Introduction
Lung cancer is one of the most common malignancies,
with a high incidence and mortality rate worldwide. The 5-year
survival rate is 15% for Americans, 10% for Europeans and 8.9% in
developing countries. In China, there are ~400,000 new cases and
360,000 fatalities from lung cancer per year, accounting for
one-third of the overall incidence of this cancer worldwide
(1). Although certain mechanisms
have been identified to underlie the development of lung cancer,
current research is insufficient with regard to what is required to
significantly improve the current diagnosis and treatment
practices. Therefore, it is crucial that additional lung
cancer-related genes are identified to provide new markers or
targets for the diagnosis, treatment and prognosis of this
disease.
The use of gene arrays, as powerful tools for the
gene expression profile analysis of the whole genome, is a
preferred strategy for the identification of
differentially-expressed genes in cancer. They have been widely
used to identify tumor-associated genes in various types of cancer,
including lung carcinomas. However, the majority of gene
array-based expression studies of lung cancer have ignored the
differences between the clinical features of patients, including
the tumor-node-metastasis (TNM) stage (2,3). The
identification of differentially-expressed genes in lung cancers of
various clinical statuses may provide new opportunities to improve
the diagnosis and treatment of this disease.
The main histological type of lung cancer is
non-small cell lung carcinoma (NSCLC), which consists of squamous
cell carcinoma, adenocarcinoma and large cell lung cancer. Based on
clinical practice and the published data in China, lung
adenocarcinoma is an aggressive paradigm with a high chance of
relapse and early metastasis. The condition has become one of the
most common subtypes of lung cancer in the country, accounting for
30–40% of the overall cases.
In the present study, a 35K oligo gene array was
used to identify the differentially-expressed genes that were
associated with the various TNM stages of lung adenocarcinomas. The
results may provide new information to decode the molecular
pathogenesis of lung cancer and offer new targets for the
diagnosis, treatment and prognosis of this disease.
Materials and methods
Specimens
Lung adenocarcinoma specimens were collected from
240 patients who underwent thoracic surgery at Southwest Hospital.
All patients were primary cases with no prior history of other
malignancies, and no patient had been administered chemo- or
radiotherapy. In addition to the cancer tissue, the surrounding
normal lung tissue was collected to be used as a control. A
1.0-cm3 volume of tissue was further cut into
0.5-cm3 blocks and placed into 5-fold volumes of
RNAlater. Subsequent to being frozen for 30 min in liquid nitrogen,
the tissues were kept at −80°C for later use. A section of each
cancer tissue was prepared for routine hematoxylin and eosin
staining to confirm the percentage of cancer cells. Only the
samples in which the proportion of cancer cells was >80% were
used to prepare the RNA. The specimens were reviewed pathologically
to confirm the diagnosis of lung adenocarcinoma. A total of 90
samples (30 each for stages I, II and IIIA) were used for the gene
array, and 150 samples (50 each for stages I, II and IIIA) were
used for the validation by quantitative (q)PCR. Approval for the
present study was obtained from the ethics committee of the
Affiliated South-West Hospital (Chonqing, China). The study
conforms to the provisions of the Declaration of Helsinki in 1995
(as revised in Tokyo 2004) and informed consent was obtained from
all patients.
Preparation of total RNA
The total RNA from each tissue sample was extracted
using a Tripure™ Isolation Reagent kit (Ambion Inc., Foster City,
CA, USA) according to the manufacturer's instructions. The RNA
samples were purified with a Nucleospin RNA clean-up kit
(Machery-Nagel Inc., Bethlehem, PA, USA), according to the
manufacturer's instructions. The concentration and purity of the
RNA samples were assayed with a Nanodrop spectrophotometer (Thermo
Fisher Scientific Inc., Waltham, MA, USA). The integrity of the RNA
was confirmed using electrophoresis in agarose gel containing
formaldehyde.
Gene array hybridization
35K oligo gene array
The Jingxin® 35K oligo gene array
(CapitalBio Inc., Beijing, China) involved 35,000 70-mer
oligonucleotide probes (human genome-wide oligo library Version
4.0; Operon Inc., Huntsville, AL, USA), covering ~25,100 genes.
