Introduction
Gestational trophoblastic disease (GTD) is known to
be associated with increased maternal age and is more commonly
observed in Asian subjects compared with non-Asian subjects
(1). GTD may be subcategorized into
hydatidiform moles [complete hydatidiform moles (CHMs), partial
hydatidiform moles (PHMs) and invasive moles (IMs)] and gestational
trophoblastic tumors [gestational choriocarcinomas (GTTs),
placental site trophoblastic tumors (PSTT) and epithelioid
trophoblastic tumors (ETTs)] (1)
After malignant transformation of the mole,
chemotherapy is indicated. IMs are divided into low-risk or
high-risk based on each prognostic factor, including age, human
chorionic gonadotropin (hCG) levels, antecedent pregnancy, interval
from antecedent pregnancy to chemotherapy, metastases, largest
tumor mass diameter and previous chemotherapy. This was intensively
revised by Seckl et al(1)
and Tse et al(2) that how to
estimate the IM risk. In cases of low-risk disease (score 0–6),
therapy is continued for six weeks (one to two cycles of
chemotherapy). In cases of high-risk disease (score ≥7), therapy
lasts for eight weeks (two to three cycles of chemotherapy) if poor
prognostic features such as metastases to the liver or brain are
present (1). However, in a small
number of patients with IMs, Doppler ultrasonography (DU) indicates
blood-flow signals within the mass if one to two cycles of
chemotherapy have been completed after their hCG levels have
decreased back to normal. The dilemma is which treatment option
should be pursued, a simple follow-up, continued chemotherapy or a
resection of the mass. The present study describes three patients
in whom DU indicated blood-flow signals in the tumor mass after one
to two cycles of chemotherapy when the patients’ hCG concentrations
had returned to a normal level. This study was approved by the
Medical Ethics Committee of Hubei University of Medicine (Hubei,
China) and the patients all provided informed written consent.
Case reports
Case 1
Case 1 was that of a 38-year-old female (gravida 4,
para 1) who experienced vaginal bleeding 12 weeks into pregnancy.
Transvaginal ultrasonography (TVS) indicated that the uterine
cavity was filled with cystic material. The attending physician
suggested that this was a molar pregnancy. Aspiration was
performed. The pathological diagnosis was of a CHM. The hCG level
increased from 2,130 mIU/ml to 4,321 mIU/ml (normal range, 0–5
mIU/ml) continually for three weeks after TVS. Rich blood-flow
signals within the myometrium of the uterus were noted. The patient
was subsequently diagnosed as having an IM (score, 5). The patient
underwent five cycles of chemotherapy consisting of 5-fluorouracil
(5-FU; 26 mg/kg/day, 8 days/cycle) and actinomycin (KSM; 6
μg/kg/day, 8 days/cycle); the interval between each cycle was three
weeks. At the beginning of the fourth cycle, the hCG level
decreased to 0.1 mIU/ml. Subsequent to this cycle, menstruation
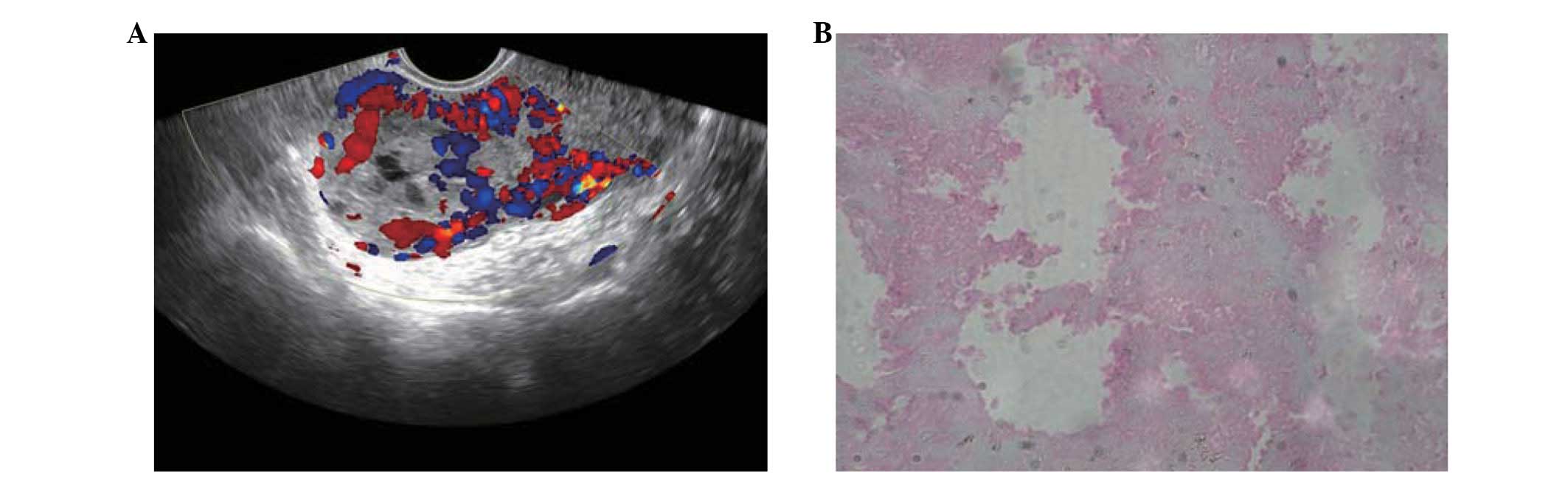
started again. At the fifth cycle, DU (5–7.5 MHz transvaginal
transducer with spectral Doppler) indicated that rich blood-flow
signals were present in the mass within the myometrium (Fig. 1). The resistive index (RI) was 0.24
and the number of blood-flow signals had sharply decreased compared
with the start of chemotherapy. The patient requested resection of
