Introduction
Nodular hyperplasia is the most common form of
thyroid disease (1), while sporadic
(nodular) goiter is the most common type to be observed in the
United States. Although the pathogenesis of nodular goiter remains
unknown (2), a mild dietary
deficiency of iodine, a slight impairment of hormone synthesis, an
increased level of iodine clearance by the kidneys, the presence of
thyroid-stimulating immunoglobulins and an increased production of
insulin-like growth factor have all been suggested as causes
(3–5). The incidence of the disease in the
general adult population is 3–5% clinically and 50% at autopsy
(6,7). Histopathologically, a wide range of
appearances may be observed in the form of secondary degenerative
changes, including hemorrhages, calcification, hyalinization,
fibrosis and cystic degeneration. Occasionally, osseous metaplasia
may occur (1). However, mature bone
formation in a thyroid nodule is a rare occurrence (8,9). The
present study describes the cases of three female patients with
thyroid nodules showing osseous metaplasia with mature bone
formation. The Insititional Review Board of Chosun University
Hospital waived the requirement for written informed consent due to
the nature of the study.
Case reports
Case 1
A 41-year-old female was examined in the Department
of Endocrinology of Chosun University Hospital (Gwangju, South
Korea) for thyroidal nodules due to the presence of thyroidal
lesions that had been detected in a routine health examination
three months prior to admittance to the hospital. The patient had
