and
infants. However, to the best of our knowledge, the effect of
matrine on pancreatic cancer has not been previously reported
(24,25).
Uncontrolled proliferation is a significant
biological feature of cancer cells, and inhibiting cell
proliferation may achieve the arrest of tumor growth (17,26).
The present study investigated whether matrine was able to reduce
the proliferation rate of tumor cells. As shown in the MTT assay,
the growth of the HT29 cells was inhibited in a dose- and
time-dependent manner when treated with 4–16 mg/ml matrine. FCM
revealed that matrine markedly arrested the HT29 cells in the
G0/G1 phase of the cell cycle (Fig. 3), indicating that retardation of
cell cycle progression may be a mechanism that underlies the
anti-proliferative effect of matrine.
Apoptosis is not only a significant phenomenon in
normal cells, but it is also associated with the development of
numerous diseases. An imbalance of apoptosis and proliferation is a
significant cause for the development and progression of a tumor.
The inhibition of proliferation and the induction of apoptosis in
tumor cells are main treatment strategies in combined anticancer
therapy (27–33) To determine the anticancer mechanisms
of matrine, the pro-apoptotic properties of the compound were
assessed in the present study. The results from the Annexin
V-FITC/PI double staining suggested that matrine was able to induce
apoptosis in a dose-dependent manner in the range of 4–16 mg/ml.
The results of the present study suggest that matrine, by a
reduction in tumor cell proliferation combined with an induction of
tumor cell apoptosis, may be useful for the two aspects of the
anticancer treatment strategy.
Since the mechanisms through which these compounds
induce cell death are not completely understood, the changes in the
expression and localization of several apoptosis-related proteins
were examined in HT29 cells in the present study.
The Bcl-2 family of proteins, including Bcl-2 and
Bax, function to control cell proliferation, differentiation and
programmed cell death, and consist of pro- and anti-apoptotic
family members. One of the main regulatory steps of apoptotic cell
death is controlled by the ratio of anti- and pro-apoptotic members
of the Bcl-2 family of proteins, which determines the
susceptibility to apoptosis. Bax, a pro-apoptotic factor of the
Bcl-2 family, is located in a monomeric form in the cytosol or
loosely attached to the membranes under normal conditions.
Following a death stimulus, cytosolic and monomeric Bax translocate
to the mitochondria, where they become integral membrane proteins,
which are able to cross-link as homodimers, allowing for the
release of factors from the mitochondria, including Cyto C, to
propagate the apoptotic pathway. Bcl-2 and its associated proteins
control the release of Cyto C from the mitochondria. Hallmarks of
the apoptotic process include the activation of cysteine proteases,
which represent initiators and executors of cell death. In the
cytosol, Cyto C activates caspase-9, which in turn activates
effector caspases, including caspase-3 (34–37).
In the present study, matrine reduced the
anti-apoptotic/pro-apoptotic Bcl-2/Bax ratio. In addition to this,
matrine caused the release of Cyto C from the mitochondria and
subsequently increased caspase-3 activity. Caspase-3 is synthesized
as a 32-kDa inactive precursor, which is proteolytically cleaved to
produce a mature enzyme composed of 17-kDa fragments. In the
present study, the procaspase-3 protein was reduced and the
caspase-3 in a cleaved form was increased in the apoptotic HT29
cells following treatment with matrine at concentrations of 4–16
mg/ml (38,39).
In summary, the present results demonstrated that
matrine was able to suppress HT29 cell proliferation and induce
apoptosis and cell cycle arrest at the G0/G1
phase by targeting the Cyto C, caspase-9, caspase-3, Bcl-2 and Bax
mitochondrial apoptotic pathway. These findings suggest that
matrine may have wide therapeutic and/or adjuvant therapeutic
application in the treatment of human colon cancer.
Acknowledgements
This study was supported by the Hubei Provincial
Natural Scientific Foundation of China (grant no.
2010CDB071401).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Siegel R, DeSantis C, Virgo K, Stein K,
Mariotto A, Smith T, et al: Cancer treatment and survivorship
statistics, 2012. CA Cancer J Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
4
|
Bosetti C, Levi F, Rosato V, et al: Recent
trends in colorectal cancer mortality in Europe. Int J Cancer.
129:180–191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Møller H, Sandin F, Robinson D, et al:
Colorectal cancer survival in socioeconomic groups in England:
variation is mainly in the short term after diagnosis. Eur J
Cancer. 48:46–53. 2012.PubMed/NCBI
|
|
6
|
Malvezzi M, Arfé A, Bertuccio P, et al:
European cancer mortality predictions for the year 2011. Ann Oncol.
22:947–956. 2011.PubMed/NCBI
|
|
7
|
Kopetz S, Chang GJ, Overman MJ, et al:
Improved survival in metastatic colorectal cancer is associated
with adoption of hepatic resection and improved chemotherapy. J
Clin Oncol. 27:3677–3683. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coleman MP, Quaresma M, Berrino F, Lutz
JM, De Angelis R, et al; CONCORD Working Group. Cancer survival in
five continents: a worldwide population-based study (CONCORD).
Lancet Oncol. 9:730–756. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jayne DG, Thorpe HC, Copeland J, et al:
Five-year follow-up of the Medical Research Council CLASICC trial
of laparoscopically assisted versus open surgery for colorectal
cancer. Br J Surg. 97:1638–1645. 2010.
|
|
10
|
Puls R, Langner S, Rosenberg C, et al:
Laser ablation of liver metastases from colorectal cancer with MR
thermometry: 5-year survival. J Vasc Interv Radiol. 20:225–234.