the mass. After entering the abdomen, it was observed that the mass
was on the convex surface of the uterus. The color of the surface
of the mass was identical to that of the normal tissue. The
pathological diagnosis of the mass was necrosis of the placental
tissue (Fig. 1). Another cycle of
chemotherapy was administered following the surgery. The patient’s
hCG level remained with the normal range during the 17 months of
follow-up.
Case 2
Case 2 was that of a 22-year-old female (gravida 2,
para 0) at the 16th week of pregnancy. The patient was diagnosed as
having a hydatidiform mole due to vaginal bleeding, high hCG levels
and indication of a cystic pregnancy by TVS. Subsequent to
aspiration, the pathological diagnosis was of a CHM. The patient’s
hCG level decreased for one week, then constantly increased over
the next three weeks. DU revealed rich blood-flow signals within
the myometrium of the uterus. The patient was diagnosed as having
an IM (score, 8) and subsequently underwent four cycles of
chemotherapy with etoposide (100 mg/m2), methotrexate
(200 mg/m2), KSM (6 μg/kg), cyclophosphamide (100
mg/m2) and vincristine (1 mg/m2; EMA-CO
regimen). The interval between each cycle was three weeks. The hCG
level decreased to within the normal range at the beginning of the
third cycle. Following the fourth cycle of chemotherapy, the
patient opted to be managed by follow-up appointments even though
DU showed that blood-flow signals were present within the mass (RI,
0.21). The patient’s hCG level was monitored through the follow-up
appointments and remained normal. Repeated DU indicated that the
blood-flow signals had disappeared after six months. After one year
of chemotherapy, the patient became pregnant again and the
pre-natal diagnosis was normal. The patient delivered a healthy boy
at 39 weeks of pregnancy and demonstrated a normal hCG level six
months after the delivery.
Case 3
Case 3 was that of a 34-year-old female (gravida 2,
para 1) who was known to have pelvic inflammatory disease. The
patient underwent in vitro fertilization-embryo transfer
(IVF-ET) subsequent to being diagnosed with a blockage of the
fallopian tubes. The patient experienced vaginal bleeding at 16
weeks of pregnancy. Repeat TVS revealed two viable fetuses,
although one placenta was identified as cystic. After careful
consideration, the patient and her partner decided to terminate the
pregnancy and delivery was induced with rivanol. A histological
examination confirmed the clinical impression of one normal
placenta and a second PHM (69, XXY). Three weeks after curettage,
the serial serum hCG concentrations increased continually and TVS
indicated that the trophoblast had invaded the uterine myometrium.
CT of the chest indicated that the trophoblast had metastasized to
the lung (score, 8). Following three cycles of 5-FU with KSM, the
hCG level decreased into the normal range. Subsequent to the next
two cycles of chemotherapy, CT of the chest indicated that the
metastasis had disappeared. However, TVS revealed blood-flow
signals within the mass (RI, 0.31). The patient decided to
temporarily stop therapy and wished to be managed by follow-up
appointments. Two months after ceasing chemotherapy, DU did not
reveal any blood-flow signals within the mass. The patient’s hCG
level remained normal for the following 12 months.
