Introduction
Esophageal cancer may result in stenosis and
obstruction or a fistula combined with stenosis (1). Older patients who decline to have
surgery and patients with post-operative stenosis comprise ~50% of
all patients with advanced esophageal cancer (2–4).
Dysphagia is the predominant symptom exhibited by patients with
inoperable esophageal cancer. To relieve the dysphagia and improve
the quality of life of patients with esophageal cancer, stent
placement is a widely-accepted option for palliation of the
symptoms that are caused by esophageal strictures (5–8).
The conventional metal stent only provides
palliative treatment through mechanical support to improve the
eating ability of a patient. In recent years, an esophageal stent
loaded with iodine 125 (125I) seeds has been developed
(9,10). This form of irradiation stent
inhibits tumor growth by administering continuous low-dosage
irradiation from the 125 seeds. However, the
125I seeds must be installed in the esophageal stent
every time. To make the treatment easier to use, the iodine-eluting
esophageal stent was developed. This is a new type of esophageal
nitinol stent with a polyurethane membrane uniformly covered with
125I. In the present study, the conventional stent alone
was compared with the iodine-eluting stent in treating malignant
esophageal strictures in esophageal cancer.
Materials and methods
Patients
A total of 71 consecutive patients with malignant
esophageal strictures were enrolled for selective intraluminal
stent placement between April 2008 and December 2010. However, four
patients were lost to follow-up. The specific inclusion criteria
for stent placement was as follows: i) A histopathological
diagnosis using an endoscopic biopsy confirming that the tumor was
esophageal cancer; ii) tumor invasion or compression resulting in
esophageal luminal stenosis or occlusion; iii) an expected survival
time of more than one month; iv) physical fitness (Karnofsky) score
≥50; v) no serious heart, lung, hematological, nervous system,
liver or kidney dysfunction; and vi) no acute infection. No
patients received chemotherapy or radiotherapy treatment prior to,
concurrently with or following stent placement. Approval for the
study was obtained from the ethics committee of Tianjin Cancer
Institute and Hospital, and informed consent was obtained from all
patients. The exclusion criteria included acute infection, severe
cardiovascular or mental illness and evidence of multiple
small-bowel obstructions. Four patients were lost to follow-up. The
remaining patients were randomly assigned into two groups, those
who received a conventional stent (group A; n=36) and those who
received an iodine-eluting esophageal stent (group B; n=31). No
significant differences were observed in gender, age, vital signs
or pain and dysphagia grades between the two groups prior to the
stent placement (Table I).
 | Table IBackground characteristics of patients
prior to stent placement. |
Table I
Background characteristics of patients
prior to stent placement.
| Characteristic | Group A | Group B | P-value |
|---|
| Age (years)a | 71.26±8.93 | 68.13±10.44 |
0.191b |
| Gender | | |
0.529c |
| Male | 28 | 26 | |
| Female | 8 | 5 | |
| Heart rate
(bpm)a | 77.40±7.04 | 75.03±9.17 |
0.241b |
| Respiratory rate
(bpm)a | 18.61±1.63 | 18.06±1.77 |
0.192b |
| SBP (mmHg)a | 118.67±17.83 | 117.74±12.19 |