Fluorescence-labeling of RNA
samples
The Jingxin cRNA linear amplification and labeling
kit (CapitalBio) was used. The RNA was first reverse transcribed to
single stranded cDNA, which was used as a template to synthesize
the second cDNA strand. The second strand of cDNA was used as a
template to synthesize the cRNA using T7 Enzyme Mix. The cRNA was
reverse transcribed to cDNA using Random Primer. The purified cDNA
was used as a template to synthesize the fluorescence-labeled cDNA
chain using the Klenow enzyme. Cy5 dCTP and Cy3 dCTP were used to
label the test sample (tumor or surrounding normal tissue) and the
Universal Human Reference RNA, respectively. The final labeled
products were purified using a PCR NucleoSpin Extract II kit
(Machery-Nagel Inc.). A Nanodrop spectrophotometer was used to
determine the concentration and labeling efficiency of the
products. The fluorescence intensities of Cy3 and Cy5 should be
comparable.
Hybridization
Subsequent to being mixed with a hybridization
buffer, the labeled products were added to the gene arrays, which
were then incubated in the CapitalBio® BioMixer™ II
microarray hybridizing incubator overnight at 42°C. Following
hybridization, the gene arrays were washed and scanned using
CapitalBio LuxScan™10K-A scanners. The data was extracted and
analyzed by LuxScan 3.0, BoaoAnalyzer6_step1.pl and
BoaoAnalyzer6_step2.pl software products (CapitalBio Inc.), and the
fluorescence intensity was normalized prior to analysis.
Identification of
differentially-expressed genes
The genes that were differentially-expressed between
the lung adenocarcinoma and surrounding normal lung tissues, and
those between the various TNM stage tumors, were analyzed using
significance analysis of microarrays (SAM) software (Stanford
University, Stanford, CA, USA).
qPCR
To verify the data from the gene array, the human
zinc finger-containing, Miz1, PIAS-like protein on chromosome 7
(Zimp7), GINS complex subunit 2 (GINS2) and NSAID activated gene 1
(NAG-1) were chosen as candidates for the qPCR in an alternative
set of tumor samples to the gene array assay. A total of 50 samples
were used per TNM stage. The primers were designed and synthesized
by Takara Inc. (Dalian, China; Table
I). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used
as the internal control (Takara Inc.). The amplification was
performed according to the instructions outlined in the
SYBR® Premix Ex Taq™ Real Time RT-PCR kit manual (Takara
Inc.). The 25 μl amplification included 1 μl cDNA, 0.5 μl each of
forward and reverse primers, 12.5 μl 2X SYBR Premix, 0.5 μl ROX
Reference Dye I and 10 μl dH2O. Each sample underwent
amplification three times. The reactions were performed on Rotor
gene 6300 (Corbett Life Science, Australia) and the conditions were
as follows: predenaturation at 95°C for 10 sec, followed by 40
cycles at 95°C for 5 sec, 52.5°C (Zimp7), 59°C (GINS2) and 62°C
(NAG-1) for 15 sec and finally, 72°C for 20 sec. The relative
expression was quantified using the 2(−ΔΔCt) method
(4,5).
 | Table IForward and reverse primers of Zimp7,
GINS2 and NAG-1 genes for qPCR amplification. |
Table I
Forward and reverse primers of Zimp7,
GINS2 and NAG-1 genes for qPCR amplification.
