Introduction
Upper urinary tract urothelial carcinoma (UTUC)
accounts for ~5% of all urothelial tumors and 10% of all renal
tumors (1). Since the disease
recurrence and progression rates are high in patients with UTUC
(2), an improved understanding of
the prognostic parameters may lead to the identification of
patients who may benefit from intensified therapy and
monitoring.
The classical risk factors for the development of
UTUC include smoking, abuse of analgesics, chronic urinary tract
infection, urolithiasis and oncological agents, such as
cyclophosphamide (3). A significant
prognostic factor of UTUC is the disease stage. The five-year
survival rate for low stage tumors is ~90%, which decreases to
<30% in cases of regional nodal metastases and to <10% in
cases of distant metastases (4).
To date, several contemporary, single-center studies
of patients who were treated with radical nephroureterectomy (RNU)
for UTUC have been published (5–9), and
several risk factors for developing UTUC have been reported,
including a delay in the RNU (10)
and tumor necrosis (11). Although
the studies have largely contributed to our understanding of the
disease, they were limited by small and heterogeneous populations.
To overcome this limitation and to improve our understanding of the
natural history of UTUC, a comprehensive database [the Upper Tract
Urothelial Carcinoma Collaboration (UTUCC)] incorporating the
clinicopathological characteristics and outcomes of >1,300
patients treated with RNU for UTUC at 13 academic centers worldwide
was created in 2008 (12). It was
concluded that an RNU provided durable local control and
cancer-specific survival (CSS) in patients with a localized UTUC,
and that the pathological tumor grade, T stage, lymph node status,
tumor architecture and lymphovascular invasion (LVI) were
significant prognostic variables that were associated with
oncological outcomes, which may potentially be used to select
patients for adjuvant systemic therapy.
However, there have been no studies that considered
the prognostic value of urothelial recurrence in patients with UTUC
of the kidney and ureter that is managed by surgery. Consequently,
the present study focused on the prognostic impact of urothelial
recurrence in comparison with non-urothelial recurrence.
Patients and methods
Patient selection
The present study was a retrospective analysis of
153 consecutive patients with UTUC, who underwent surgery between
1996 and 2009 at Hirakata City Hospital (Osaka, Japan). Of the 153
patients that were screened for the study, 103 patients were
included in the analysis. The inclusion criteria consisted of a
diagnosis of non-metastatic UTUC (any T stage, N0–1 and M0) and
receipt of an RNU with an ipsilateral bladder cuff as the primary
treatment. No patient had an invasive bladder tumor (BT) at the
time of the RNU. Written informed consent was obtained from the
patient. This study was approved by the ethics committee of
Hirakata City Hospital (Osaka, Japan).
In the past, open RNU using an open excision of the
distal ureter with a bladder cuff has been performed to dissect the
kidney, with the entire length of the ureter and an adjacent
segment of the bladder cuff. From June 2003 to date, the approach
that has been used is one of conventional four-trocar nephrectomy.
Once the nephrectomy is completed, the ureter is dissected and the
intact specimen is moved into the pelvis. Next, a semi-Pfannenstiel
incision is made in the lower abdomen, which assists in retrieving
the specimen, eases the dissection of the lower ureter and
facilitates the excision of the bladder cuff. The hilar and
regional lymph nodes that are adjacent to the ipsilateral great
vessel are then resected, if possible.
Study design
The following clinical and pathological variables
were evaluated: Gender, age, tumor side, presence of a BT at
diagnosis, serum level of C-reactive protein, hemoglobin,
histological type, pathological grade, adjuvant chemotherapy,
microvascular invasion, lymphatic invasion, urothelial recurrence,
non-urothelial recurrence and pathological stage (2002 TNM system).
