Introduction
Osteosarcoma is the most common primary tumor of the
bone. Patients with the condition had a poor prognosis until the
1970s. Major progress was made with the introduction of neoadjuvant
chemotherapy, which combines doxorubicin (ADM), cisplatin,
methotrexate and/or ifosfamide, resulting in a 5-year survival rate
of 60–70% (1–4). However, the rates have not improved
further (5). Certain patients,
including 74% with lung metastases at the time of diagnosis, have a
poor response to neoadjuvant chemotherapy (6), in which treatment-related toxicity and
mortality are the main limiting factors allowing no further
treatment intensification. Furthermore, a higher dosage of
chemotherapy has been shown to result in more long-term
complications, including cardiac failure (7,8). Human
osteoskeletal non-neoplastic and neoplastic disorders are diseases
in which gender differences are markedly noted in the incidence and
development. The highest incidence of osteosarcoma is observed in a
younger patient group with high levels of sex hormone and sex
steroid receptor activity. Estrogen receptors (ERs) have been
sporadically reported in human osteosarcoma or its cell lines. Sex
steroids and receptors play significant roles in the regulation of
cell proliferation in human osteosarcoma.
Tamoxifen (TAM), a selective ER modulator (SERM),
has been used widely as the first-line drug of breast cancer
chemotherapy with little side effects (9). Studies have shown that TAM may have
the ability to enhance the relative sensitivity of tumors of
non-sex-hormone-targeted organs to chemotherapeutics (10). As TAM and ADM have antitumoral
properties, a combination of these medications may be an option in
the therapy of osteosarcoma. However, no data regarding their
potential synergistic effects are currently available. Therefore,
the present study examined ER expression in the MG63 human
osteosarcoma cell line and compared the combined effect of TAM and
ADM with the individual effects of TAM and ADM in MG63 human
osteosarcoma cells.
Materials and methods
Cells and cell culture
The MG63 human osteosarcoma cell line was provided
by the Chinese Academy of Science (Shanghai, China). The cells were
cultured in Dulbecco’s modified Eagle’s medium (DMEM), supplemented
with 10% fetal calf serum and antibiotics, at 37°C in a humidified
atmosphere with 5% CO2. All cell culture reagents were
purchased from Gibco Co., Ltd. (Carlsbad, CA, USA).
Analysis of ER mRNA expression
Due to the low expression of ER in osteoskeletal
cells (11) and the difficulty in
examining ER through immunohistochemistry, reverse transcription
(RT) PCR for ERα and ERβ was performed on the MG63 cells. Total RNA
was extracted using TRIzol (Invitrogen Life Technologies, Carlsbad,
CA, USA) from the MG63 cells according to the manufacturer’s
instructions. First-strand cDNA was synthesized from 1 μg total RNA
by reverse transcription using oligo-dT primers and reverse
transcriptase (Invitrogen Life Technologies) according to the
manufacturer’s instructions. cDNA was utilized as a template for
the amplification of full-length ERα and ERβ using the following
primers: 5′-TTGCTATGTTACTAAGCGTGAG-3′ for ERα and
5′-GATGCTTTGGTTTGGGTGATT-3′ for ERβ. PCR was performed in 50 μl
volumes containing 1 μl cDNA, 1 μl (10 μM) of each primer and 4 μl
(2.5 mM) of each dNTP in a reaction buffer containing 1 μl (2.5
u/μl) Taqplus (Invitrogen Life Technologies). The thermocycling
conditions consisted of an initial incubation step at 95°C to
activate the polymerase enzyme, followed by 35 cycles consisting of
45 sec at 95°C, 45 sec at 60°C and 45 sec at 72°C. The PCR products
were separated in 2% agarose gels and visualized by ethidium
bromide staining. The housekeeping gene, β-actin
(5′-AGGGGCCGGACTCGTCATACT-3′), was used as a control.
Drugs and drug treatment
The MG63 cells were divided into three groups
according to the incubation time (24, 48 and 72 h). Each group was
treated with various concentrations of TAM (First Pharmaceuticals,
Hangzhou, China) and ADM (Farmitalia Carlo Erba, Milan, Italy).
According to the clinical serum concentrations of the two drugs,
concentrations of 0.1, 1, 5 or 10 μg/ml ADM and 1, 2, 5 or 10
μmol/l TAM were used for the individual groups, while 5 μg/ml ADM
and 5 μmol/l TAM were used for the AT (ADM and TAM) combination
group.