no clinical symptoms, including endocrinological manifestations,
compressive symptoms or palpable lumps. The serological thyroidal
hormone levels were within the normal limits. Ultrasonography (US)
of the thyroid revealed a 3-mm nodule in the right upper pole and a
10-mm nodule in the right lower lobe. The remaining thyroid gland
was normal. A right lobectomy was performed. On gross examination,
the cut surface revealed a 10×10-mm lesion, which was gray-yellow
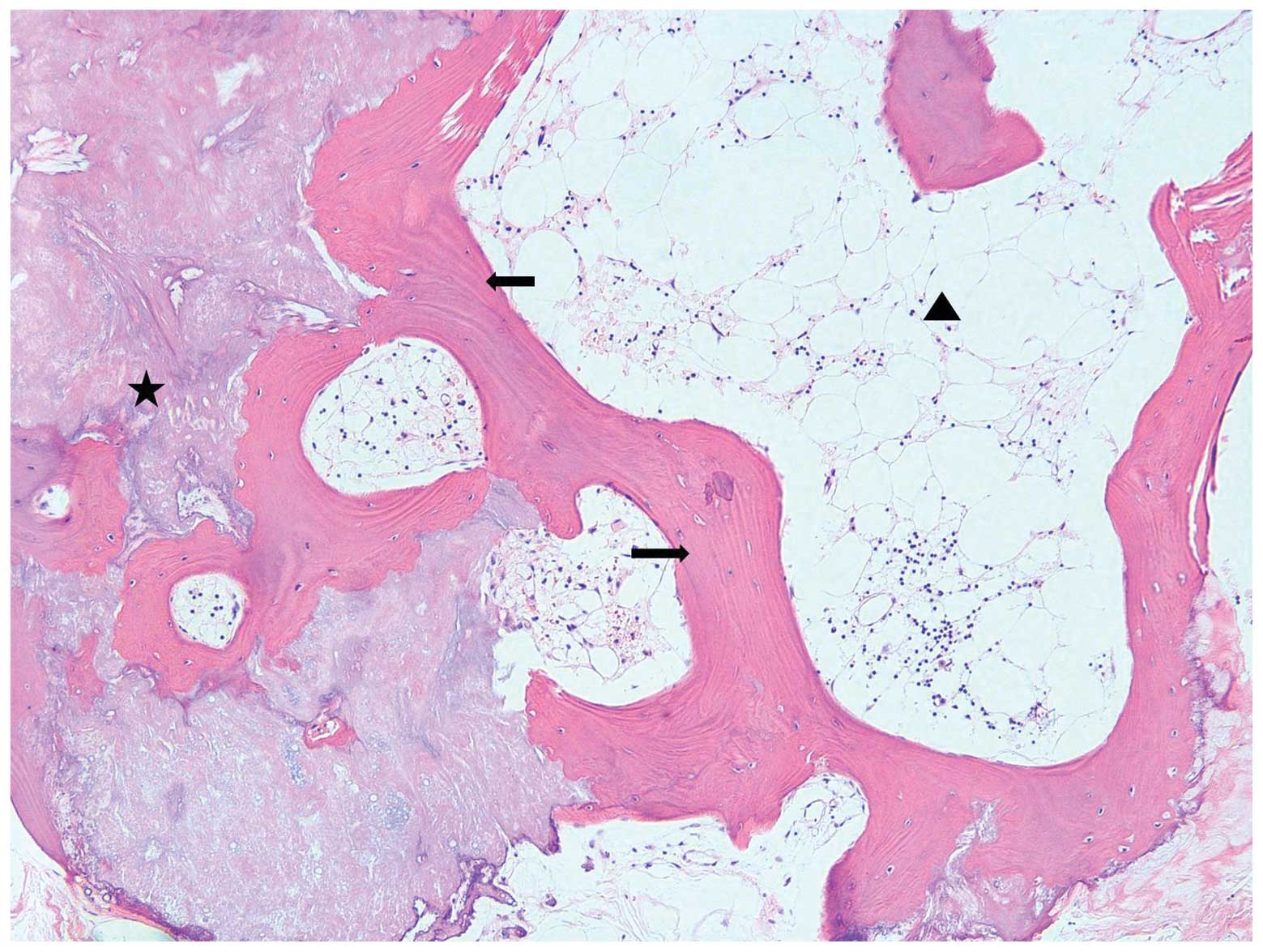
in color and had a bony-hard consistency. Microscopically, this
lesion was a circumscribed mass composed of hyperplastic thyroidal
follicles and mature bony trabeculae filled with mature fatty
marrow (Fig. 1). The remaining
thyroid gland presented with the appearance of nodular hyperplasia.
The final diagnosis was nodular hyperplasia with mature bone
formation.
Case 2
A 49-year-old female was admitted to the Surgery
Clinic of Chosun University Hospital with a neck mass that had been
present for several years. All the laboratory test results,
including the thyroid hormone levels, were within the normal
limits. The patient had not been previously treated with any form
of irradiation and had no other disease present in the neck area.
US examination of the thyroid revealed multiple thyroidal nodules
with diameters measuring 40 mm in the left lobe, 16 mm in the
isthmus, 7.5 mm in the right upper pole, 10 mm in the right
mid-pole and 8.5 mm in the right lower lobe. A near total
thyroidectomy was performed once the clinical diagnosis of a
suspicious malignancy had been made. Grossly, multiple
