Introduction
Lung cancer is currently one of the most common
malignancies in the world. In the year 2002, 1.35 million new cases
were diagnosed, which represented 12.4% of all the newly-diagnosed
cancers worldwide. Metastatic disease is observed in ~40% of
patients with lung cancer, with the most common sites of metastasis
being the bone, liver, brain and adrenal glands.
Peritoneal carcinomatosis (PC) is defined as the
progression of the primary cancer to the peritoneum. Although the
condition may be diagnosed by surgical procedures, including a
laparoscopic peritoneal biopsy, the majority of patients are
diagnosed with PC using imaging techniques, such as computed
tomography (CT), or by the presence of malignant cells in the
ascitic fluid.
PC is a rare clinical event in lung cancer patients,
with autopsy results showing an incidence of 2.7–16%. In a study by
McNeill et al(1), peritoneal
and small bowel metastases were observed in 46 of 431 patients with
primary lung cancer who underwent autopsies during an 11-year
period. These patients had an average of 4.8 metastatic sites.
Small bowel metastases were present in 12 of 31 (39.0%) patients
with large cell carcinoma, 13 of 108 (12.3%) patients with
adenocarcinoma, six of 73 (8.0%) patients with small cell
carcinoma, 15 of 199 (7.5%) patients with squamous cell carcinoma
and none of the 20 (0.0%) patients with undifferentiated carcinoma
(1).
The clinical manifestations of these metastases are
rare, with intestinal perforation and obstruction being the most
common (2).
A review of the literature identified only clinical
case studies. The present study reviewed four cases of patients
that were diagnosed with PC secondary to primitive lung carcinoma,
in order to describe the characteristics of this rare complication.
Written informed consent was obtained from the patients.
Cases reports
Case 1
A 64-year-old male, who had been a heavy smoker for
30 years (>60 pack-years), was studied in the Department of
Pulmunology (Infanta Sofía University Hospital San Sebastián de los
Reyes, Madrid, Spain) due to a progressive dyspnea. The chest X-ray
revealed a massive left pleural effusion. A thoracocentesis was
performed for evacuation, which resulted in a significant
symptomatic improvement. The bronchoscopy revealed an extrinsic
compression secondary to the pleural effusion. A cytological
examination showed positive staining for malignant cells, which is
consistent with trefoil factor (TFF)-1 adenocarcinoma metastases. A
hydropneumothorax was observed on a body CT as a complication of
the biopsy, which used the fine-needle aspiration procedure. The
chest CT revealed pleural effusion and pathological mediastinal
nodes. A chest tube was required for the drainage of the pleural
effusion. An epidermal growth factor receptor (EGFR) activating
mutation was detected in the cytological examination (deletion in
exon 19).
At the time that the patient presented to the
hospital, anti-EGFR treatment in the first-line setting for
patients with EGFR-activating mutations was not approved.
Therefore, the patient was treated using chemotherapy. A biweekly
regimen was administered based on a combination of cisplatin (50
mg/m2) and docetaxel (50 mg/m2). The patient
underwent eight courses of chemotherapy with a partial response.