| Primer | Sequence (5′→3′) |
|---|
| Zimp7 |
| Forward |
CGGGTCACCATTTCCTCCAGTC |
| Reverse |
GGGGCAACGCTCACACCAGATAC |
| GINS2 |
| Forward |
CAGACGAATGGCATGGCTTTTAC |
| Reverse |
GCGGGTGCTCTTAGGCTCTC |
| NAG-1 |
| Forward |
GCCCGCCAGCTACAATCC |
| Reverse |
GGCAGGAATCGGGTGTCTCA |
Statistical analysis
The numbers of Zimp7, GINS2 and NAG-1 transcripts
between the tumor samples at the various TNM stages were compared
using the Fisher's least significant difference (LSD) test using
SPSS software version 18.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Screening the differentially-expressed
genes
A two-class unpaired method from the SAM software
was adopted to screen the differentially-expressed genes between 90
tumor samples of lung adenocarcinoma and their surrounding normal
lung tissues. The criteria to define the difference were as
follows: i) q-value of <5%; and ii) the ratio of the
fluorescence intensity of Cy5 to Cy3 (i.e. fold change) as ≥2 or
≤0.5. Overall, a total of 640 candidate genes were identified to be
differentially-expressed in the tumor tissues compared with the
normal tissues, among which, 289 genes were upregulated and 351
were downregulated. A multiclass method from the SAM software was
used to identify the differentially-expressed genes among the tumor
samples at the various TNM stages (stages I, II and IIIA) from the
640 candidates with criteria of: i) q-value of <15%; and ii)
fold change of ≥2 or ≤0.5. Finally, 10 genes were obtained
(Table II), among which, four
genes, dual-specificity mitogen activated protein (MAP) kinase
phosphatase 1 (DUSP1), Zimp7, EST NP_937824 and XM_498632, were
expressed at a higher level in the stage I adenocarcinoma samples
than in those of stages II and IIIA. A further two genes showed a
higher expression in the stage II samples than in those of stages I
and IIIA (GINS2 and lymphocyte antigen 6 complex, locus K, LY6K),
and four genes exhibited higher expression in the stage II and IIIA
samples than in those of stage I [NAG-1, melanoma-associated
antigen 3 (MAGE-A3), metastasis associated lung adenocarcinoma
transcript-1 (MALAT-1) and matrix metalloproteinase 12
(MMP-12)].
 | Table IITen differentially-expressed genes
identified to be associated with various lung adenocarcinoma TNM
stages. |
Table II
Ten differentially-expressed genes
identified to be associated with various lung adenocarcinoma TNM
stages.
| Symbol | GB. accession | Description | Fold change | Contrast-1 | Contrast-2 | Contrast-3 |
|---|
| DUSP1 | NM_004417 | Dual specificity MAP
kinase phosphatase 1 | 0.41a | 1.91 | −1.50 | −0.30 |
| Zimp7 | NM_031449.3 | Human zinc
finger-containing, Miz1, PIAS-like protein on chromosome 7 | 2.39b | 2.66 | −1.30 | −1.01 |
| EST | NP_937824 | 34 kDa protein | 2.36b | 2.16 | −1.52 | −0.47 |
| EST | XM_498632 | Hypothetical gene
supported by BC022385; BC035868; BC048326 | 3.67b | 2.10 | −1.18 | −0.20 |
| GINS2 | NM_016095.2 | GINS complex subunit
2 | 5.44b | −2.08 | 2.02 | 0.04 |
| LY6K | NM_017527.3 | Lymphocyte antigen 6
complex, locus K | 12.96b | −0.96 | 1.50 | −0.40 |
| NAG-1 | NM_004864.2 | NSAID activated gene
1 | 4.81b | −1.03 | 0.56 | 1.64 |
| MAGE-A3 | NM_005362.3 | Melanoma-associated
antigen 3 | 6.30b | −1.02 | 0.87 | 1.97 |
| MALAT-1 | NR_002819.2 | Metastasis associated
lung adenocarcinoma transcript 1 | 4.03b | −1.44 | 0.74 | 1.90 |
| MMP-12 | NM_002426.4 | Matrix
metalloproteinase-12 | 5.42b | −2.08 | 0.94 | 2.02 |
Confirming the expression of certain
candidate genes by qPCR
Using GAPDH as an internal control, the ratio of the
candidate gene transcript between the tumor and the matched normal
tissues [tumor/normal (T/N)] was calculated. If the ratio equaled
more than two, the target gene was considered to be upregulated in
the tested tumor sample compared with its matched normal sample.
The results showed that the Zimp7, GINS2 and NAG-1 transcripts were
upregulated in lung adenocarcinoma and that the mean ratio (T/N;
mean ± SD) of the three genes in 150 matched tumor and normal
samples were 3.86±2.09, 6.80±4.55 and 10.80±5.61, respectively,
with an upregulation positive percentage of 85.33% (128/150),
88.67% (133/150) and 96.67% (145/150), respectively. The results of
the qPCR were consistent with those from the gene array.