well-demarcated nodules were observed throughout the thyroid; a
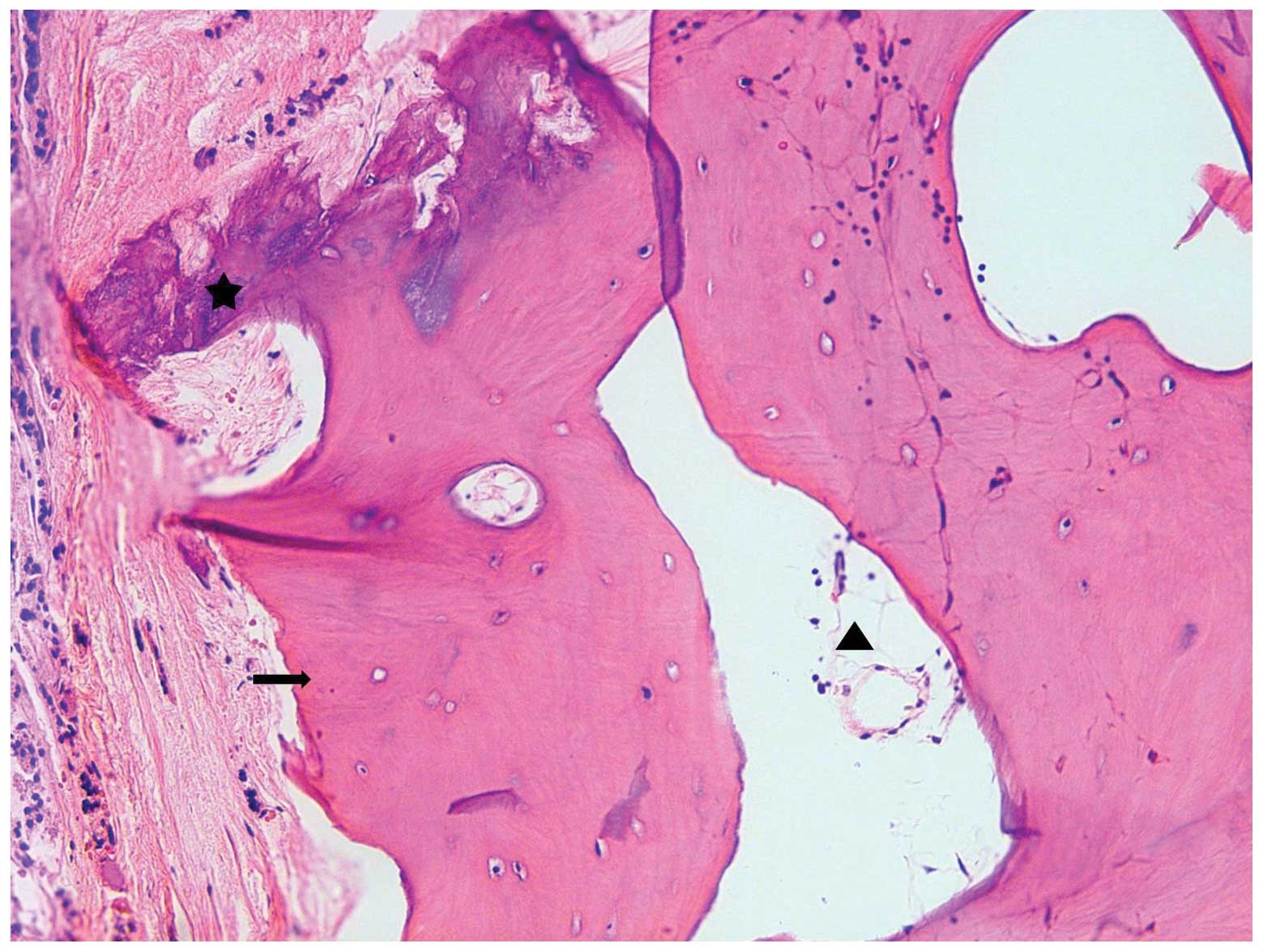
section from the right lobe demonstrated the presence of a mass
with a hard consistency accompanied by calcification, which was
microscopically shown to be a mature bone formation with fatty
marrow and osseous metaplasia, as shown in Fig. 2. The remaining thyroid gland
presented nodular hyperplasia showing dense hyalinization, fibrosis
and cystic changes. The outcome of the patient following resection
was unremarkable.
Case 3
The last case was of a 72-year-old female with a
multinodular non-toxic goiter, which was detected by US of the
thyroid. The laboratory test results, including the thyroid hormone
levels, were within the normal limits. A total thyroidectomy was
performed in the Department of ENT of Chosun University Hospital
following the clinical diagnosis of a large-sized multinodular
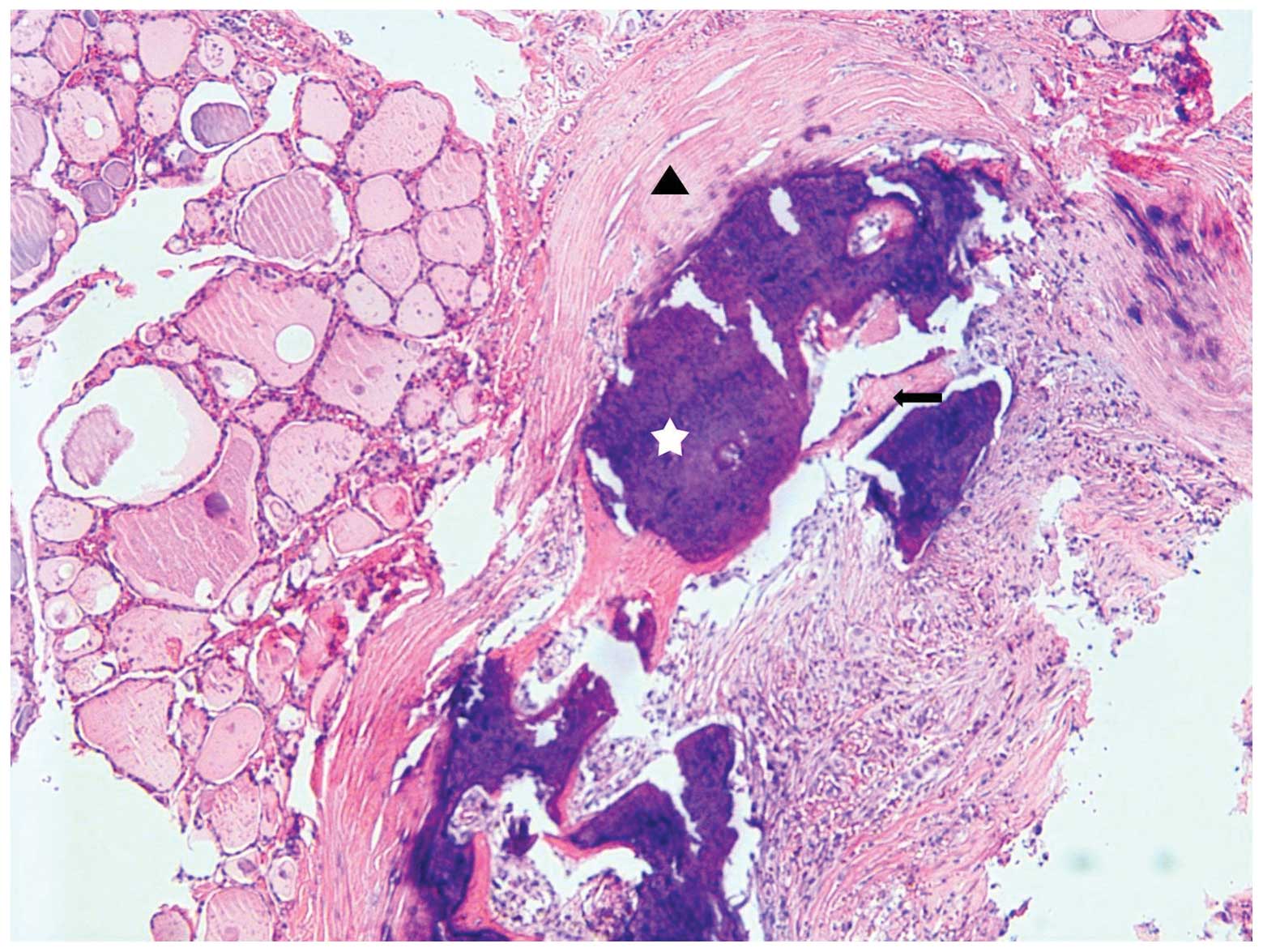
goiter. Histopathologically, there were multiple nodules showing
secondary changes, including calcification, ossification and
fibrosis in the resected specimen(Fig.
3).
Discussion
Multinodular goiters, which are mainly caused by
iodine deficiency, represent the most common form of thyroid
disease in Europe and the United States, and degenerative changes
are frequently observed (10,11). A
rupture of the follicles leads to a granulomatous reaction to the
colloid with the appearance of histiocytes and foreign body-type
giant cells. Areas of fresh and old hemorrhages, coarse fibrous