Subsequent to terminating the chemotherapy, the patient presented
with an asymptomatic pulmonary embolism in a follow-up CT scan and
anticoagulant treatment was consequently administered. Progression
of pleural effusion and an increased number of mediastinal nodes
was identified 10 months later in a routine chest CT. A new regimen
treatment with erlotinib was administered, resulting in a stable
disease state for one year. In a body scan, a pleural progression
was described. In the abdomen, a marked thickness of the epiplon
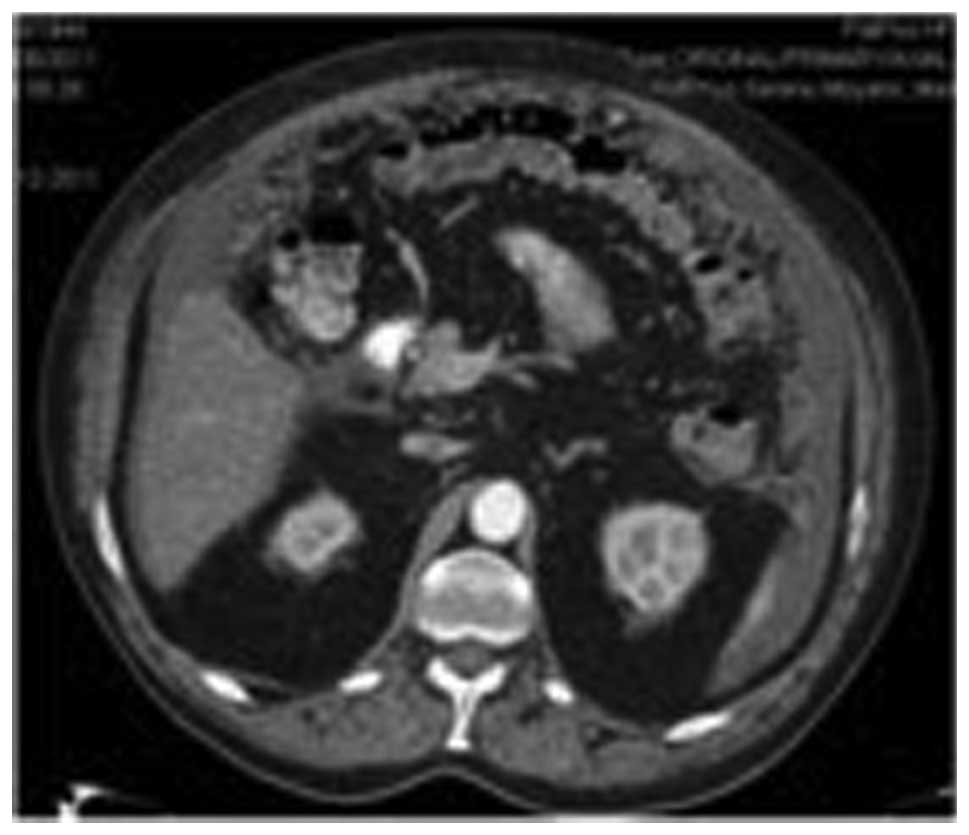
and the mesentery was observed with ascitis (Fig. 1). All these findings were suggestive
of PC. A guided CT biopsy was obtained from the peritoneum that
confirmed lung adenocarcinoma metastasis. A new EGFR analysis was
performed to determine the acquired resistant mutations. A double
mutation was detected consisting of an activating mutation (exon 19
deletion) and an acquired resistance mutation (exon 20 T790M).
The patient underwent a third-line treatment and was
stable following eight courses of pemetrexed and six of carboplatin
and paclitaxel following the previous regimen. A partial response
was observed in the last CT.
The patient is no longer undergoing chemotherapy and
has not exhibited any symptoms of progression while waiting for a
new evaluation.
Case 2
A 52-year-old female who was a heavy smoker (30/day,
for 20 years) with no medical conditions was admitted to the
emergency room (ER) due to an intensive and progressive pain in the
left shoulder. In the chest X-ray, a pulmonary mass was detected in
the left upper lobe. The chest CT demonstrated a 4.9×1.6-cm mass in
the left upper lobe, which was consistent with the radiological
findings of emphysema. A contralateral 2.5-cm lesion was observed
in the right upper lobe and was associated with the pleural
thickness in addition to smaller lesions in the two lungs, which
were suggestive of metastatic lesions. In the abdomen, several
nodules were identified in the greater omentum (the larger lesion
was ~1.6 cm in diameter), which were consistent with PC. A
fine-needle aspiration puncture of the lesion in the left upper
lobe was performed. The pathological findings were consistent with
TFF1-positive lung adenocarcinoma and no EGFR activating mutations
or echinoderm microtubule-associated protein like 4-anaplastic
lymphoma kinase (EML4-ALK) translocations were detected. The
patient was administered chemotherapy based on cisplatin (75
mg/m2) and pemetrexed (500 mg/m2). Following
six courses of this regimen, a radiological evaluation was
scheduled. A partial response was obtained and maintenance with
pemetrexed was initiated. At five months post-treatment, the
patient presented with worsening dyspnea and a body scan revealed a
pulmonary progression. Therefore, a second-line regimen based on
docetaxel was administered. Following nine courses of chemotherapy,
a clinical and radiological response was observed. Since asthenia
grade I, mild nail toxicity and neuropathy grade I were observed,
the chemotherapy was stopped and the patient was administered a
maintenance regimen with erlotinib. The patient is currently on
treatment and is clinically and radiologically stable.