0.803b |
| DBP (mmHg)a | 74.56±8.72 | 76.35±9.34 |
0.418b |
| Temperature
(ºC)a | 36.49±0.43 | 36.60±0.26 |
0.235b |
| Dysphagia grade | | |
0.327d |
| 1 | 2 | 0 | |
| 2 | 4 | 3 | |
| 3 | 25 | 23 | |
| 4 | 5 | 5 | |
| Pain grade, n
(%) | | |
0.070d |
| 0 | 11 (52.4) | 17 (77.3) | |
| I | 9 (42.9) | 5 (22.7) | |
| II | 1 (4.8) | 0 (0.0) | |
| III | 0 (0.0) | 0 (0.0) | |
Stent
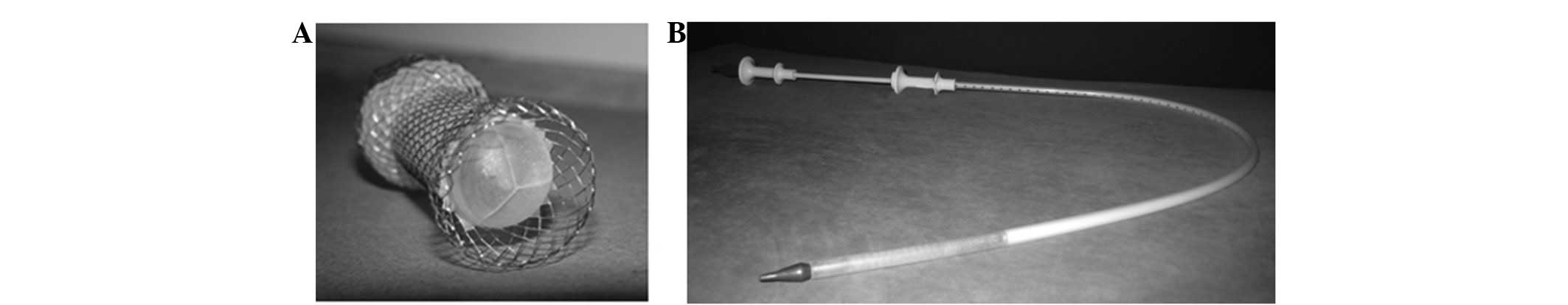
The iodine-eluting esophageal stent was composed of
two parts: An esophageal nitinol stent and a polyurethane membrane
that was uniformly covered with 125I. All esophageal
stents were produced by Anhui Jinmin Medical Instruments Co., Ltd.
(Tianchang, Anhui, China; Fig. 1).
The half-life of the 125I seeds was 59.6 days. The
radiation dose was determined on the basis of the size of the
individual tumor, according to clinical studies, and the activity
for clinical use was 5–13.5 mCi (9–10).
Stent placement
Prior to stent placement, the site, degree and
length of the obstruction were assessed using a conventional upper
gastrointestinal (GI) endoscope, computerized tomography (CT)
and/or a water-soluble contrast fluoroscopic study. The type, size
and length of the stent were chosen according to the measured
length of the obstruction. The length of the stent was chosen to be
at least an additional 2 cm on either side of the proximal and
distal extent of the strictures or fistula. The two types of stents
were placed in the same way and the esophagus was able to
selectively expand according to the extent of the esophageal
strictures.
It should be noted that nickel-titanium shape-memory
alloy stents will soften when encountering cold temperatures. The
temperature of the stent should be >37ºC for one week in order
to allow it to form a shape. After one week, the stent does not
change in response to cold temperatures. To avoid the shifting or
dropping of the stent, cold or rough foods were prohibited for one
week following the stent placement.
Observation
The patients from groups A and B underwent an
esophagography 1–3 days after the stent placement in order to
verify the position and patency of the stent. The patients were
instructed not to eat solid food until the stent had fully
expanded. Following the procedure, routine blood tests, thyroid
function examinations, barium meal tests, endoscopies, chest CT
scans and plain X-rays were ordered at regular intervals to check
for complications.
The scores of the dysphagia and pain grades and the
condition of the granulation tissue were recorded for all patients
pre- and post-stent placement. To assess the clinical improvement,
the dysphagia score prior to and following the procedure was graded
on a scale of 0–4, according to the CIRSE guidelines (11) as follows: Grade 0, normal diet;
grade 1, ability to swallow solid food; grade 2, ability to swallow
semi-solids only; grade 3, ability to swallow liquids only; and
grade 4, complete dysphagia. The pain score was graded on a scale
of 0-III as follows: 0, feeling no pain; I, mild pain and
uninterrupted sleep; II, moderate pain that is not tolerable and
requires pain medication and sleep disturbance; and III, severe
pain and severe sleep disturbance that requires the treatment to be
stopped. The condition of the granulation tissue was graded as no
proliferation, mild proliferation or significant proliferation.