Discussion
The combined use of grayscale and color DU is able
to depict the patency and flow dynamics of larger vessels in
tumors. The analysis of spectral waveforms has met with variable
success with regard to differentiating between benign and malignant
lesions (3–5). A few studies have reported that a
reduction in the number of blood-flow signals indicates the
efficiency of tumor therapy in malignant lesions in mice (6,7). The
use of contrast-enhanced ultrasound with microbubbles is capable of
improving the accuracy of the diagnosis. However, limitations in
spatial resolution, operator dependence, the short time window
available for imaging and the limited field of view restrict the
widespread use of microbubble ultrasound. Positron emission
tomography (PET) with 18F-fluorodeoxyglucose may aid in
the identification of the site of active disease to aid in a
resection and cure (8,9).
An HM results in pregnancies with excessive
proliferation of placental villi, but severely stunted or absent
embryonic development. HM should be regarded as a pre-malignant
lesion since 15–20% of CHMs and 1% of PHMs undergo malignant
transformation into IMs, choriocarcinomas or, rarely, PSTTs
(1). The most significant problem
is that it is difficult to predict which HMs are likely to undergo
malignant transformation. Patients must wait a number of weeks or
months to find out if they require chemotherapy after the diagnosis
of a molar pregnancy. Certain genetic disorders have been
identified as being expressed in moles and they indicated the
malignant transformation of HMs (10,11).
However, it is unknown whether residual CHMs acquire additional
genetic changes after evacuation; these mutations may promote
malignant transformation. IMs are usually diagnosed clinically
rather than pathologically, based on the persistent elevation of
hCG levels following molar evacuation, and are frequently treated
with chemotherapy without a histopathological diagnosis.
Combination chemotherapy consisting of 5-FU and KSM or EMA-CO has
been used as to treat gestational trophoblastic neoplasia (GTN) in
China. Patients with GTN who progress during or after primary
chemotherapy have excellent outcomes, with ~100% of low-risk and
~84% of high-risk patients being cured.
In the present cases, the hCG levels returned to
normal and TVS indicated blood-flow signals within the masses.
Subsequent to reviewing these cases, we suggest that IM patients
should continue to be followed up if they complete regular
chemotherapy.
References
|
1
|
Seckl MJ, Sebire NJ and Berkowitz RS:
Gestational trophoblastic disease. Lancet. 376:717–729. 2010.
View Article : Google Scholar
|
|
2
|
Tse KY, Chan KKL, Tam KF and Ngan H: An
update on gestational trophoblastic disease. Obstet Gynaecol Reprod
Med. 22:7–14. 2012. View Article : Google Scholar
|
|
3
|
Korpanty G, Carbon JG, Grayburn PA,
Fleming JB and Brekken RA: Monitoring response to anticancer
therapy by targeting microbubbles to tumor vasculature. Clin Cancer
Res. 13:323–330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nishida M, Koito K, Hirokawa N, Hori M,
Satoh T and Hareyama M: Does contrast-enhanced ultrasound reveal
tumor angiogenesis in pancreatic ductal carcinoma? A prospective
study. Ultrasound Med Biol. 35:175–185. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Balleyguier C, Opolon P, Mathieu MC, et
al: New potential and applications of contrast-enhanced ultrasound
of the breast: Own investigations and review of the literature. Eur
J Radiol. 69:14–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gee MS, Saunders HM, Lee JC, et al:
Doppler ultrasound imaging detects changes in tumor perfusion
during antivascular therapy associated with vascular anatomic
alterations. Cancer Res. 61:2974–2982. 2001.
|
|
7
|
Goertz DE, Yu JL, Kerbel RS, Burns PN and
Foster FS: High-frequency Doppler ultrasound monitors the effects
of antivascular therapy on tumor blood flow. Cancer Res.
62:6371–6375. 2002.PubMed/NCBI
|
|
8
|
Champion L, Brain E, Giraudet AL, et al:
Breast cancer recurrence diagnosis suspected on tumor marker
rising: value of whole-body 18FDG-PET/CT imaging and impact on
patient management. Cancer. 117:1621–1629. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dhillon T, Palmieri C, Sebire NJ, et al:
Value of whole body 18FDG-PET to identify the active site of
gestational trophoblastic neoplasia. J Reprod Med. 51:879–887.
2006.PubMed/NCBI
|
|
10
|
Zhang XW, Zhu HB, Wu SY, et al:
Distribution of the alleles at loci D16S539, D7S820, and D13S317 in
hydatidiform mole genome from Chinese women and its relationship
with clinical prognosis. Cancer Genet Cytogenet. 164:133–136. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yazaki-Sun S, Daher S, de Souza Ishigai
MM, Alves MT, Mantovani TM and Mattar R: Correlation of c-erbB-2
oncogene and p53 tumor suppressor gene with malignant
transformation of hydatidiform mole. J Obstet Gynaecol Res.
32:265–272. 2006. View Article : Google Scholar : PubMed/NCBI
|