The tumor grading was assessed according to the 1998 World Health
Organization/International Society of Urologic Pathology consensus
classification (13). All surgical
specimens were processed according to the standard pathological
procedures at the Hirakata City Hospital. UTUC was defined as a
urothelial carcinoma located in the renal pelvis or calices, as
well as tumors located within the ureter. C-reactive protein was
pre-operatively obtained from the blood of the UTUC patients and
collected in a serum-separating tube. The C-reactive protein level
was expressed in units of mg/dl. Microvascular invasion was defined
as tumor cells in an endothelium-lined space observed using routine
light microscopy in whole-mounted UTUC specimens. The oncological
follow-up schedule included a physical examination, cystoscopy and
CT imaging between the chest and pelvis twice per year during the
first five years and annually thereafter.
Statistical analysis
The continuous parametric variables were reported as
mean ± SD and range. The continuous non-parametric variables were
presented as median values and interquartile ranges. The F test was
used to assess whether the standard deviations of two populations
were equal. χ2 tests were conducted to assess the
differences in the covariate distributions between the urothelial
and non-urothelial recurrence categories. CSS was defined as the
primary endpoint of the study. The survival interval was defined as
the time elapsed between the surgery and the last clinical
evaluation or cancer-specific mortality. Survival curves were
estimated using the Kaplan-Meier method. The patients who remained
alive or succumbed to other causes were censored. The log-rank test
was used to compare the survival curves. A Cox proportional hazards
regression model was used to verify the clinicopathological
variables that independently predicted CSS. In all statistical
analyses, a two-sided value of P<0.05 was considered to indicate
a statistically significant difference. All data were analyzed
using the PASW Statistics version 17 statistical program (SPSS
Japan Inc., Tokyo, Japan).
Results
A total of 103 patients with comprehensive
clinicopathological data, who fulfilled the inclusion criteria,
were included in the analysis (Table
I). The mean age was 68.6 years (interquartile range, 62–75
years). During the follow-up period, 45 patients (43.7%) succumbed
to UTUC, 12 (11.7%) succumbed to other causes and 38 (36.9%)
displayed evidence of disease recurrence. The median follow-up
period for the surviving patients was 29 months (interquartile
range, 14–63 months). The tumor was located on the right side in 55
patients (53.4%) and on the left side in 48 (46.6%). A BT was
identified at the time of the UTUC diagnosis in 28 patients
(27.2%). The histological type was urothelial in 92 patients
(89.3%) and non-urothelial in 11 (10.7%). The pathological stage
was divided into three groups: Superficial (pT0/pTis/pTa/pT1),
muscle-invasive (pT2) and non-organ confined (pT3/pT4), which were
identified in 43 (41.8%), 13 (12.6%) and 47 (45.6%) patients,
respectively.
 | Table IClinicopathological characteristics
grouped by non-urothelial or urothelial recurrence in 103 patients
treated with an RNU and ipsilateral bladder cuff for UTUC. |
Table I
Clinicopathological characteristics
grouped by non-urothelial or urothelial recurrence in 103 patients
treated with an RNU and ipsilateral bladder cuff for UTUC.