trabeculation and foci of calcification are common. Occasionally,
osseous metaplasia is observed (1).
Although dystrophic calcification may often be detected in a
nodular goiter, maturation of this calcified tissue to mature bone
is extremely rare (12).
The etiopathogenesis of osseous metaplasia remains
unclear, although various theories have been proposed. A specific
morphogenetic factor, bone morphogenetic factor (BMP), plays a
significant role in bone formation, inducing local ossification and
synthesizing a ground substance and collagen. However, the final
step in bone formation depends on the presence of adequate
concentrations of calcium and phosphate (12,13).
The BMP family has at least 30 members, among which BMPs 1–7, which
were initially isolated from demineralized bone matrix, are capable
of inducing ectopic bone formation (14). Of these BMPs, BMP-1 is a type of
metalloproteinase with conserved domains that is able to convert a
variety of precursor proteins into mature or active forms that are
involved in extracellular matrix formation (15–18).
BMP-1 converts procollagen types I, II, III and VII into their
mature forms and also mediates the C-terminal processing of the
procollagen V homotrimer (16–18). A
study by Basbug et al(19),
which was conducted by the Tumor Research Center, showed that
calcified thyroid tissue has a significantly higher expression of
BMP-2 compared with that of normal thyroid tissue.
In conclusion, the present study reports the notable
and unusual cases of multinodular goiter with osseous metaplasia
and mature bone formation in three female patients.