Statistical analysis
The statistical analyses were performed using SAS
software, version 9.0 (SAS Institute, Cary, NC, USA). The numeric
data of the ages of the patients were examined using the Student’s
t test, whereas other characteristics of the patients prior to the
stent placement were analyzed using the χ2 test. The
comparison of the side-effects and complications associated with
the stent placement between the two groups was also analyzed using
the χ2 test. The log-rank test was used for the
evaluation of the patient survival time. P<0.05 was considered
to indicate a statistically significant difference.
Results
Relief of esophageal obstruction
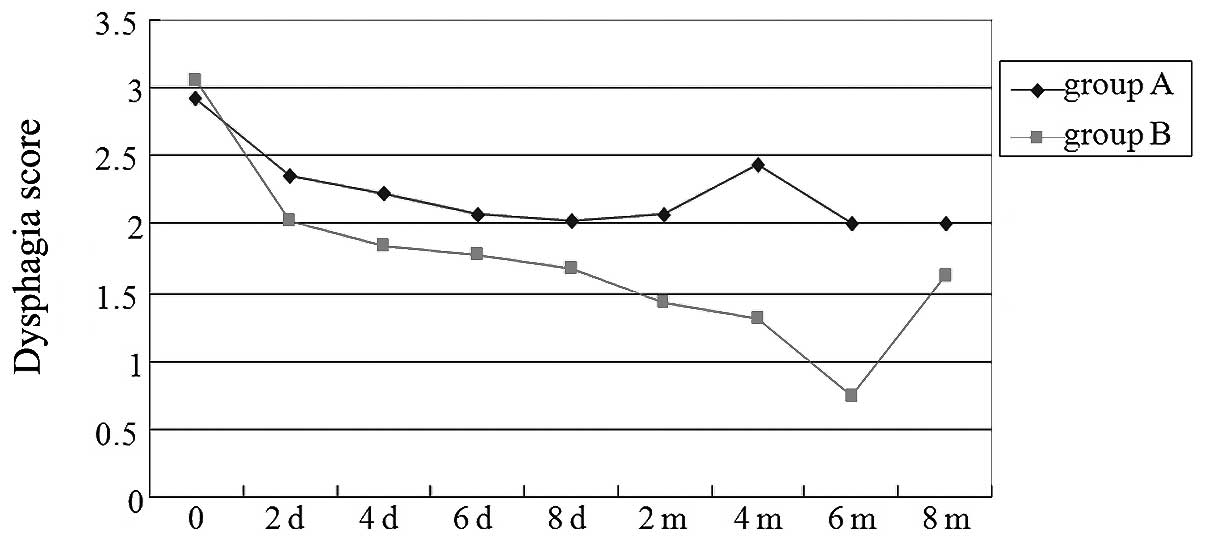
The dysphagia scores improved in groups A and B, and
eight days after the stent placement, there were no significant
differences between the two groups (P=0.212, Student’s t-test;
Fig. 2) At two months
post-procedure, the mean value that the dysphagia score had
decreased by was 0.83 in group A and 1.65 in group B, and a
significant difference was observed between the two groups
(P=0.002; Student’s t-test). At two months after the stent
placement, 16 patients in group A and 11 in group B were evaluated
using a gastroendoscopy examination. Hyperplasia of the granulation
tissue was noted at each end of the stent, but particularly the
proximal end, in all 27 patients. Hyperplasia of the granulation
tissue was more evident in the patients in group A than in those of
group B (P=0.007; Cochran-Mantel-Haenszel test).