| | Non-urothelial
recurrence | Urothelial
recurrence |
|---|
| |
|
|
|---|
| Characteristics | Total | Yes | No | P-value | Yes | No | P-value |
|---|
| Number of
patients | 103 | 32 | 71 | | 38 | 65 | |
| Gender, n | | | | 0.819 | | | 0.511 |
| Male | 71 | 23 | 48 | | 28 | 43 | |
| Female | 32 | 9 | 23 | | 10 | 22 | |
| Age, years | | | | 0.788 | | | 0.138 |
| Mean | 68.6 | 70.3 | 67.8 | | 67.3 | 71.0 | |
| SD | 10.1 | 9.3 | 10.4 | | 10.2 | 9.4 | |
| Median | 69.0 | 70.5 | 69.0 | | 69.0 | 71.0 | |
| Range | 23–91 | 51–91 | 23–87 | | 23–87 | 54–91 | |
| Tumor side, n | | | | 0.400 | | | 0.221 |
| Right | 55 | 15 | 40 | | 17 | 38 | |
| Left | 48 | 17 | 31 | | 21 | 27 | |
| BT at diagnosis,
n | | | | 0.399 | | | 0.255 |
| Yes | 28 | 11 | 17 | | 13 | 15 | |
| No | 75 | 21 | 54 | | 25 | 50 | |
| C-reactive protein
(mg/ml), n | | | | 0.282 | | | 0.432 |
| <0.3 | 60 | 16 | 44 | | 23 | 37 | |
| ≥0.3 | 43 | 16 | 27 | | 15 | 28 | |
| Hemoglobin (mg/ml),
n | | | | 0.599 | | | 0.614 |
| ≤NR | 75 | 14 | 61 | | 27 | 48 | |
| >NR | 28 | 18 | 10 | | 11 | 17 | |
| Histological type,
n | | | | 0.166 | | | 0.865 |
| Urothelial | 92 | 24 | 68 | | 32 | 60 | |
|
Non-urothelial | 11 | 8 | 3 | | 6 | 5 | |
| Pathological grade,
n | | | | 0.008 | | | 0.278 |
| 1 | 20 | 3 | 17 | | 4 | 16 | |
| 2 | 28 | 5 | 23 | | 12 | 16 | |
| 3 | 55 | 24 | 31 | | 22 | 33 | |
| Adjuvant
chemotherapy, n | | | | 0.508 | | | 0.756 |
| Yes | 12 | 5 | 7 | | 5 | 7 | |
| No | 91 | 27 | 64 | | 33 | 55 | |
| Microvascular
invasion, n | | | | 0.001 | | | 0.214 |
| Absent | 69 | 13 | 56 | | 23 | 46 | |
| Present | 34 | 19 | 15 | | 15 | 19 | |
| Lymphatic invasion,
n | | | | 0.005 | | | 0.654 |
| Absent | 71 | 17 | 54 | | 24 | 47 | |
| Present | 32 | 15 | 17 | | 14 | 18 | |
| pT classification,
n | | | | 0.001 | | | 0.209 |
|
pT0/pTis/pTa/pT1 | 43 | 6 | 37 | | 15 | 28 | |
| pT2 | 13 | 2 | 11 | | 2 | 11 | |
| pT3/pT4 | 47 | 24 | 23 | | 21 | 26 | |
The median ages of the patients at the time of the
surgery in the groups with non-urothelial recurrence (n=32) and
without non-urothelial recurrence (n=71) were 70.5 and 69 years,
respectively. The median ages in the groups with urothelial
recurrence (n=38) and without urothelial recurrence (n=65) were 69
and 71 years, respectively. When comparing the risk parameters
between the non-urothelial recurrence categories, the factors of
pathological grade, microvascular invasion, lymphatic invasion and
pT classification demonstrated significant differences. However,
there were no significant differences observed between the
urothelial recurrence categories.