Morphological changes of cell growth
inhibition
The MG63 osteosarcoma cells were cultured to the
exponential phase of growth and the cell number was adjusted to
1.0×105 cells/ml and seeded in 25-cm3 tissue
culture flasks. Subsequent to the cells being incubated for 24 h,
the medium was replaced by a culture medium that contained the
various concentrations of drugs, and the cells were incubated for
another 24, 48 and 72 h. The morphological changes during cell
growth inhibition were observed using an inverted microscope.
3-(4,5-Dimethy1-2-thiazol-2-yl)-2,
5-diphenyl-etrazolium bromide (MTT) activity measurement
The MTT assay is a colorimetric measurement of MTT
reduction to a blue formazan product by mitochondrial
dehydrogenases of viable cells (12). The cells were seeded in 96-well
plates at a density of 5×103 cells/well and incubated
for 24 h. Thereafter, the medium was replaced and incubation
continued for a further 24, 48 and 72 h in the presence or absence
of the test compounds of the various concentrations of the drugs.
The medium was replaced and 50 μl MTT (Sigma Chemical Co., St.
Louis, MO, USA) working solution was added to each well at a final
concentration of 0.5 mg/ml. The cells were incubated for 4 h at
37°C and the medium was changed to 100 μl dimethyl sulfoxide to
dissolve the formazan. Optical density (A) was measured at 492 nm
of the wave length using a Tecan Spectrafluor Plus microplate
spectrophotometer (Esbe Scientific Industries Inc., Ontario,
Canada). Each experiment was performed in triplicate. The medium of
the control group was replaced by DMEM supplemented with 10% fetal
calf serum and antibiotics. The quantity of viable cells was
reflected by the cell growth inhibition rate that was calculated
using the following formula: Cell growth inhibition rate (%) = (1 −
optimum A of experimental group/optimum A of control) × 100.
Statistical analysis
The results are expressed as the mean ± standard
error. The data were evaluated by a multivariate analysis of single
variance with repeated measures designs. Multiple comparisons were
performed using one way analysis of variance (ANOVA) by a
Student-Newman-Keuls test. For each variable, at least three
independent experiments were performed. All analyses were performed
using SPSS 20.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
ER expression in MG63 cells
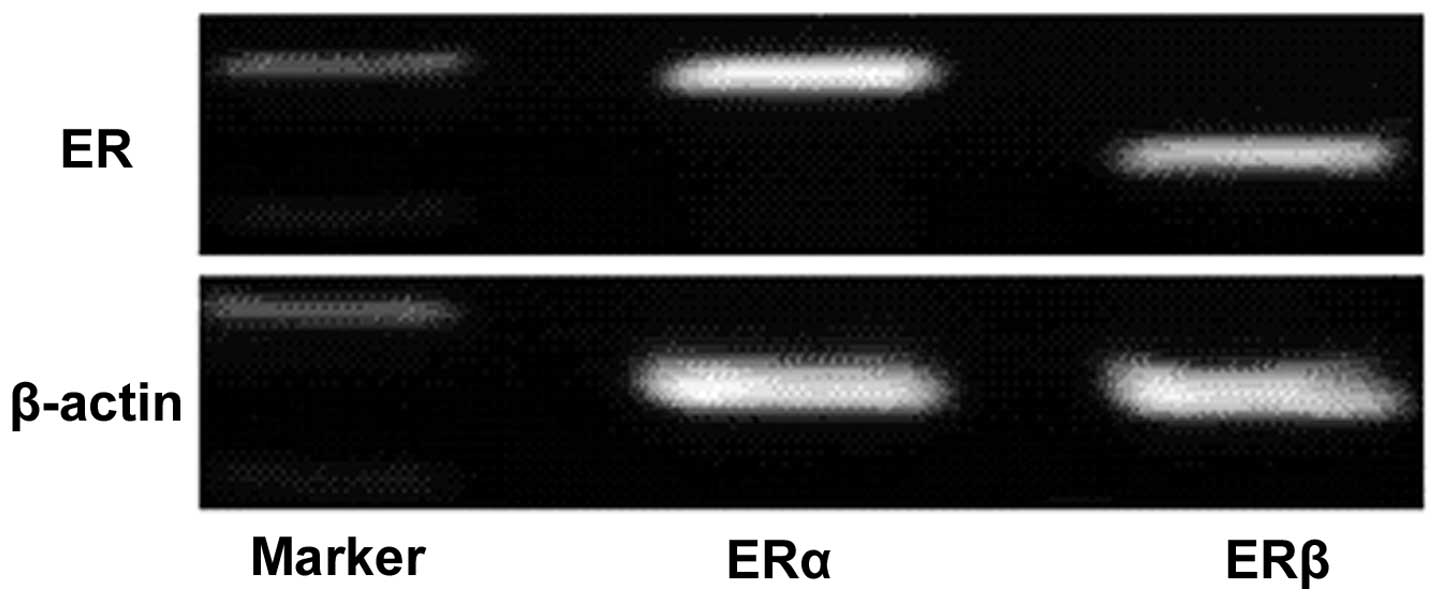
RT-PCR analysis of mRNA that was isolated from the
MG63 human osteosarcoma cell line indicated the presence of ERα and
ERβ mRNA in the MG63 cells, suggesting that ERα and ERβ are
constitutively expressed by the MG63 human osteosarcoma cell lines
(Fig. 1).