Case 3
A 63-year-old male was admitted to the ER due to
shortness of breath, a cough and chest pain. The X-ray of the chest
revealed a right pleural effusion. A diagnostic thoracocentesis was
performed and a sample of the pleural effusion was sent for
pathological examination. Malignant cells that were positive for
TFF1 and carcinoembryonic antigen (CEA) expression were detected,
which was suggestive of adenocarcinoma. The CT showed a compressive
atelectasis involving the right lower lobe and an extensive pleural
effusion. In the abdomen, a malignant 2-cm nodule was observed in
the left adrenal gland. A positron emission tomography (PET)-CT
scan confirmed the CT findings, which depicted pathological uptakes
in the right lower lobe and several uptakes in the right pleural
cavity and the adrenal nodule. A pleural biopsy was performed and
the pathologist confirmed the diagnosis of adenocarcinoma. However,
the immunohistochemical analysis of the pleural tissue was
inconsistent with the analysis of the ion pleural effusion sample.
The biopsy revealed that the tissue was negative for TFF1 and
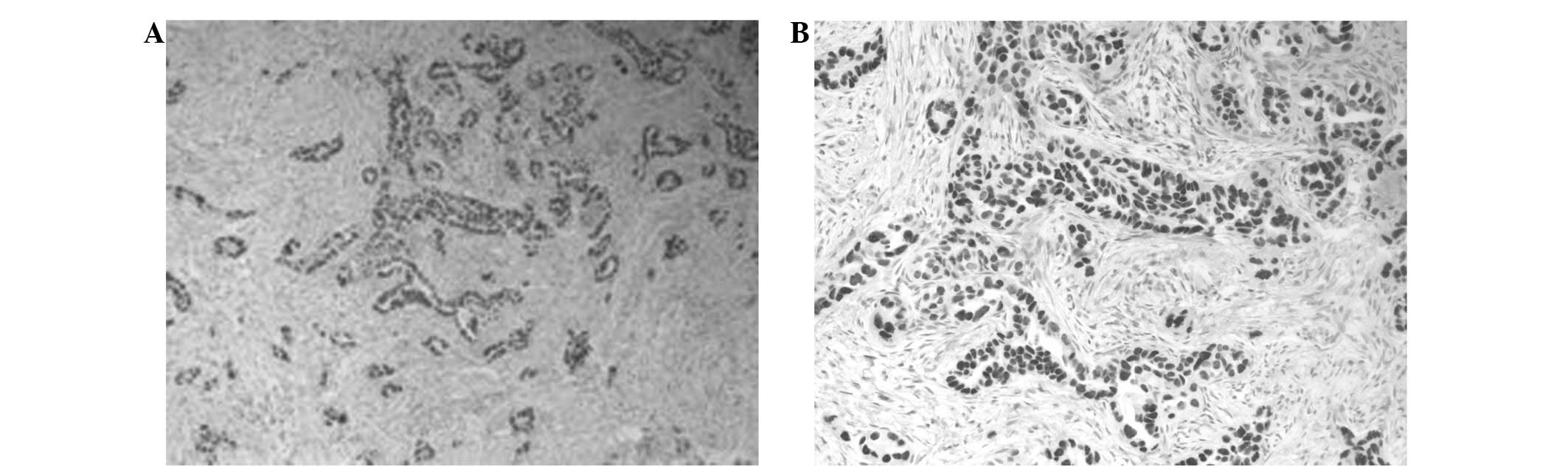
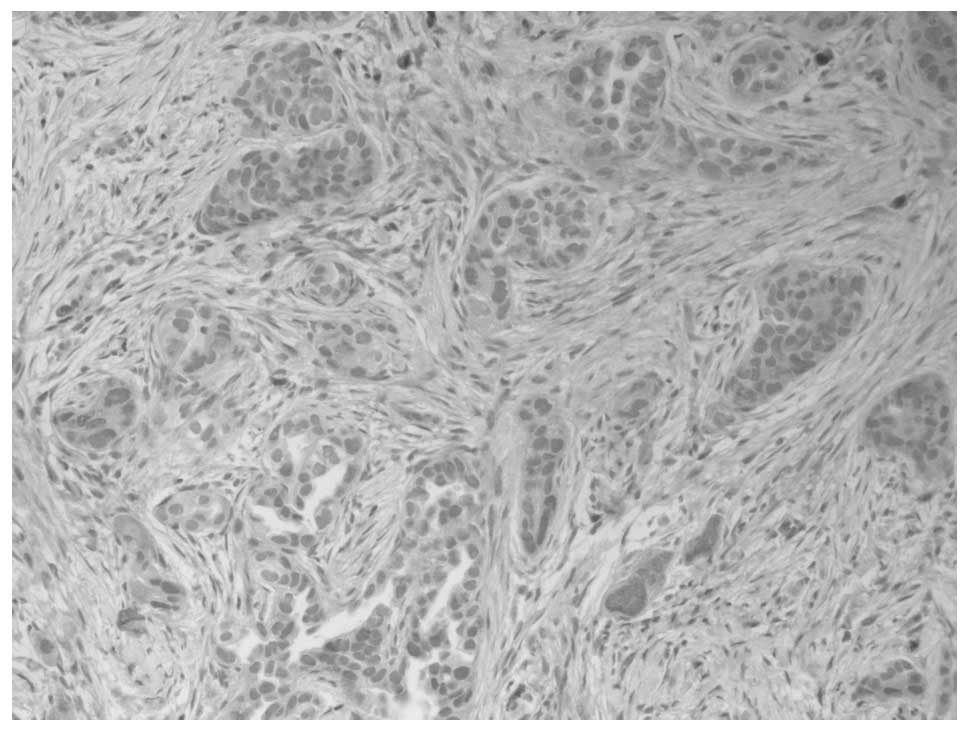
calretinin, but positive for CEA and cytokeratin (CK)-7 (Figs. 2 and 3). The pleural liquid sample analysis
revealed positive results for TFF1, CEA and CK7. The colonoscopy
and stomach endoscopy did not indicate any lesions.
The final diagnosis was of pleural metastasis from
lung adenocarcinoma. The EGFR analysis did not detect any
activating mutations. Therefore, the patient was administered a
chemotherapy regimen based on carboplatin-docetaxel-bevacizumab,
but presented with grade III [World Health Organization (WHO)
classification] hematological toxicity following the first course
(1). Consequently, a dose reduction
was required. The patient underwent six courses, with a partial
response, and continued with bevacizumab as a maintenance
treatment. Following three months of treatment, the CT scans showed
an increased pleural effusion, pulmonary nodules and several images
that were suggestive of PC. A demonstrative biopsy of the
peritoneum was performed and the pathological findings were