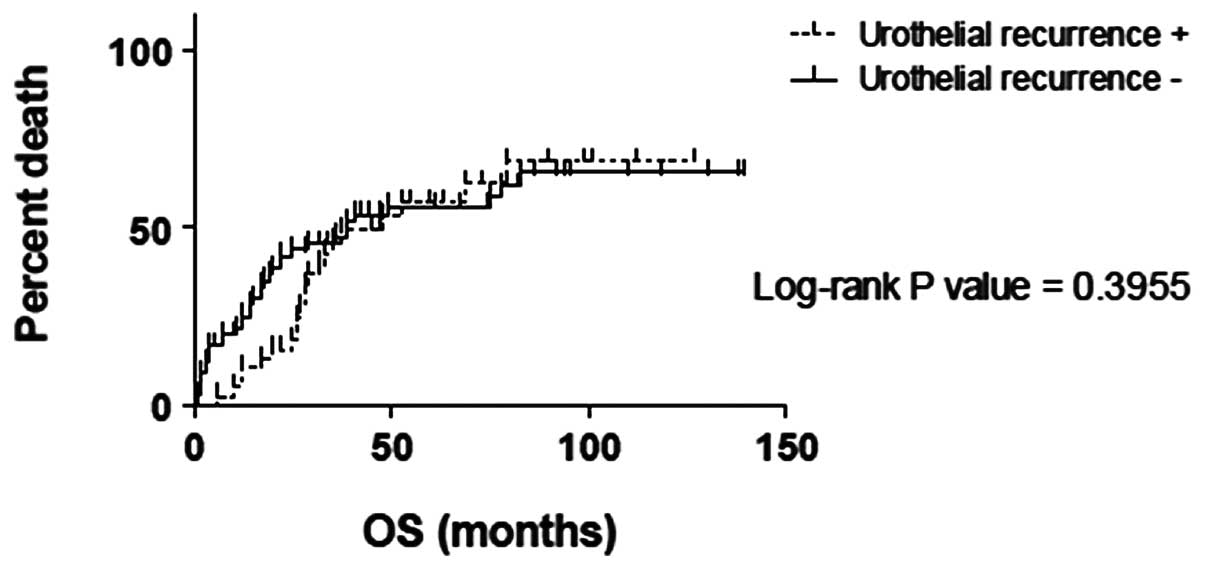
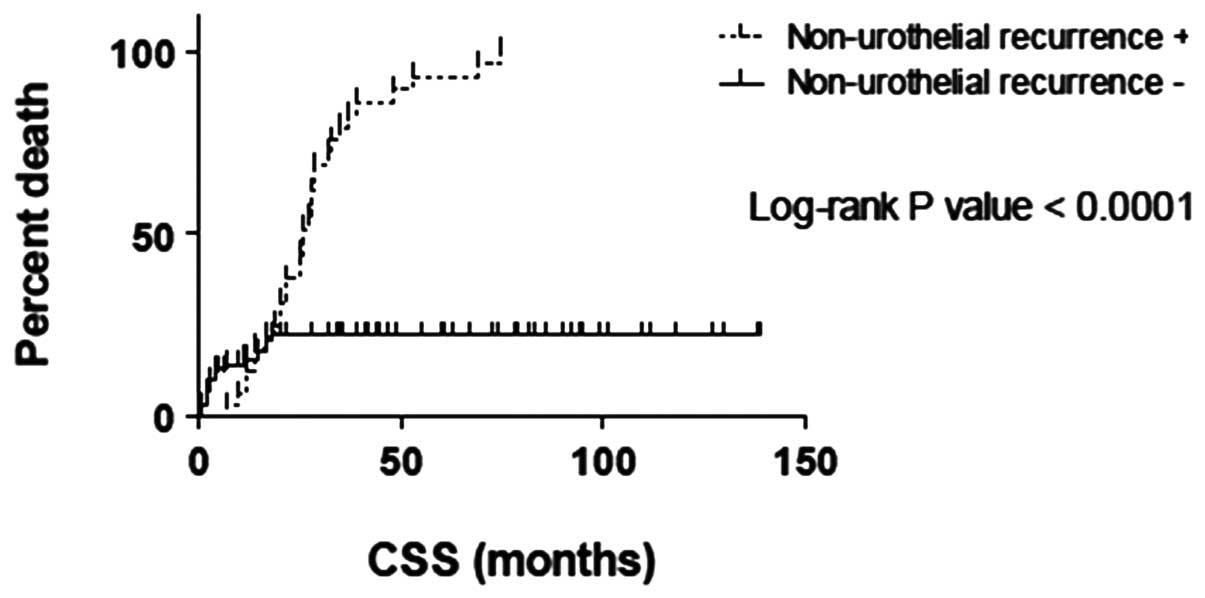
The OS and CSS times between the urothelial
recurrence categories showed no significant differences (P=0.3955
and P=0.05891, respectively), while a significant difference was
observed within the non-urothelial recurrence categories
(P<0.0001 and P<0.0001, respectively; Figs. 1–4).