Morphological analysis of MG63 cells with
various drug intervention
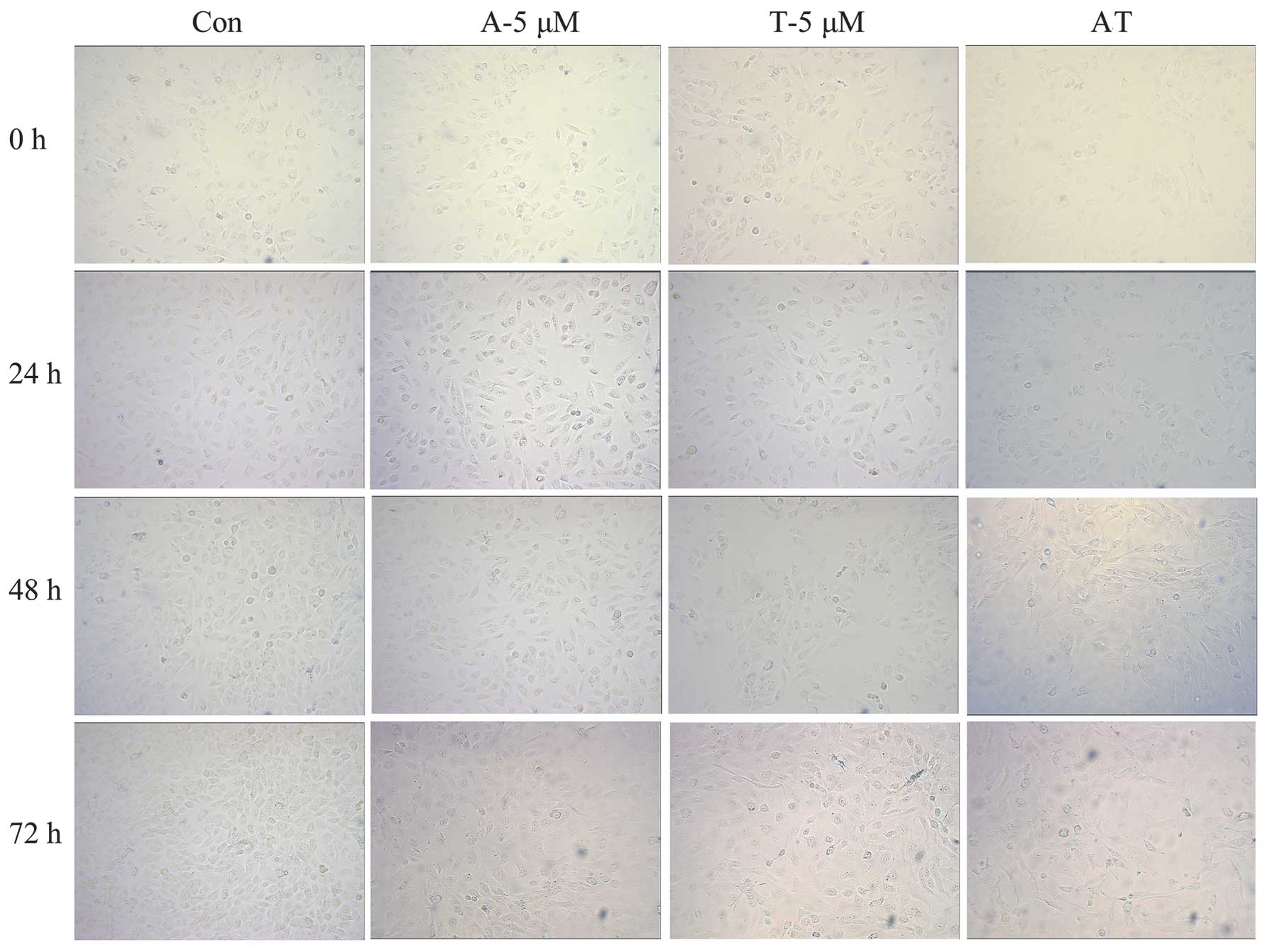
Typical apoptotic cell morphology was observed in
all the groups, with the exception of the control group. The images
show that the cells grew well in the control group, and were mostly
spindle-shaped and large, with uniform round nuclei and clear
cytoplasm. It was identified that 24 h following the administration
of TAM (5 μmol/l), a small number of cells became round-shaped and
multiplied slowly. With a prolonged amount of time and an increased
drug concentration, more cells became round-shaped and dark.