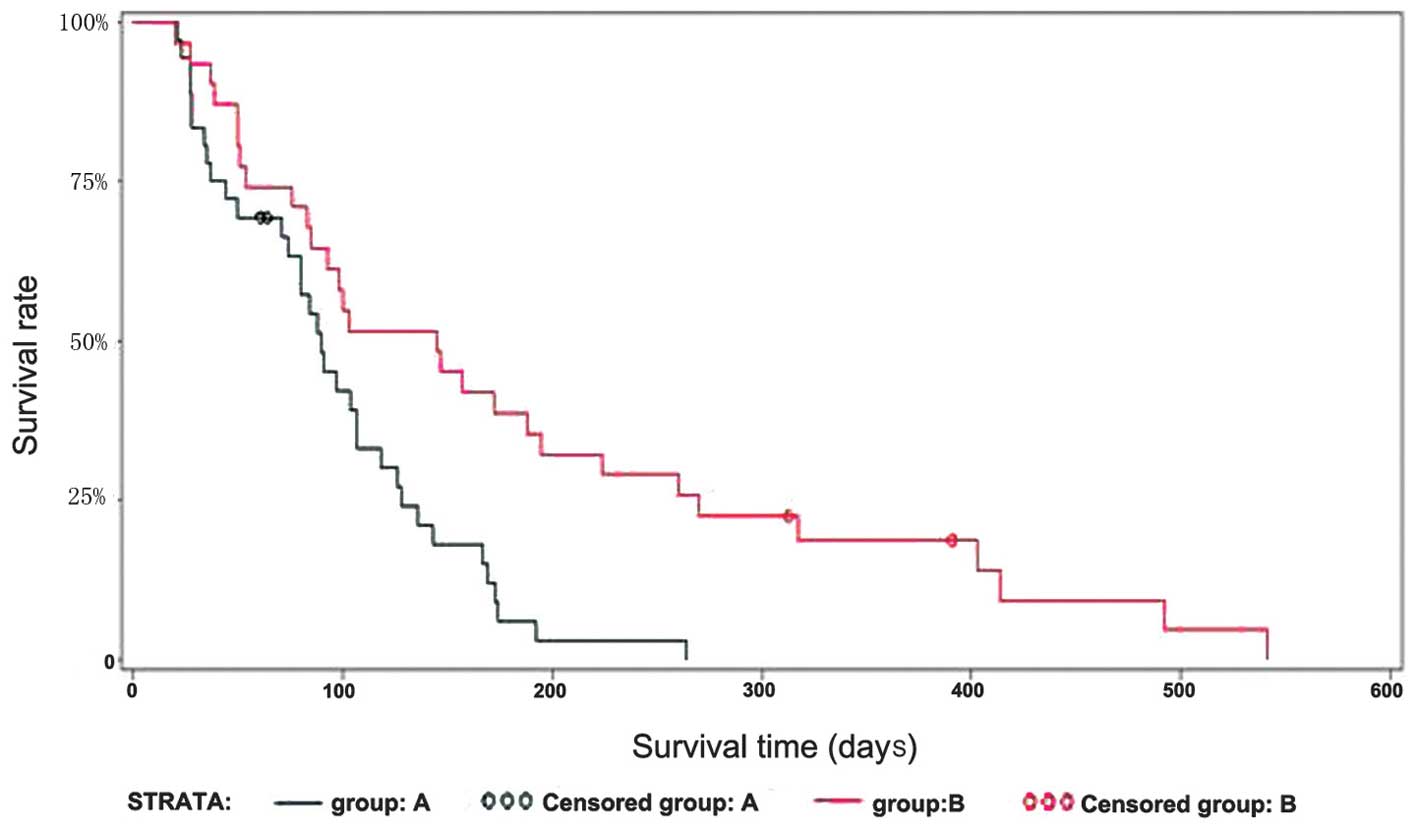
Survival
The median survival time was longer for group B
patients than group A patients [145 days (95% CI, 85–195) vs. 90
days (95% CI, 74–107), respectively], which demonstrated a
significant difference between the two groups (P=0.0022, log-rank
test; Fig. 3).
Side-effects, complications and security
assessment
No severe procedure-related complications occurred
in any of the cases. Severe complications occurred in 11 patients
in group A (28.9%) and 4 in group B (12.1%), and no significant
differences were identified between the two groups (P=0.144;
Fisher’s exact test). The majority of patients felt pain in the
rear of the sternum following the stent placement and 25 patients
(16 patients in group A and nine patients in group B) complained of
severe chest pain, which was palliated using narcotic analgesics.