The univariate analyses using the
clinicopathological characteristics, including C-reactive protein,
hemoglobin, pathological grade, non-urothelial recurrence,
microvascular invasion, lymphatic invasion and pT classification,
were associated with the CSS. In the multivariate analysis for the
clinicopathological characteristics, only non-urothelial recurrence
was associated with a worse CSS (P=0.002, HR 9.512; Table II).
 | Table IIUnivariate and multivariate Cox
regression models for clinicopathological characteristics
predicting CSS in 103 patients treated with RNU and ipsilateral
bladder cuff for UTUC. |
Table II
Univariate and multivariate Cox
regression models for clinicopathological characteristics
predicting CSS in 103 patients treated with RNU and ipsilateral
bladder cuff for UTUC.
| Univariate | Multivariate |
|---|
|
|
|
|---|
|
Characteristics | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Gender |
| Male | Reference | | Reference | | | |
| Female | 0.906 | 0.467–1.758 | 0.771 | 0.945 | 0.318–2.805 | 0.919 |
| Age |
| Continuous | 1.001 | 0.971–1.033 | 0.928 | 0.998 | 0.922–1.080 | 0.954 |
| Tumor side |
| Right | Reference | | Reference | | | |
| Left | 0.686 | 0.379–1.241 | 0.213 | 0.475 | 0.172–1.312 | 0.151 |
| BT at
diagnosis |
| Yes | Reference | | Reference | | | |
| No | 1.014 | 0.498–1.952 | 0.968 | 2.494 | 0.089–1.794 | 0.232 |
| C-reactive protein
(mg/ml) |
| Continuous | 1.184 | 1.095–1.281 | 0.000 | 1.189 | 0.933–1.516 | 0.161 |
| Hemoglobin
(mg/ml) |
| Continuous | 1.203 | 0.732–0.944 | 0.004 | 1.133 | 0.685–1.138 | 0.337 |
| Histological type,
n |
| Urothelial | Reference | | Reference | | | |
|
Non-urothelial | 2.036 | 0.924–4.486 | 0.078 | 1.951 | 0.409–9.320 | 0.402 |
| Pathological grade,
n |
| 1/2 | Reference | | Reference | | | |
| 3 | 4.918 | 1.984–12.191 | 0.001 | 2.288 | 0.266–19.705 | 0.451 |
| Adjuvant
chemotherapy |
| Yes | Reference | | Reference | | | |
| No | 1.183 | 0.333–2.144 | 0.723 | 1.499 | 0.149–2.989 | 0.597 |
| Urothelial
recurrence |
| No | Reference | | Reference | | | |
| Yes | 0.929 | 0.511–1.690 | 0.809 | 0.648 | 0.215–1.956 | 0.442 |
| Non-urothelial
recurrence |
| No | Reference | | Reference | | | |
| Yes | 5.750 | 3.018–10.954 | 0.000 | 9.512 | 2.293–39.464 | 0.002 |
| Microvascular
invasion, n |
| Absent | Reference | | Reference | | | |
| Present | 8.299 | 3.909–17.618 | 0.000 | 3.551 | 0.585–21.558 | 0.168 |
| Lymphatic invasion,
n |
| Absent | Reference | | Reference | | | |
| Present | 3.953 | 1.966–7.947 | 0.000 | 1.205 | 0.319–4.553 | 0.784 |
| pT classification,
n |
|
pT0/pTis/pTa/pT1 | Reference | | Reference | | | |
| pT2/pT3/pT4 | 2.619 | 1.676–4.092 | 0.000 | 0.996 | 0.275–3.611 | 0.995 |
Discussion
UTUC is a relatively rare malignancy. Although
affected patients may benefit from endoscopic or nephron-sparing
approaches, an RNU with an ipsilateral bladder cuff excision
remains the standard treatment for patients with large, multifocal
or high-grade tumors. However, despite definitive surgery, UTUC
remains a malignancy with a high potential for local and distant
recurrence, particularly in patients with advanced diseases
(14). The outcomes of patients
with UTUC following an RNU are heterogeneous and, therefore,
difficult to predict. Multi-institutional collaborative studies
have identified several potential factors that predict the outcome
following an RNU for UTUC, supplementing the traditional
pathological staging system (15–17).