The relative expression levels of Zimp7 mRNA in the
lung adenocarcinoma patients of stages I, II and IIIA were
4.04±0.86, 1.35±0.32 and 1.67±0.40, respectively, and for the GINS2
mRNA, the levels were 2.15±0.95, 12.23±3.27 and 5.01±1.02,
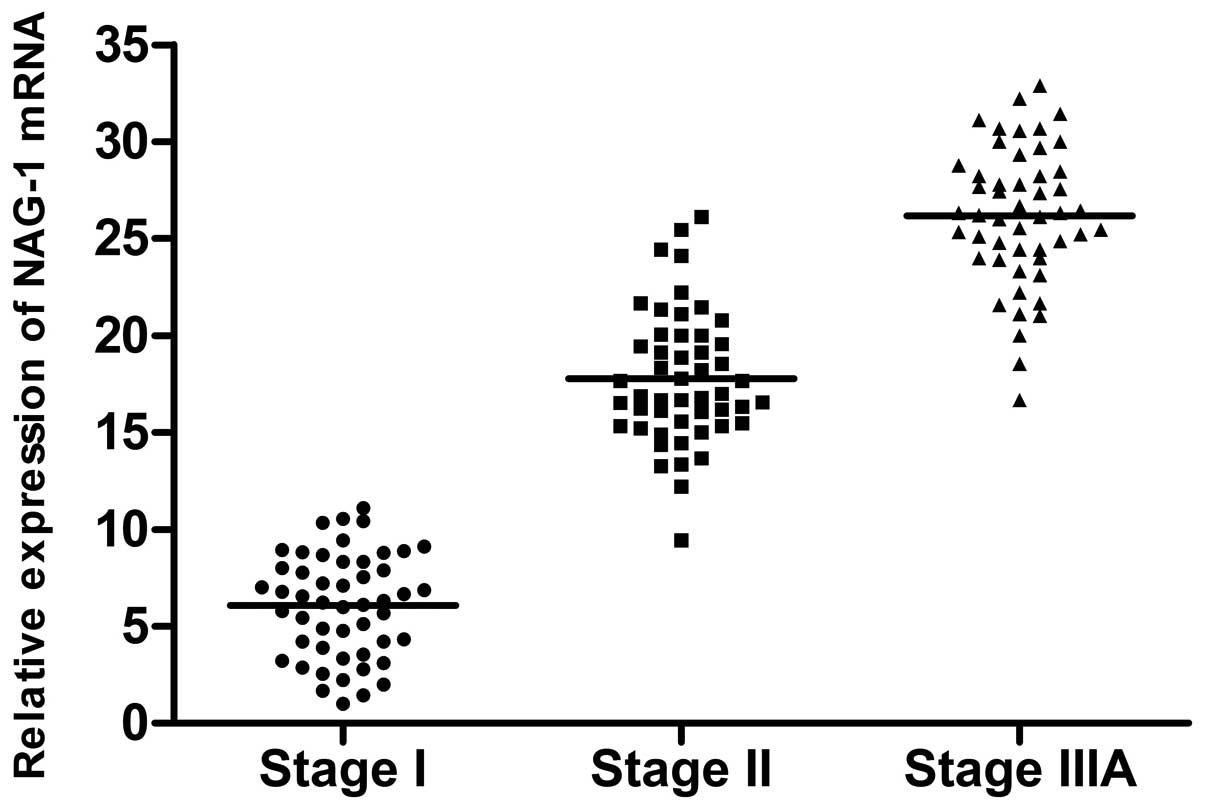
respectively. The mRNA levels of NAG-1 were 6.08±2.67, 17.77±3.40
and 26.16±3.55, respectively (Figs.
1–3). Zimp7 showed a
significantly higher expression level in the stage I samples than
in those of stages II and IIIA, while GINS2 showed a higher level
in stage II samples than in those of stages I and IIIA. NAG-1
expression was significantly increased in stages II and IIIA
compared with stage I. These results were consistent with the gene
array.
Discussion
The present study identified 10
differentially-expressed genes among 90 lung adenocarcinoma tumor
samples of various TNM stages (30 cases per stage) using a 35K
oligo gene array. Of the 10 candidates, DUSP1 was downregulated in
lung adenocarcinoma and the other nine were upregulated.
Compared with the stage II and stage IIIA samples,
the stage I lung adenocarcinoma samples were marked by four
differentially-expressed genes, DUSP1, Zimp7, EST NP_937824 and
XM_498632.