Only one patient felt severe pain and ceased stent treatment. The
degree of chest pain between the two groups was not significantly
different. Tracheoesophageal fistulae occurred in three patients
(two patients in group A and one in group B). Hemorrhages occurred
in seven patients (five patients in group A and two in group B),
but no patients succumbed due to an acute massive hemorrhage.
Prior to the stent placement, routine blood tests
and thyroid function examinations were performed on the patients in
groups A and B and there were no significant differences at this
point or at two months post-procedure between the two groups
(Table II).
 | Table IIBlood regular and thyroid function
examination at two months post-stent insertion |
Table II
Blood regular and thyroid function
examination at two months post-stent insertion
| Examination | Group A | Group B | P-value |
|---|
| Hg (g/l)a | 117.82±14.53 | 127.44±31.50 |
0.398b |
| WBC
(x109/l)a | 7.81±2.00 | 7.90±2.89 |
0.924b |
| Plt
(x109/l)a | 278.59±84.56 | 295.00±127.57 |
0.697b |
| TT3 | | |
0.580c |
| Normal | 5 | 5 | |
| Abnormal, no
clinical significance | 4 | 1 | |
| Abnormal, clinical
significance | 0 | 0 | |
| TT4 | | |
1.000c |
| Normal | 9 | 6 | |
| Abnormal, no
clinical significance | 0 | 0 | |
| Abnormal, clinical
significance | 0 | 0 | |
| TSH | | |
0.967d |
| Normal | 14 | 7 | |
| Abnormal, no
clinical significance | 0 | 1 | |
| Abnormal, clinical
significance | 1 | 0 | |
| FT4 | | |
1.000c |
| Normal | 10 | 7 | |
| Abnormal, no
clinical significance | 1 | 0 | |
| Abnormal, clinical
significance | 0 | 0 | |
| FT3 | | |
1.000c |
| Normal | 7 | 4 | |
| Abnormal, no
clinical significance | 4 | 2 | |
| Abnormal, clinical
significance | 0 | 0 | |
Discussion
Since malignant esophageal cancer has no specific
symptoms in its early stage, 60–80% of esophageal cancers are
diagnosed at the middle or advanced stage of the disease (12). Surgery is not a viable option for
these patients, but a metal stent may be used as a treatment option
to relieve the dysphagia, thus improving the quality of life of the
patient. Compared with other treatments for esophageal strictures,
the metal stent placement procedure has shown favorable
characteristics. The procedure is relatively simple, rapidly
effective and generally well-tolerated (13,14).
Conventional stent placement alone does not offer
any therapeutic effects on the esophageal cancer itself and is used
only for mechanical support and obstruction relief. Certain studies
have shown that a self-expandable stent loaded with 125I
seeds is a safe and effective treatment for esophageal cancer
(9,10). However, the 125I seeds
must be installed in the esophageal stent every time the procedure
is performed. Furthermore, although the 125I seeds are
uniformly placed in the stent, the irradiation caused by the seeds
is not distributed evenly. To overcome these shortcomings, the
iodine-eluting esophageal stent was developed.
In the present study, the dysphagia score was
improved greatly in groups A and B, and eight days following the
stent placement, there were no significant differences between the
two groups. At two months post-procedure, the mean value that the
dysphagia score decreased by was 0.83 in group A and 1.65 in group
B, and a significant difference was observed between the two
groups. The dysphagia score improved significantly in group B. The
reason for restenosis may be due to the tumor tissue and/or
hyperplasia of the granulation tissue growing into the stent from
the superior margin at the two ends of the stent. A total of 16
patients in group A and 11 in group B underwent a gastroendoscopy
examination at two months post-procedure. Restenosis due to the
regrowth of the tumor tissue into the stent did not occur in any of
the patients. However, hyperplasia of the granulation tissue was
noted at each end of the stent, particularly at the proximal end,
in all 27 patients. Hyperplasia of the granulation tissue was more
evident in patients of group A than those of group B. The
endoscopic examinations demonstrated that the iodine-eluting
esophageal stent had partial inhibitory effects on the tumor and
the hyperplasia of the granulation tissue. Furthermore, there was a
significant improvement in the survival of the patients, with a
median survival time of 145 days in group B vs. 90 days in group A.