Certain papers have examined the prognostic value of
urothelial recurrence (particularly intravesical recurrence)
following the treatment of an RNU for UTUC. Koda et al
reported that intravesical recurrence following surgery for UTUC
was not associated with the mode of surgery (i.e.
laparoscopy-assisted or open surgery), and that the only risk
factor for intravesical recurrence was a history of bladder cancer
(18). Several other studies
reported that it may be important to perform careful follow-up
appointments that target intravesical recurrence for patients,
particularly males and those with low-stage tumors and/or
multifocal tumors, following RNU (19, 20).
Concomitant carcinoma in situ (CIS) and the tumor size were
predictors for bladder cancer recurrence (21). In a series of 196 patients, bladder
recurrence was lower in those who received mitomycin C or
epirubicin compared with those who did not received anything (29.0,
25.9 and 41.3%, respectively) (22). Novara et al observed that
only a history of bladder cancer prior to an RNU was an independent
risk factor for metachronous recurrence, which was identified in 6%
of patients (23). Youssef et
al underlined the prognostic impact of previous bladder cancer
(24); patients with a positive
bladder cancer (CIS) history had a greater risk of recurrence and
mortality from UTUC following RNU (24).
The common locations for the spread of UTUC,
depending on the site of the primary tumor, include para-aortic,
paracaval, ipsilateral common iliac and pelvic lymph nodes.
Hematogenous seeding also occurs in the liver, lungs and bone,
which are common sites for metastases. Once a distant metastasis is
diagnosed, the prognosis for the patient is extremely poor, in
spite of chemotherapy. Certain publications suggest a benefit from
the surgical removal of urothelial carcinoma metastases for a
subgroup of patients (25). In a
large German retrospective study, only 44 patients with distant
metastases of the bladder or upper urinary tract underwent a
complete resection of all the detectable metastases and were
analyzed. The resected metastatic sites included the
retroperitoneal lymph nodes (56.8%), distant lymph nodes (11.3%),
lung (18.2%), bone (4.5%), adrenal gland (2.3%), brain (2.3%),
small intestine (2.3%) and skin (2.3%). Pre- and/or
post-metastasectomy systemic chemotherapy was administered in 35 of
44 patients (79.5%). Since no significant prognostic factors were
determined due to the limited patient numbers, it was concluded
that the metastasectomy in the patients with disseminated
urothelial carcinoma metastases remained investigational and there
are a limited number of disease types for which a combined-modality
approach with systemic chemotherapy would be successful (26).
Lymph node dissection (LND) appears to have an
impact on node-positive patients (27). In one previous study, 76 out of 293
patients developed disease relapse. Regional lymph node recurrence
was the most common type of relapse (34 patients). In the
multivariate analyses that adjusted for the effect of tumor stage
and grade, pNx (skipping LND) was an adverse factor for
locoregional recurrence and distant relapse (28). However, in the study by Lughezzani
et al, which analyzed 2,824 patients from the Surveillance,
Epidemiology and End Results (SEER) database, LND showed no benefit
in patients with an N0 status compared with those with an Nx status
(29). Roscigno et al
suggested that LND should be performed in patients with suspected
T2–4 stage diseases, to improve the prediction of the natural
history of surgically treated UTUC and to use this information for
possible adjuvant chemotherapy (30). Thus, the method of defining the
right patient, LND template and the extent of the LND remains
unclear.