Additionally, the number of fragments increased and the sizes and
amount of the plasma granules within the cells changed. Cell
shrinkage and the formation of apoptotic bodies were observed. In
the ADM group (5 μg/ml, 24 h), the typical early stage of cell
apoptosis changes, including cell membrane budding and swollen
organelles, were observed. The same morphological changes as
previously described were observed in the combination group (AT)
and the rate of apoptosis in the combination group was higher than
that in the single drug groups under the same treatment times
(Fig. 2).
MTT colorimetric analysis
It is well-known that the MTT reagent directly
reacts with the mitochondria (mitochondrial dehydrogenase) of
metabolically active cells. Therefore, the reaction of MTT
reduction is directly proportional to the number of growing cells.
The measured optical density is directly proportional to the number
of viable cells in the culture medium. Therefore, MTT is regarded
as a quantitative assay to determine the cytotoxicity of the
materials and the viability/proliferation of the cells in solution
in various groups.
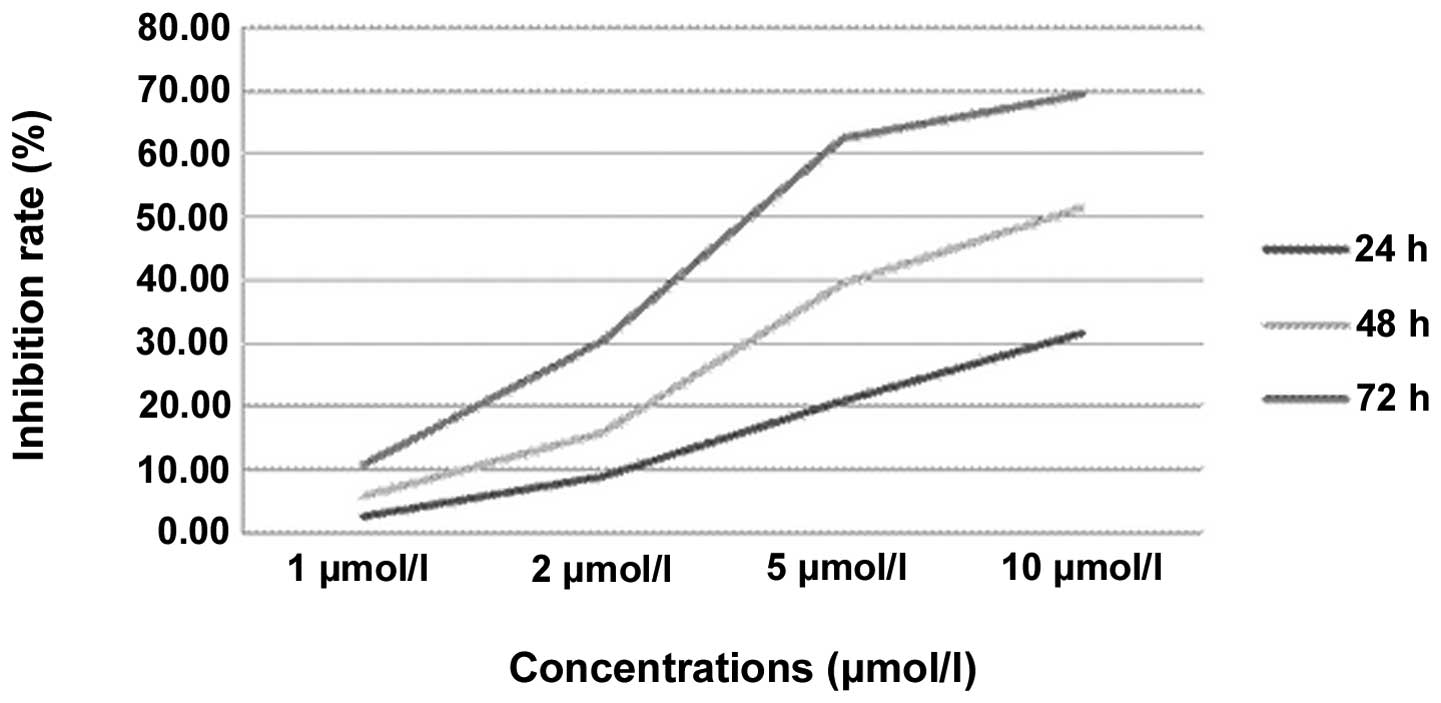
Table I and Fig. 3 show the effect of TAM on the cell
growth inhibition rate. According to the statistical analysis, the
cell growth inhibition rates in the TAM groups gradually increased
with a prolonged treatment time and increased drug concentrations.