DUSP1, also known as MKP1, negatively regulates the
activity of extracellular regulated kinases (ERKs) and MAP kinases
(MAPKs). MKP1 expression has been shown to progressively decrease
with the development of epithelial carcinomas (6), and is one of the 20 most significantly
downregulated genes in colorectal cancers (7). Studies indicate that the induction of
MKP1 may significantly suppress the proliferative and metastatic
abilities of NSCLC in vitro and in vivo. Therefore,
MKP1 may be considered to be a potential therapeutic target in
NSCLC therapy (8). In the present
study, DUSP1 mRNA was significantly downregulated in the lung
adenocarcinoma tissues, and the levels declined progressively with
the development of this cancer, which was consistent with the
results reported with regard to epithelial carcinomas. The analysis
of DUSP1 expression may be useful to evaluate the disease extent
and prognosis of lung adenocarcinoma.
Zimp7, also named Zmiz2, is a novel PIAS-like
protein that functions as a transcriptional co-activator. Transient
transfection into prostate epithelial cells showed that human Zimp7
augments the transcriptional activity of the androgen receptor
(AR), which is known to be of importance in the survival of
prostate cancer cells (9). The
present study was the first to report the higher expression of
Zimp7 mRNA in stage I lung adenocarcinomas. Whether the consequence
of its high expression is also to augment the nuclear hormone
receptor-regulated transcription similar to that found in prostate
cancer requires further study.
Furthermore, two more genes were identified bearing
unknown functions, which were expressed at higher levels in the
stage I lung adenocarcinomas (EST NP_937824 and XM_498632). The
potential value of these genes in the early diagnosis of lung
adenocarcinoma required further evaluation later in the study.
Compared with the stage I and IIIA samples, the
stage II lung adenocarcinoma samples were marked by two
differentially-expressed genes (GINS2 and LY6K).
GINS2 is a member of a tetrameric complex, GINS,
composed of GINS1, GINS2, GINS3 and GINS4, which most likely serves
as the replicative helicase. As it has been reported that DNA
replication-associated proteins exhibit diverse functions in
various cells, GINS components, particularly GINS2, have been
suggested as possessing a function in cell division, and more
precisely in the chromosome segregation of cancer cells (10). A high level of GINS2 expression has
been observed among metastasizing breast tumors. Bioinformatic
analyses of published gene expression and DNA copy number studies
of clinical breast tumors suggested that GINS2 was associated with
the aggressive characteristics of a subgroup of breast cancers
in vivo(11). In the present
study, GINS2 mRNA was observed to be significantly highly expressed
in stage II lung adenocarcinoma for the first time. Together with
its expression in breast cancer, GINS2 is speculated to be a
potential metastasis-promoting gene, and its overexpression may
participate in lung adenocarcinoma metastasis.
LY6K is a cancer testis antigen located on
chromosome 8q24.3. Gene expression profile analyses of NSCLC
revealed that LY6K was specifically expressed in the testis and
transactivated in the majority of NSCLCs. Immunohistochemical
staining confirmed that LY6K overexpression was associated with a
poor prognosis for patients with NSCLC (12). Cell viability assays demonstrated
that the significant inhibition of cell growth, migration and
invasion occurred in LY6K-knockdown bladder cancer cell lines
(13). The present data show that
the LY6K transcript was significantly overexpressed in the stage II
lung adenocarcinoma samples, suggesting that the upregulation of
the LY6K gene may contribute to the development of this cancer, and
therefore, that LY6K may potentially be used for predicting the
prognosis of lung adenocarcinoma, as previously reported for NSCLC
(12).
Compared with the stage I samples, NAG-1, MAGE-A3,
MALAT-1 and MMP-12 were significantly upregulated in stage II and
IIIA lung adenocarcinoma samples.
NAG-1 was identified as a divergent member of the
TGF-β superfamily. Studies of NAG-1 expression in tumors have been
inconsistent. Using immunohistochemistry, a study by Kim et
al(14) showed that NAG-1
expression is downregulated in colon tumors. NAG-1 expression has
also been shown to be absent in squamous metaplastic tracheal
epithelium, whereas positive expression has been observed in the
normal tracheobronchial epithelia (15). In contrast, a high expression of
NAG-1 is also frequently observed in certain tumors. NAG-1 has been
identified as highly expressed in melanoma, and metastatic melanoma
biopsies have displayed a strong expression of MIC-1 compared with
the primary melanoma biopsies (16). Brown et al(17) reported that NAG-1 expression was
markedly upregulated in colorectal cancers and that serum NAG-1
levels were higher in patients with a higher TNM stage. The
apparent dichotomy of NAG-1 expression in tumors raises the
possibility that NAG-1 plays distinctly differing roles at various
stages of tumor progression. The gene suppresses tumorigenesis at
the early stages of cancer development and promotes tumor
invasiveness and survival at more advanced stages of the disease.