The present study examined the prognostic value of
urothelial and non-urothelial recurrence in patients with UTUC of
the kidney and ureter that was managed by surgery. The OS and CSS
times between the urothelial recurrence categories showed no
significant differences, while significant differences were
observed in the OS and CSS between the non-urothelial recurrence
categories. The factors of pathological grade, microvascular
invasion, lymphatic invasion and pT classification significantly
affected the non-urothelial recurrence. However, no factors were
observed to significantly affect the urothelial recurrence. In the
multivariate analysis for clinicopathological characteristics, only
non-urothelial recurrence remained associated with a worse CSS. The
present data are limited by the retrospective nature of the study
and the relatively small cohort. Prospective studies are required
to confirm these findings. However, it may be concluded that
non-urothelial recurrence significantly affected the prognosis in
patients with UTUC managed by RNU with an ipsilateral bladder cuff
compared with those with urothelial recurrence. The findings of the
present study underscore the requirement for the careful follow-up
and management of urothelial recurrence in patients with UTUC
managed by RNU, which aids in lowering the risk of mortality due to
cancer.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Margulis V, Youssef RF, Karakiewicz PI, et
al; Upper Tract Urothelial Carcinoma Group. Preoperative
multivariable prognostic model for prediction of nonorgan confined
urothelial carcinoma of the upper urinary tract. J Urol.
184:453–458. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oosterlinck W, Solsona E, van der Meijden
AP, et al; European Association of Urology. EAU guidelines on
diagnosis and treatment of upper urinary tract transitional cell
carcinoma. Eur Urol. 46:147–154. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raman JD and Scherr DS: Management of
patients with upper urinary tract transitional cell carcinoma. Nat
Clin Pract Urol. 4:432–443. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hall MC, Womack S, Sagalowsky AI, Carmody
T, Erickstad MD and Roehrborn CG: Prognostic factors, recurrence,
and survival in transitional cell carcinoma of the upper urinary
tract: a 30-year experience in 252 patients. Urology. 52:594–601.
1998.PubMed/NCBI
|
|
6
|
Langner C, Hutterer G, Chromecki T,
Winkelmayer I, Rehak P and Zigeuner R: pT classification, grade,
and vascular invasion as prognostic indicators in urothelial
carcinoma of the upper urinary tract. Mod Pathol. 19:272–279. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Novara G, De Marco V, Gottardo F, et al:
Independent predictors of cancer-specific survival in transitional
cell carcinoma of the upper urinary tract: multi-institutional
dataset from 3 European centers. Cancer. 110:1715–1722. 2007.
View Article : Google Scholar
|
|
8
|
Secin FP, Koppie TM, Salamanca JI, et al:
Evaluation of regional lymph node dissection in patients with upper
urinary tract urothelial cancer. Int J Urol. 14:26–32. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brown GA, Busby JE, Wood CG, et al:
Nephroureterectomy for treating upper urinary tract transitional
cell carcinoma: Time to change the treatment paradigm? BJU Int.
98:1176–1180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Waldert M, Karakiewicz PI, Raman JD, et
al: A delay in radical nephroureterectomy can lead to upstaging.
BJU Int. 105:812–817. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zigeuner R, Shariat SF, Margulis V, et al:
Tumour necrosis is an indicator of aggressive biology in patients
with urothelial carcinoma of the upper urinary tract. Eur Urol.
57:575–581. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Margulis V, Shariat SF, Matin SF, et al;
The Upper Tract Urothelial Carcinoma Collaboration. Outcomes of
radical nephroureterectomy: a series from the Upper Tract
Urothelial Carcinoma Collaboration. Cancer. 115:1224–1233. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Epstein JI, Amin MB, Reuter VR and Mostofi
FK: The World Health Organization/International Society of
Urological Pathology consensus classification of urothelial
(transitional cell) neoplasms of the urinary bladder. Bladder
Consensus Conference Committee. Am J Surg Pathol. 22:1435–1448.
1998. View Article : Google Scholar
|
|
14
|
Eng MK and Shalhav AL: Laparoscopic
nephroureterectomy: long-term outcomes. Curr Opin Urol. 18:157–162.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kikuchi E, Margulis V, Karakiewicz PI, et
al: Lymphovascular invasion predicts clinical outcomes in patients
with node-negative upper tract urothelial carcinoma. J Clin Oncol.