2009.PubMed/NCBI
|
|
11
|
Jung KW, Park S, Kong HJ, et al: Cancer
statistics in Korea: incidence, mortality, survival, and prevalence
in 2008. Cancer Res Treat. 43:1–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li DR and Lin HS: Safety and effectiveness
of large dose compound Sophora flavescens Ait injection in
the treatment of advanced malignant tumors. Zhonghua Zhong Liu Za
Zhi. 33:291–294. 2011.(In Chinese).
|
|
13
|
Lin Z, Huang CF, Liu XS and Jiang J: In
vitro anti-tumour activities of quinolizidine alkaloids derived
from Sophora flavescens Ait. Basic Clin Pharmacol Toxicol.
108:304–309. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meng XH, Lu CF, Zang GH, et al:
Spermicidal effect of alcohol extracts from different ratios of
Sophora flavescens Ait/Chinese Bulbul in vitro. Zhonghua Nan
Ke Xue. 18:83–87. 2012.(In Chinese).
|
|
15
|
Chen S, Peng X and Liu J: Effect of
Sophora flavescens Ait on cultured beating myocardial cells
of Coxsackie B3 virus infected newborn rat. Zhonghua Shi Yan He Lin
Chuang Bing Du Xue Za Zhi. 14:137–140. 2000.(In Chinese).
|
|
16
|
Sun M, Cao H, Sun L, Dong S, et al:
Antitumor activities of kushen: literature review. Evid Based
Complement Alternat Med. 2012:3732192012.PubMed/NCBI
|
|
17
|
Liu T, Song Y, Chen H, et al: Matrine
inhibits proliferation and induces apoptosis of pancreatic cancer
cells in vitro and in vivo. Biol Pharm Bull. 33:1740–1745. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu HB, Zhang HF, Li DY, et al: Matrine
inhibits matrix metalloproteinase-9 expression and invasion of
human hepatocellular carcinoma cells. J Asian Nat Prod Res.
13:242–250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li H, Tan G, Jiang X, et al: Therapeutic
effects of matrine on primary and metastatic breast cancer. Am J
Chin Med. 38:1115–1130. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Taylor LA, Pletschen L, Arends J, et al:
Marine phospholipids - a promising new dietary approach to
tumor-associated weight loss. Support Care Cancer. 18:159–170.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu R, Zhang Y, Ye X, et al: Inhibition
effects and induction of apoptosis of flavonoids on the prostate
cancer cell line PC-3 in vitro. Food Chem. 138:48–53. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thomas S, Quinn BA, Das SK, et al:
Targeting the Bcl-2 family for cancer therapy. Expert Opin Ther
Targets. 17:61–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Low IC, Kang J and Pervaiz S: Bcl-2: a
prime regulator of mitochondrial redox metabolism in cancer cells.
Antioxid Redox Signal. 15:2975–2987. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mukhtar H: Chemoprevention: making it a
success story for controlling human cancer. Cancer Lett.
326:123–127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adhami VM and Mukhtar H: Human cancer
chemoprevention: hurdles and challenges. Top Curr Chem.
329:203–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Naviglio S, Caraglia M, Abbruzzese A, et
al: Protein kinase A as a biological target in cancer therapy.
Expert Opin Ther Targets. 13:83–92. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rasheva VI and Domingos PM: Cellular
responses to endoplasmic reticulum stress and apoptosis. Apoptosis.
14:996–1007. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ghobrial IM, Witzig TE and Adjei AA:
Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin.
55:178–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Circu ML and Aw TY: Reactive oxygen
species, cellular redox systems, and apoptosis. Free Radic Biol
Med. 48:749–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eisenberg-Lerner A, Bialik S, Simon HU and
Kimchi A: Life and death partners: apoptosis, autophagy and the
cross-talk between them. Cell Death Differ. 16:966–775. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brunelle JK and Letai A: Control of
mitochondrial apoptosis by the Bcl-2 family. J Cell Sci.
122:437–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cotter TG: Apoptosis and cancer: the
genesis of a research field. Nat Rev Cancer. 9:501–507. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen HW and Huang HC: Effect of curcumin
on cell cycle progression and apoptosis in vascular smooth muscle
cells. Br J Pharmacol. 124:1029–1040. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang C and Youle RJ: The role of
mitochondria in apoptosis. Annu Rev Genet. 43:95–118. 2009.
View Article : Google Scholar
|
|
35
|
Kang MH and Reynolds CP: Bcl-2 inhibitors:
targeting mitochondrial apoptotic pathways in cancer therapy. Clin
Cancer Res. 15:1126–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chipuk JE, Moldoveanu T, Llambi F, et al:
The BCL-2 family reunion. Mol Cell. 37:299–310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Autret A and Martin SJ: Emerging role for
members of the Bcl-2 family in mitochondrial morphogenesis. Mol
Cell. 36:355–363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shamas-Din A, Kale J, Leber B and Andrews
DW: Mechanisms of Action of Bcl-2 Family Proteins. Cold Spring Harb
Perspect Biol. 5:a0087142013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Weyhenmeyer B, Murphy AC, Prehn JH and
Murphy BM: Targeting the anti-apoptotic Bcl-2 family members for
the treatment of cancer. Exp Oncol. 34:192–199. 2012.PubMed/NCBI
|