consistent with malignant mesothelioma of epithelioid subtype. The
immunophenotype demonstrated positive calretinin staining and was
negative for CK7, CK20, prostate specific antigen (PSA), TTF1, p63,
CD10 and α-fetoprotein (FP). Ki67 was expressed in ~50% of the
cells.
A new line of chemotherapy was administered based on
pemetrexed. The patient received three courses, presenting with
toxicity grade II. The CT scans showed a stable disease. A
pulmonary embolism was detected following the fourth administration
and the patient succumbed several days later.
Case 4
A 67-year-old male was admitted to the ER with
breathlessness and chest pain. The patient suffered from diabetes
and hyperuricemia, which was kept under control using alopurinol
and metformina.
The chest X-ray revealed a large right pleural
effusion and the CT showed a compressive atelectasis and a right
hilar mass. The pathological analysis of the pleural effusion was
consistent with the malignant cells from the immunohistochemical
pattern, suggesting a diagnosis of adenocarcinoma (TFF1-positive,
CEA-positive, CK7-positive, CK20-negative and calretinin-negative).
The pleural biopsy described a pleura that was infiltrated by a
primary lung adenocarcinoma. The patient was diagnosed with stage
IVB lung adenocarcinoma due to the positive pleural effusion. A
chemotherapy regimen based on carboplatin, paclitaxel and
bevacizumab was initiated. A partial response was observed, but the
treatment had to be stopped due to hematological toxicity. EGFR
mutations were identified involving a mutation in exon 18, G719S.
The patient underwent treatment with erlotinib, which was well
tolerated. Erlotinib was discontinued due to a pleural and
mediastinal progression and a new chemotherapy regimen based on
pemetrexed was administered. Following nine courses of chemotherapy
resulting in stabilization, the patient suffered a new progression
in the peritoneum and liver metastasis. The patient was admitted to
the ER with a bowel obstruction that was resolved with supportive
treatment. Docetaxel was administered every two weeks, which
improved the clinical status. The radiological evaluation revealed
a stable disease.