27:612–618. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Remzi M, Haitel A, Margulis V, et al:
Tumour architecture is an independent predictor of outcomes after
nephroureterectomy: a multi-institutional analysis of 1363
patients. BJU Int. 103:307–311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Otto W, Shariat SF, Fritsche HM, et al:
Concomitant carcinoma in situ as an independent prognostic
parameter for recurrence and survival in upper tract urothelial
carcinoma: a multicenter analysis of 772 patients. World J Urol.
29:487–494. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koda S, Mita K, Shigeta M and Usui T: Risk
factors for intravesical recurrence following urothelial carcinoma
of the upper urinary tract: no relationship to the mode of surgery.
Jpn J Clin Oncol. 37:296–301. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kusuda Y, Miyake H, Terakawa T, Kondo Y,
Miura T and Fujisawa M: Gender as a significant predictor of
intravesical recurrence in patients with urothelial carcinoma of
the upper urinary tract following nephroureterectomy. Urol Oncol.
Aug 6–2011.(Epub ahead of print).
|
|
20
|
Terakawa T, Miyake H, Muramaki M, Takenaka
A, Hara I and Fujisawa M: Risk factors for intravesical recurrence
after surgical management of transitional cell carcinoma of the
upper urinary tract. Urology. 71:123–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pieras E, Frontera G, Ruiz X, Vicens A,
Ozonas M and Pizá P: Concomitant carcinoma in situ and tumour size
are prognostic factors for bladder recurrence after
nephroureterectomy for upper tract transitional cell carcinoma. BJU
Int. 106:1319–1323. 2010. View Article : Google Scholar
|
|
22
|
Wu WJ, Ke HL, Yang YH, Li CC, Chou YH and
Huang CH: Should patients with primary upper urinary tract cancer
receive prophylactic intravesical chemotherapy after
nephroureterectomy? J Urol. 183:56–61. 2010. View Article : Google Scholar
|
|
23
|
Novara G, De Marco V, Dalpiaz O, et al:
Independent predictors of contralateral metachronous upper urinary
tract transitional cell carcinoma after nephroureterectomy:
multi-institutional dataset from three European centers. Int J
Urol. 16:187–191. 2009. View Article : Google Scholar
|
|
24
|
Youssef RF, Shariat SF, Lotan Y, et al:
Prognostic effect of urinary bladder carcinoma in situ on clinical
outcome of subsequent upper tract urothelial carcinoma. Urology.
77:861–866. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Remzi M, Shariat S, Huebner W, Fajkovic H
and Seitz C: Upper urinary tract urothelial carcinoma: what have we
learned in the last 4 years? Ther Adv Urol. 3:69–80.
2011.PubMed/NCBI
|
|
26
|
Lehmann J, Suttmann H, Albers P, et al:
Surgery for metastatic urothelial carcinoma with curative intent:
the German experience (AUO AB 30/05). Eur Urol. 55:1293–1299. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bolenz C, Shariat SF, Fernández MI, et al:
Risk stratification of patients with nodal involvement in upper
tract urothelial carcinoma: value of lymph-node density. BJU Int.
103:302–306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abe T, Shinohara N, Muranaka M, et al:
Role of lymph node dissection in the treatment of urothelial
carcinoma of the upper urinary tract: multi-institutional relapse
analysis and immunohistochemical re-evaluation of negative lymph
nodes. Eur J Surg Oncol. 36:1085–1091. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lughezzani G, Jeldres C, Isbarn H, et al:
A critical appraisal of the value of lymph node dissection at
nephroureterectomy for upper tract urothelial carcinoma. Urology.
75:118–124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roscigno M, Shariat SF, Margulis V, et al:
Impact of lymph node dissection on cancer specific survival in
patients with upper tract urothelial carcinoma treated with radical
nephroureterectomy. J Urol. 181:2482–2489. 2009. View Article : Google Scholar : PubMed/NCBI
|