A significant difference was observed between the various groups
with various times and concentrations. However, in the multiple
comparisons, there were no significant differences between the 24-
and 48-h, 1- and 2-μmol/l or 5- and 10-μmol/l groups (P>0.05).
There were obvious dose-effect and time-effect associations in the
MG63 cells that were treated with TAM.
 | Table ICell growth inhibition rate of various
concentrations of TAM (%). |
Table I
Cell growth inhibition rate of various
concentrations of TAM (%).
| Concentrations of TAM
(μmol/l) |
|---|
|
|
|---|
| Time (h) | 0 | 1 | 2 | 5 | 10 |
|---|
| 24 | 0 | 2.49±0.21 | 8.80±0.56 | 20.85±1.15 | 31.52±1.54 |
| 48 | 0 | 5.68±0.32 | 15.67±0.62 | 39.57±0.61 | 51.56±1.85 |
| 72 | 0 | 10.64±0.44 | 30.38±0.76 | 62.51±1.24 | 69.40±2.24 |
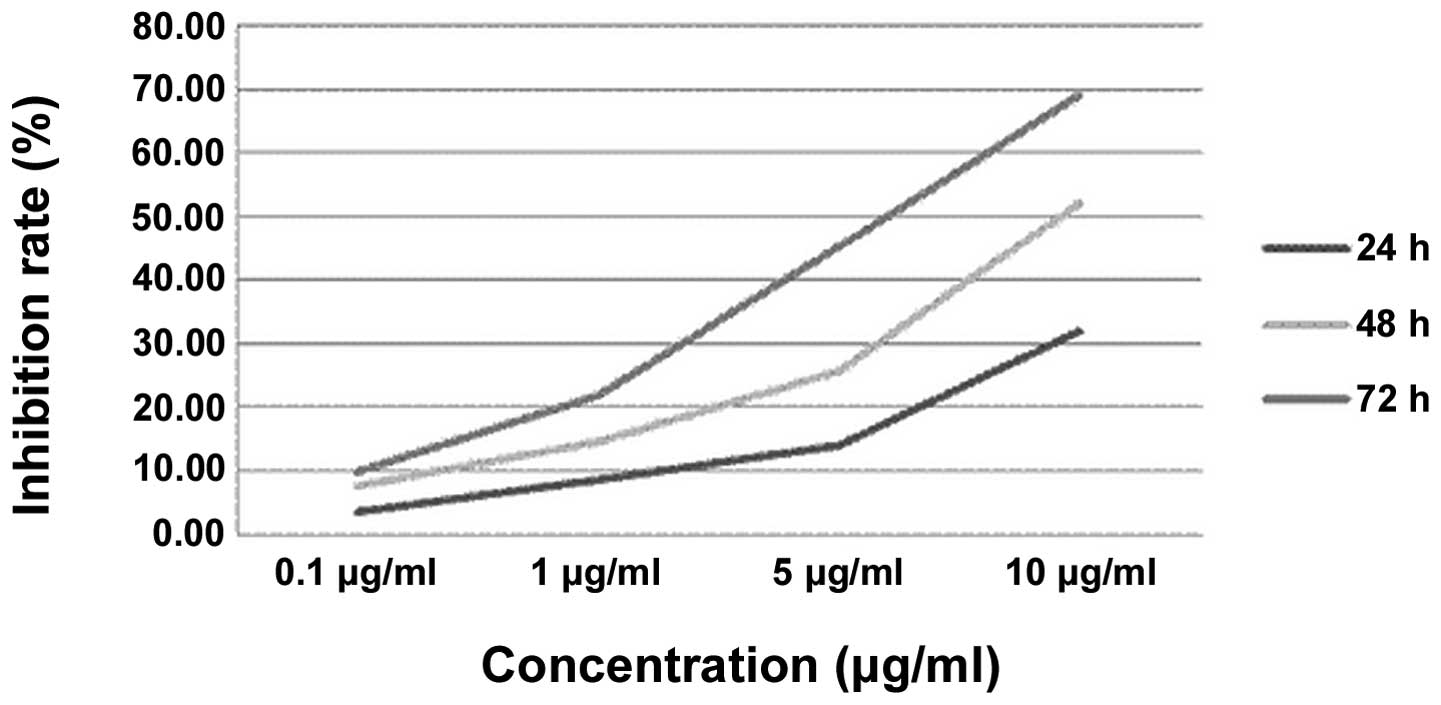
Table II and
Fig. 4 show the effect of ADM on
the cell growth inhibition rate. The statistical analysis shows
that the cell growth inhibition rates in the ADM groups gradually
increased with a prolonged treatment time and increased drug
concentrations. A significant difference was observed between the
groups with the various times and concentrations. However, in the
multiple comparisons, there were no significant differences between
the 24- and 48-h, 72- and 48-h or 0.1- and 1-μg/ml groups
(P>0.05). There were obvious dose-effect and time-effect
associations in the MG63 cells that were treated with ADM.