Discussion
Lung carcinoma is the leading cause of
cancer-related mortality and ~50% of cases exhibit distal
metastasis at the time of diagnosis (1). The preferential sites of
extrapulmonary metastasis are the lymph nodes, liver, adrenal
gland, bone and brain.
Peritoneal metastasis of primary lung carcinoma is
considered to be very rare, although it is identified in 2.7–16% of
all lung cancer patients (3).
Clinical studies concerning this distant metastasis are rare. Satoh
et al reviewed 1,041 lung cancer patients over a 26-year
period and 8 cases (0.77%) developed clinical PC. In the present
study, the most common histological type of lung cancer that was
associated with PC was adenocarcinoma, accounting for >80% of
the cases with PC, and unlike the study by Satoh et al, no
patient with large cell lung cancer was diagnosed with PC, which
was relatively rare in patients with small cell and squamous cell
lung cancer (4). In a previous
study, the median survival from the diagnosis of PC was
approximately two months and half of the patients developed liver
or abdominal lymph node metastases (2).
Occasionally, PC causes a reconsideration of the
initial pathological diagnosis and requires the consideration of
alternative diagnoses, such as that of mesothelioma. This is a not
a rare situation, particularly when the initial diagnosis has been
established using cytology. A patient in one of the previously
mentioned case studies (3) was
initially diagnosed with pleural metastasis from lung
adenocarcinoma according to the pleural biopsy and pleural effusion
sample: TTF1 and calretinin was negative and CEA and CK7 were
positive in the biopsy results. However, in the pleural liquid
sample, the TTF1, CEA and CK7 results were positive. This pattern
was suggestive of lung adenocarcinoma, and the patient underwent
treatment according to this diagnosis. However, when the patient
later presented with a peritoneal progression, a new and larger
biopsy was taken and the pathological diagnosis was consistent with
malignant mesothelioma of epithelioid subtype. The immunophenotype
demonstrated positive calretinin staining and was negative for CK7,
CK20, PSA, TTF1, P63, CD10 and α-FP. The new diagnosis may have
been due to the small size of the previous pleural biopsy, or due
to an incomplete pattern of antibodies (5). The distinction between epithelioid
mesothelioma and lung adenocarcinoma remains a significant
diagnostic challenge for surgical pathologists. Kushitani et
al demonstrated that for distinguishing between epithelioid
mesothelioma and lung adenocarcinoma, the combination of CEA,
calretinin and either WT1 or thrombomodulin would form the best
panel of immunohistochemical markers (5). Therefore, in a patient with PC and
primary lung adenocarcinoma, a possible metastatic pleural
mesothelioma must first be ruled out by checking the
immunohistochemical pattern of the antibodies that are used in the
pathological examination.
In the present study, using a cytological
examination, the patient from Case 1 was identified to harbor an
EGFR-activating mutation (deletion in exon 19). Currently, there is
no published evidence with regard to the frequency of
EGFR-activating mutations in patients with lung adenocarcinoma and
secondary PC. In a multivariate analysis of the incidence of EGFR
mutations, including the presence or absence of brain or bone
metastases, Rosell et al identified no association between
the prognosis and the location of the metastasis (6). In this study, we have presented the
first case to be published in the literature that describes a
primary lung adenocarcinoma with peritoneal metastasis and an EGFR
mutation (deletion in exon 19) (Case 1). In patients with advanced
non-small cell lung cancer, activating mutations in the EGFR gene
confer hypersensitivity to the tyrosine kinase inhibitors,
gefitinib and erlotinib. The patient was first administered
erlotinib and maintained a good response for one year, according to
the literature data (14 months) (7). The patient then presented with a
peritoneal progression and a second biopsy was planned to rule out
acquired resistant EGFR mutations. A double mutation was detected,
consisting of an activating mutation (exon 19 deletion) and an
acquired resistance mutation (exon 20 T790M). Due to these
findings, a new chemotherapy regimen was administered, resulting in
a stabilization of the peritoneal mass and a considerable
improvement in the ascitis and abdominal perimeter. Case 4 also
involved an EGFR mutation in exon 18, but the patient responded
poorly to anti-EGFR.