This is not unexpected since NAG-1 is a member of the TGF-β
superfamily, but an explanation for this change in biological
activity is not clearly understood at the present time. The present
data first revealed that the NAG-1 transcript was upregulated in
lung adenocarcinomas in contrast with data from a previous report
on squamous metaplastic tracheal epithelia, in which no expression
was identified. The expression level was also markedly increased
with the progression of the disease, similar to that in colorectal
cancer as reported by Brown et al(17). Thus, NAG-1 may be used as a
potential marker to asses the prognosis of lung adenocarcinoma.
MAGE-A3 is a cancer testis antigen that is expressed
in cancer cells, but not in normal tissues. MAGE-A3 is a promising
target for anticancer immunotherapy as it is exclusively presented
on the cell surface of cancer cells and may be associated with an
aggressive cancer phenotype. Sienel et al(18) compared the expression of MAGE-A3 in
stage I and II NSCLCs and observed that in comparison with stage I
tissues, the rate of MAGE-A3-positive tumors was significantly
increased in stage II tissues, which was in agreement with its
possible role in tumor metastasis. Consistent with the literature,
the expression of MAGE-A3 mRNA was significantly increased in the
present study in stage II and IIIA lung adenocarcinoma tissues
compared with those of stage I. MAGE-A3 may also be a candidate
target for the immunotherapy of lung adenocarcinoma.
MALAT-1 is a novel non-coding RNA of >8,000
nucleotides that is expressed on chromosome 11q13.
Short-interfering RNA-mediated MALAT-1 silencing has been shown to
impair the in vitro cell motility of lung adenocarcinoma
cells (19). MALAT-1 has been
demonstrated to be significantly associated with metastasis in
NSCLC patients, and may be used as prognosis-predicting parameter
for early-stage NSCLC (20). The
present study identified that the expression of MALAT-1 was
significantly higher in the stage II and IIIA samples than in stage
I lung adenocarcinoma, which was consistent with the published
data. The present results demonstrate the significance of MALAT-1
for the prediction of the metastasis and prognosis of lung
adenocarcinoma.
MMP-12, also known as macrophagic elastase, is a
member of the MMP family that binds to certain substrates,
including elastin, type IV collagen and fiber junction protein.
Extensive studies are available with regard to the role of MMPs in
the invasion and metastasis of different cancers (21). We compared the MMP-12 mRNA levels
between cancer tissues and matched surrounding normal tissues,
between TNM stage I and stage II/III, as well as between tumors
with lymph node metastasis and without, in cases of NSCLC. The data
showed that MMP-12 was present in all the tested cancer tissues,
but not in the normal tissues. There was a significantly higher
expression level in the stage II/III samples than in those of stage
I, and a higher level in the cancers with lymph node metastasis
than in those without. Consistent with published data (22), the present gene array-based study of
various TNM stages of lung adenocarcinoma further confirmed the
role of MMP-12 in the metastasis of NSCLC and its value as a
potential marker for predicting the prognosis of the disease.
In summary, the present study presents the
association between the genome-wide gene expression profiles and
the various TNM stages of lung adenocarcinoma in a study
population, thus providing a preliminary analysis of the TNM
stage-related genes that are present in lung adenocarcinomas. The
majority of the 10 genes that were identified in the present study
are present in lung cancer, and the results further demonstrate
their essential roles in the development of lung adenocarcinoma.
However, the three remaining genes, which have not been reported
previously in lung cancer, have been indicated to be involved in
cancers at other levels. Further study with regard to the roles of
these genes in lung cancer would be of great value.
Acknowledgements
This study was supported by two grants from the
Chongqing Local Natural Sciences Foundation of China (nos.