This difference was statistically significant, indicating the
therapeutic advantages of the iodine-eluting esophageal stent.
The possible complications following the
implantation of the esophageal stent include hemorrhage,
perforation and tracheoesophageal fistulae (15–17).
Severe complications occurred in 11 patients in group A (35.5%) and
four in group B (11.1%), and there were no differences between the
two groups. Hemorrhaging has been reported in 3–8% of all stent
patients and is usually self-limited (1). Guo et al(9) reported that hemorrhaging occurred in
16 patients (30%) in two groups studied during implantation and
follow-up. In the present study, hemorrhaging occurred in seven
patients (five patients in group A and two in group B), but no
patients succumbed due to an acute massive hemorrhage. Esophageal
perforations or tracheoesophageal fistulae have been shown to occur
in 2.7–7.3% of patients following stent placement (18–20).
Although the occurrence of such a complication may be increased
with an iodine-eluting stent due to the radiation effect on the
esophageal wall, tracheoesophageal fistulae occurred in three
patients in the present study (two patients in group A and one in
group B) with no significant differences between the two groups,
indicating that an esophageal perforation or a tracheoesophageal
fistula is mainly caused by the shearing action of the edge of the
esophageal stent and necrosis of the tumor.
The radiation effect of the iodine-eluting
esophageal stent may have an effect on thyroid function and blood
characteristics. In the present study, no significant differences
were observed between the two groups in the evaluation of the
routine blood tests and thyroid function examinations prior to the
stent placement or at two months later. This indicated that the
radioactivity of the iodine-eluting esophageal stent has little
effect on thyroid function and routine blood results.
There are certain limitations to the present study.
First, the irradiation dose of the iodine-eluting esophageal stent
was only selected using the length of the tumor due to the lack of
sophisticated measuring techniques for esophageal cancer. Second,
the quality of life, which is a significant measure of outcome for
the palliative treatment of malignancies, including inoperable
esophageal cancer, was not measured in the present study.
In conclusion, the present data show the
iodine-eluting esophageal stent to be a relatively safe, feasible
and effective treatment for esophageal stenosis caused by advanced
esophageal carcinoma. Treatment is believed to be improving
continuously with the development of advanced materials and
techniques, and the long-term prognosis and effectiveness of the
iodine-eluting stent requires further evaluation through further
observation and research.
References
|
1
|
Katsanos K, Sabharwal T and Adam A:
Stenting of the upper gastrointestinal tract: current status.
Cardiovasc Intervent Radiol. 33:690–705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park JG, Jung GS, Oh KS and Park SJ:
Double-layered PTFE-covered nitinol stents: experience in 32
patients with malignant esophageal strictures. Cardiovasc Intervent
Radiol. 33:772–779. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim KR, Shin JH, Song HY, Ko GY, Kim JH,
Yoon HK and Sung KB: Palliative treatment of malignant
esophagopulmonary fistulas with covered expandable metallic stents.
AJR Am J Roentgenol. 193:W278–W282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
White RE, Parker RK, Fitzwater JW, Kasepoi
Z and Topazian M: Stents as sole therapy for oesophageal cancer: a
prospective analysis of outcomes after placement. Lancet Oncol.
10:240–246. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Knyrim K, Wagner HJ, Bethge N, Keymling M
and Vakil N: A controlled trial of an expansile metal stent for
palliation of esophageal obstruction due to inoperable cancer. N
Engl J Med. 329:1302–1307. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song HY, Do YS, Han YM, Sung KB, Choi EK,
Sohn KH, Kim HR, Kim SH and Min YI: Covered, expandable esophageal
metallic stent tubes: experiences in 119 patients. Radiology.