 | Table IICell growth inhibition rate of
various concentrations of ADM (%). |
Table II
Cell growth inhibition rate of
various concentrations of ADM (%).
| Concentrations of
ADM (μg/ml) |
|---|
|
|
|---|
| Time (h) | 0 | 0.1 | 1 | 5 | 10 |
|---|
| 24 | 0 | 3.63±0.16 | 8.75±0.93 | 14.04±1.47 | 31.78±1.68 |
| 48 | 0 | 7.65±0.41 | 14.46±1.56 | 25.53±2.07 | 51.99±2.03 |
| 72 | 0 | 9.89±0.84 | 21.76±1.89 | 45.23±2.64 | 68.95±3.65 |
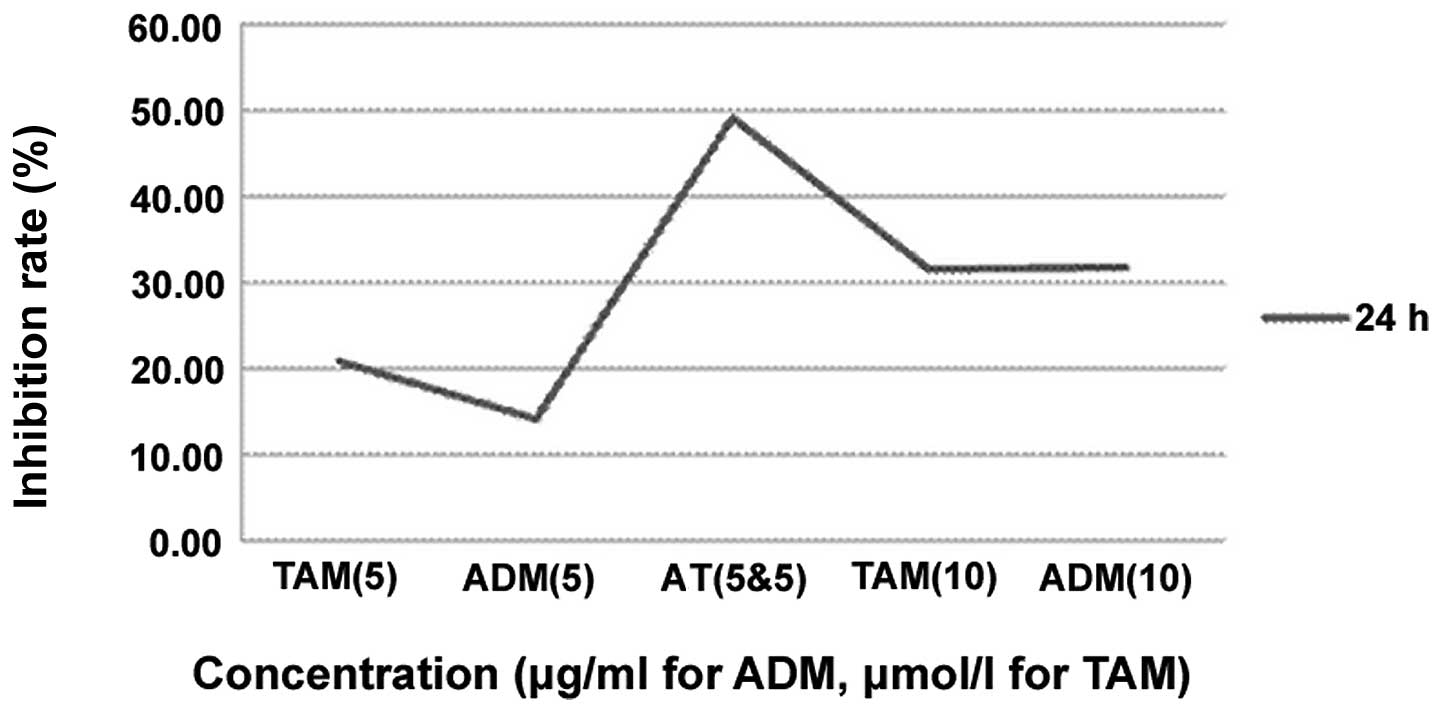
Table III and
Fig. 5 show the effects of TAM (5
and 10 μmol/l, respectively), ADM (5 and 10 μg/ml, respectively)
and AT (combination of 5 μmol/l TAM and 5 μg/ml ADM) on the cell
growth inhibition rate. By the statistical analysis, the cell
growth inhibition rates in the ADM groups gradually increased with
a prolonged treatment time. A significant difference was observed
between the group with various times and concentrations. However,
in the multiple comparisons, there were no significant differences
between the TAM (5 μmol/l) and ADM (5 μg/ml) groups, TAM (5 μmol/l)
and TAM (10 μmol/l) groups, TAM (5 μmol/l) and ADM (10 μg/ml)
groups and the TAM (10 μmol/l) and ADM (10 μg/ml) groups
(P>0.05). The inhibition rate of the combination group was
higher than that of the single drug groups, including the single
drug groups with double the concentration.
 | Table IIICell growth inhibition rates of
single and combination drugs (%). |
Table III
Cell growth inhibition rates of
single and combination drugs (%).
| Concentrations
(ADM, μg/ml; TAM, μmol/l) |
|---|
|
|
|---|
| Time (h) | 0 | TAM (5) | ADM (5) | AT (5 each) | TAM (10) | ADM (10) |
|---|
| 24 | 0 | 20.85±1.15 | 14.04±1.47 | 49.17±3.21 | 31.52±1.54 | 31.78±1.68 |
| 48 | 0 | 39.57±0.61 | 25.53±2.07 | 68.33±1.90 | 51.55±1.83 | 51.99±2.03 |
| 72 | 0 | 62.51±1.24 | 45.23±2.64 | 85.27±2.15 | 69.40±2.24 | 68.95±3.65 |
Discussion
Non-neoplastic bone diseases, including osteoporosis
and osteoarthritis, have a higher incidence in females, and
osteoporosis may be treated and prevented using 17β-estradiol and
raloxifene to a certain degree. Estrogen has been shown to play a
significant role in the proliferation of bone cells (13). Furthermore, the highest incidence of