2007AC5067 and 2010BB5192).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Singhal S, Miller D, Ramalingam S and Sun
SY: Gene expression profiling of non-small cell lung cancer. Lung
Cancer. 60:313–324. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hofmann HS, Bartling B, Simm A, et al:
Identification and classification of differentially expressed genes
in non-small cell lung cancer by expression profiling on a global
human 59.620-element oligonucleotide array. Oncol Rep. 16:587–595.
2006.PubMed/NCBI
|
|
4
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Loda M, Capodieci P, Mishra R, et al:
Expression of mitogen-activated protein kinase phosphatase-1 in the
early phases of human epithelial carcinogenesis. Am J Pathol.
149:1553–1564. 1996.PubMed/NCBI
|
|
7
|
Zhang L, Zhou W, Velculescu VE, et al:
Gene expression profiles in normal and cancer cells. Science.
276:1268–1272. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tai CJ, Wu AT, Chiou JF, et al: The
investigation of mitogen-activated protein kinase phosphatase-1 as
a potential pharmacological target in non-small cell lung
carcinomas, assisted by non-invasive molecular imaging. BMC Cancer.
10:952010. View Article : Google Scholar
|
|
9
|
Huang CY, Beliakoff J, Li X, et al:
hZimp7, a novel PIAS-like protein, enhances androgen
receptor-mediated transcription and interacts with SWI/SNF-like BAF
complexes. Mol Endocrinol. 19:2915–2929. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanissian SH, Akbar U, Teng B, et al: cDNA
cloning and characterization of a novel gene encoding the
MLF1-interacting protein MLF1IP. Oncogene. 23:3700–3707. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thomassen M, Tan Q and Kruse TA: Gene
expression meta-analysis identifies chromosomal regions and
candidate genes involved in breast cancer metastasis. Breast Cancer
Res Treat. 113:239–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ishikawa N, Takano A, Yasui W, et al:
Cancer-testis antigen lymphocyte antigen 6 complex locus K is a
serologic biomarker and a therapeutic target for lung and
esophageal carcinomas. Cancer Res. 67:11601–11611. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsuda R, Enokida H, Chiyomaru T, et al:
LY6K is a novel molecular target in bladder cancer on basis of
integrate genome-wide profiling. Br J Cancer. 104:376–386. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim KS, Baek SJ, Flake GP, Loftin CD,
Calvo BF and Eling TE: Expression and regulation of nonsteroidal
anti-inflammatory drug-activated gene (NAG-1) in human and mouse
tissue. Gastroenterology. 122:1388–1398. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Newman D, Sakaue M, Koo JS, et al:
Differential regulation of nonsteroidal anti-inflammatory
drug-activated gene in normal human tracheobronchial epithelial and
lung carcinoma cells by retinoids. Mol Pharmacol. 63:557–564. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boyle GM, Pedley J, Martyn AC, et al:
Macrophage inhibitory cytokine-1 is overexpressed in malignant
melanoma and is associated with tumorigenicity. J Invest Dermatol.
129:383–391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brown DA, Ward RL, Buckhaults P, et al:
MIC-1 serum level and genotype: associations with progress and
prognosis of colorectal carcinoma. Clin Cancer Res. 9:2642–2650.
2003.PubMed/NCBI
|
|
18
|
Sienel W, Varwerk C, Linder A, et al:
Melanoma associated antigen (MAGE)-A3 expression in Stages I and II
non-small cell lung cancer: results of a multi-center study. Eur J
Cardiothorac Surg. 25:131–134. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tano K, Mizuno R, Okada T, et al: MALAT-1
enhances cell motility of lung adenocarcinoma cells by influencing
the expression of motility-related genes. FEBS Lett. 584:4575–4580.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ji P, Diederichs S, Wang W, et al:
MALAT-1, a novel noncoding RNA, and thymosin beta4 predict
metastasis and survival in early-stage non-small cell lung cancer.
Oncogene. 22:8031–8041. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nabeshima K, Inoue T, Shimao Y and
Sameshima T: Matrix metalloproteinases in tumor invasion: role for
cell migration. Pathol Int. 52:255–264. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hofmann HS, Hansen G, Richter G, et al:
Matrix metalloproteinase-12 expression correlates with local
recurrence and metastatic disease in non-small cell lung cancer
patients. Clin Cancer Res. 11:1086–1092. 2005.PubMed/NCBI
|