References
|
1
|
Rosai J: Thyroid gland. Rosai and
Ackerman’s Surgical Pathology. 9th edition. Mosby; New York, NY:
529. 2004
|
|
2
|
Derwahl M and Studer H: Nodular goiter and
goiter nodules: Where iodine deficiency falls short of explaining
the facts. Exp Clin Endocrinol Diabetes. 109:250–260. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brown RS, Pohl SL, Jackson IMD and
Reichlin S: Do thyroid-stimulating immunoglobulins cause non-toxic
and toxic multinodular goitre? Lancet. 29:904–906. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maiorano E, Ambrosi A, Giorgino R, Fersini
M, Pollice L and Ciampolillo A: Insulin-like growth factor 1
(IGF-1) in multinodular goiters: a possible pathogenetic factor.
Pathol Res Pract. 190:1012–1016. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Studer H and Ramelli F: Simple goiter and
its variants: euthyroid and hyperthyroid multinodular goiters.
Endocr Rev. 3:40–61. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mortensen JD, Bennett WA and Woolner LB:
Incidence of carcinoma in thyroid glands removed at 1000
consecutive routine necropsies. Surg Forum. 5:659–663.
1955.PubMed/NCBI
|
|
7
|
Tunbridge WM, Evered DC, Hall R, Appleton
D, Brewis M, Clark F, et al: The spectrum of thyroid disease in a
community: the Whickham survey. Clin Endocrinol (Oxf). 7:481–493.
1977. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Visonà A, Pea M, Bozzola L, Stracca-Pansa
V and Meli S: Follicular adenoma of the thyroid gland with
extensive chondroid metaplasia. Histopathology. 18:278–279.
1991.PubMed/NCBI
|
|
9
|
Ardito G, Fadda G, Revelli L, Modugno P,
Lucci C, Ardito F, et al: Follicular adenoma of the thyroid gland
with extensive bone metaplasia. J Exp Clin Cancer Res. 20:443–445.
2001.PubMed/NCBI
|
|
10
|
Wang C and Crapo LM: The epidemiology of
thyroid disease and implications for screening. Endocrinol Metab
Clin North Am. 26:189–218. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vanderpump MP, Tunbridge WM, French JM,
Appleton D, Bates D, Clark F, et al: The incidence of thyroid
disorders in the community: a twenty-year follow-up of the Whickham
Survey. Clin Endocrinol (Oxf). 43:55–68. 1995.
|
|
12
|
Pontikides N, Botsios D, Kariki E,
Vassiliadis K and Krassas GE: Extramedullary hemopoiesis in a
thyroid nodule with extensive bone metaplasia and mature bone
formation. Thyroid. 13:877–880. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Akbulut S, Yavuz R, Akansu B, Sogutcu N,
Arikanoglu Z and Basbug M: Ectopic bone formation and
extramedullary hematopoiesis in the thyroid gland: report of a case
and literature review. Int Surg. 96:260–265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hopkins DR, Keles S and Greenspan DS: The
bone morphogenetic protein 1/Tolloid-like metalloproteinases.
Matrix Biol. 26:508–523. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amano S, Scott IC, Takahara K, Koch M,
Champliaud MF, Gerecke DR, et al: Bone morphogenetic protein 1 is
an extracellular processing enzyme of the laminin 5 gamma 2 chain.
J Biol Chem. 275:22728–22735. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee S, Solow-Cordero DE, Kessler E,
Takahara K and Greenspan DS: Transforming growth factor-beta
regulation of bone morphogenetic protein-1/procollagen C-proteinase
and related proteins in fibrogenic cells and keratinocytes. J Biol
Chem. 272:19059–19066. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rattenholl A, Pappano WN, Koch M, Keene
DR, Kadler KE, Sasaki T, et al: Proteinases of the bone
morphogenetic protein-1 family convert procollagen VII to mature
anchoring fibril collagen. J Biol Chem. 277:26372–26378. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kessler E, Fichard A, Chanut-Delalande H,
Brusel M and Ruggiero F: Bone morphogenetic protein-1 (BMP-1)
mediates C-terminal processing of procollagen V homotrimer. J Biol
Chem. 276:27051–27057. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Basbug M, Yavuz R, Dablan M and Akansu B:
Extensive osseous metaplasia with mature bone formation of thyroid
gland. J Endocrine Met. 2:99–101. 2012.
|