osteosarcoma is observed in a younger patient group with a high
level of sex hormone activity. Sex steroids and receptors play
significant roles in the regulation of cell proliferation in human
osteosarcoma.
Walker et al first reported the existence of
sex steroid receptors in four cases of osteosarcoma from a
dextran-coated charcoal assay (14). Stedman et al also identified
ER proteins in osteosarcoma from gel filtration in the same year
(15). These were the first studies
to demonstrate the potential correlation between sex steroids and
osteosarcoma. More recent studies on ER expression in osteosarcoma
cases and cell lines are rare. The majority of the studies found
that ERα and ERβ were expressed in human osteoblasts. The analysis
of the ER subtypes reported by Chen et al demonstrated the
dominance of ERβ in the MG63 cells (16). Dohi et al(17) reported that ERβ was relatively
widely distributed in the osteosarcoma cases and ERβ was the
predominant ER that was expressed in the MG63 cells. In other
osteosarcoma cell lines, including U2OS, ERα and ERβ mRNA has also
been reported to be detected at a ratio of 1:4. (18) Solakidi et al reported that
ERα was localized mainly in the nucleus of human U2OS osteosarcoma
cells and ERβ was specifically enriched at the site of the
mitochondria, but its significance has remained unknown at this
juncture (19). The results of the
present study revealed expression of ERα and ERβ mRNA in the MG63
cells, as detected by RT-PCR, which was consistent with those that
have been reported previously. Therefore, the present study
provides a basis for the endocrine therapy of osteosarcoma.
Furthermore, Dohi et al(17) reported that the proliferation of
MG63 human osteosarcoma cells was stimulated by E2 and that the
increment are significantly suppressed clinically using
well-established blockers of a corresponding steroid that had been
previously reported by Luo and Liao (20). These findings indicated the
potential role of the ER in the pathogenesis and development of
osteosarcoma. The steroid blockers have the potential to be used as
suppressors of cell proliferation of human osteosarcoma cells.