193:689–695. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Christie NA, Buenaventura PO, Fernando HC,
Nguyen NT, Weigel TL, Ferson PF and Luketich JD: Results of
expandable metal stents for malignant esophageal obstruction in 100
patients: short-term and long-term follow-up. Ann Thorac Surg.
71:1797–1802. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sabharwal T, Hamady MS, Chui S, Atkinson
S, Mason R and Adam A: A randomized prospective comparison of the
Flamingo Wallstent and Ultraflex stent for palliation of dysphagia
associated with lower third oesophageal cancer. Gut. 52:922–926.
2003. View Article : Google Scholar
|
|
9
|
Guo JH, Teng GJ, Zhu GY, He SC, Fang W,
Deng G and Li GZ: Self-expandable esophageal stent loaded with 125I
seeds: initial experience in patients with advanced esophageal
cancer. Radiology. 247:574–581. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhongmin W, Xunbo H, Jun C, Gang H, Kemin
C, Yu L and Fenju L: Intraluminal radioactive stent compared with
covered stent alone for the treatment of malignant esophageal
stricture. Cardiovasc Intervent Radiol. 35:351–358. 2012.
View Article : Google Scholar
|
|
11
|
Sabharwal T, Morales JP, Irani FG and Adam
A; CIRSE. Cardiovascular and Interventional Radiological Society of
Europe: Quality improvement guidelines for placement of esophageal
stents. Cardiovasc Intervent Radiol. 28:284–288. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang GJ, Wang LJ, Liu JS, Cheng GY, Zhang
DW, Wang GQ and Zhang RG: Surgery of esophageal carcinoma. Semin
Surg Oncol. 1:74–83. 1985. View Article : Google Scholar
|
|
13
|
McQueen AS, Eljabu W, Latimer J and Raju
PP: Thoracic discitis as a complication of self-expanding metallic
stents in esophageal carcinoma. Cardiovasc Intervent Radiol.
34(Suppl 2): S300–S302. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wenger U, Luo J, Lundell L and Lagergren
J: A nationwide study of the use of self-expanding stents in
patients with esophageal cancer in Sweden. Endoscopy. 37:329–334.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McGrath JP, Browne M, Riordan C, Ravi N
and Reynolds JV: Expandable metal stents in the palliation of
malignant dysphagia and oesophageal-respiratory fistulae. Ir Med J.
94:270–272. 2001.PubMed/NCBI
|
|
16
|
Sarper A, Oz N, Cihangir C, Demircan A and
Isin E: The efficacy of self-expanding metal stents for palliation
of malignant esophageal strictures and fistulas. Eur J Cardiothorac
Surg. 23:794–798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bartelsman JF, Bruno MJ, Jensema AJ,
Haringsma J, Reeders JW and Tytgat GN: Palliation of patients with
esophagogastric neoplasms by insertion of a covered expandable
modified Gianturco-Z endoprosthesis: experiences in 153 patients.
Gastrointest Endosc. 51:134–138. 2000. View Article : Google Scholar
|
|
18
|
Wang MQ, Sze DY, Wang ZP, Wang ZQ, Gao YA
and Dake MD: Delayed complications after esophageal stent placement
for treatment of malignant esophageal obstructions and
esophagorespiratory fistulas. J Vasc Interv Radiol. 12:465–474.
2001. View Article : Google Scholar
|
|
19
|
Baron TH: Expandable metal stents for the
treatment of cancerous obstruction of the gastrointestinal tract. N
Engl J Med. 344:1681–1687. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Siersema PD, Tan TG, Sutorius FF, Dees J
and van Blankenstein M: Massive hemorrhage caused by a perforating
Gianturco-Z stent resulting in an aortoesophageal fistula.
Endoscopy. 29:416–420. 1997. View Article : Google Scholar : PubMed/NCBI
|