Su et al(7)
have published a lung cancer and PC study in which four patients
presented with EGFR mutations and were treated with the EGFR
tyrosine kinase inhibitor, gefitinib. Two patients, who responded
to gefitinib therapy, demonstrated improved abdominal conditions
with gradually diminishing ascites and survived for 203 and 343
days, respectively. Therefore, according to these data, activating
EGFR mutations in lung carcinoma, even in cases with peritoneal
disease, are considered positive predictors of anti-EGFR therapy
(8). With the exception of the
EGFR-positive tumors, the majority of lung adenocarcinomas with PC
have poor prognoses. Aggressive systemic chemotherapy following the
diagnosis of PC in lung cancer patients is not associated with an
improved outcome. The reasons for this are the poor performance
status of these patients and the possibility of developing
undesirable complications, including perforation and obstruction.
Due to advances in the improvement of chemotherapy and supportive
care for lung cancer and the ability to extend life expectancy, an
increasing number of this kind of metastatic tumor may be
encountered in the future. Therefore, more attention should be
focused on gastrointestinal (GI) metastatic signs, including GI
bleeding, epigastric pain, nausea, vomiting, acute abdominal pain,
or less commonly, ileus. The factors that affect the development of
complications from PC are unknown. The histological type, tumor
grade and other biological parameters may play significant roles.
Contributing factors are immunosuppression, chemotherapy,
radiotherapy and the use of cortisone and other drugs (9).
Certain chemotherapeutic agents, including
antiangiogenic drugs have been suggested to contribute to the
occurrence of perforation (10).
More effort should be made to improve the management
of this clinically rare complication of lung cancer that has a poor
prognosis, avoiding critical complications, including perforation
and intestinal obstruction (8).
Acknowledgements
The authors would like to thank Dr Gutiérrez for the
pre-review of this study and the patients who provided their
authorization for the creation of this paper.
References
|
1
|
McNeill PM, Wagman LD and Neifeld JP:
Small bowel metastases from primary carcinoma of the lung. Cancer.
59:1486–1489. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meneses Grasa Z, Coll Salinas A, Macías
Cerrolaza JA, Aguayo Albasini JL, Campillo Soto A and Guillén
Paredes MP: Intestinal obstruction by metastasis in mesentery from
squamous cell lung carcinoma. Rev Esp Enferm Dig. 101:817–818.
2009.(In Spanish).
|
|
3
|
Abrams HL, Spiro R and Goldstein N:
Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer.
3:74–85. 1950. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Satoh H, Ishikawa H, Yamashita YT,
Kurishima K, Ohtsuka M and Sekizawa K: Peritoneal carcinomatosis in
lung cancer patients. Oncol Rep. 8:1305–1307. 2001.PubMed/NCBI
|
|
5
|
Kushitani K, Takeshima Y, Amatya VJ,
Furonaka O, Sakatani A and Inai K: Immunohistochemical marker
panels for distinguishing between epithelioid mesothelioma and lung
adenocarcinoma. Pathol Int. 57:190–199. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosell R, Moran T, Queralt C, Porta R,
Cardenal F, Camps C, Majem M, et al; Spanish Lung Cancer Group.
Screening for epidermal growth factor receptor mutations in lung
cancer. N Engl J Med. 361:958–967. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Su HT, Tsai CM and Perng RP: Peritoneal
carcinomatosis in lung cancer. Respirology. 13:465–467. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang CJ, Hwang JJ, Kang WY, Chong IW, Wang
TH, Sheu CC, Tsai JR and Huang MS: Gastro-intestinal metastasis of
primary lung carcinoma: clinical presentations and outcome. Lung
Cancer. 54:319–323. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamamoto M, Matsuzaki K, Kusumoto H,
Uchida H, Mine H, Kabashima A, et al: Gastric metastasis from lung
carcinoma. Case report Hepatogastroenterology. 49:363–365.
2002.
|
|
10
|
Berger A, Cellier C, Daniel C, Kron C,
Riquet M, Barbier JP, et al: Small bowel metastases from primary
carcinoma of the lung: clinical findings and outcome. Am J
Gastroenterol. 94:1884–1887. 1999. View Article : Google Scholar : PubMed/NCBI
|