Therefore, estrogen is considered to exert effects not only on
non-neoplastic bones but also on their neoplasms, particularly
osteosarcomas.
As an ER antagonist, TAM is extensively used in the
treatment of mammary adenocarcinoma. The most common side effect is
vasomotor symptoms, which have been reported in 1–4% of cases.
Toxicity and other side effects have been relatively uncommon
during the clinical use of the TAM. TAM has been demonstrated to
have biological and pharmacological activities beyond its
traditional role as an anti-estrogenic agent. Among these are the
inhibition of multidrug resistance (MDR) (21–23),
protein kinase C (PKC) (24),
calmodulin (25), insulin growth
factor (26) and transforming
growth factor-α (27). In addition,
TAM has been associated with transforming growth factor-β1
induction (28), immune reaction
modulation (29), apoptosis
induction (30,31) and a reduction in the fluidity of the
cytoplasmic membrane (32). It has
been speculated that certain activities may be responsible for the
unexpected therapeutic effect of TAM, alone or in combination with
other anti-cancer drugs, in various cancers, including malignant
melanoma (33,34), brain glioma (35) and lymphoma (36). In the present study, there were
obvious dose-effect and time-effect associations in the MG63 cells
that were treated with TAM. The therapeutic effect of TAM alone may
be mediated by its non-specific effect on cytoplasmic membranes, as
TAM, a triphenylethylene, is lipophilic and is expected to
partition into hydrophobic domains in the fluid mosaic structure of
cell membranes. Clark et al(32) have demonstrated previously that TAM,
at concentrations of >1 mM, significantly decreased the fluidity
of the plasma membrane of ER− breast cancer cells and
may have contributed to its non-ER-mediated cytotoxicity.
The analysis of sex steroid receptors in resected
specimens of osteosarcoma cases and cell lines as a potential
surrogate marker may be required in order to prepare for a
potential endocrine therapy, particularly in the instance that the
patients develop pulmonary metastasis and a poor response to the
chemotherapy in their clinical course. Ferguson et
al(37) reported that TAM
showed the ability to enhance the survival rate of advanced breast
cancer patients who had been treated with ADM in phase III. Studies
have also indicated that the drug resistance of neuroblastoma,
small cell lung cancer, gastric cancer, liver cancer, bladder
cancer, ovarian cancer and epirubicin may all be reversed using TAM
(38). The potential synergistic
cytotoxic effect between TAM and chemotherapeutic agents in
estrogen-independent solid tumors has been studied and documented
(31,39).
In an effort to increase the clinical efficacy of
ADM, the present study determined whether the antitumor effects of
this drug were able to be enhanced using a concurrent application
of other drugs that have demonstrated anti-osteosarcoma activity,
such as TAM. In the present study, the inhibition rates of the 24-h
group of the MG63 human osteosarcoma cell line by ADM (5 μg/ml),
TAM (5 μmol/l), ADM (10 μg/ml) and TAM (10 μmol/l) were 14.04,
20.85, 31.78 and 31.52%, respectively. By contrast, following the
combination of TAM and ADM, the inhibitive rates increased to
49.17% and the results were consistent with those of the other
groups, indicating that the effect of the chemotherapy drugs was
enhanced by the cooperation of the two drugs and that TAM may
enhance the effect of chemotherapeutics by comparing ADM and ADM
mixed with TAM. The present analyses demonstrated that combination
treatment of MG63 osteosarcoma cells exerts greater
antiproliferative and apoptosis-stimulatory effects than treatment
with the individual drugs alone.
Cancer cells may gradually develop a resistance to
chemotherapeutic drugs in the course of chemotherapy and there have
been numerous studies with regard to overcoming the resistance
mechanisms (40). TAM, a
cholangiocarcinoma sensitizer, may act as a P-glycoprotein (gp)
substrate by competing for the P-gp binding site with
antineoplastic agents. TAM inhibits the function of the
transmembrane transporter and lowers the velocity of drug efflux
from within cells, leading to an enhancement of the drug
concentration and the effect of the chemotherapeutic drugs. Another
underlying molecular event affecting this enhancement process
appears to be the potent downregulation of Bcl-2, a critical
anti-apoptotic and chemoprotective protein. It has been well
established that the ratio of the intracellular amount of the two
proteins, Bcl-2 (antiapoptotic) and Bax (proapoptotic), is critical
for subsequent cell survival and cell death (41,42).
Bax is the major apoptosis-promoting gene and may explain the
synergistic cytotoxicity of TAM with ADM in the treatment of
osteosarcoma. TAM also exerts a pleiotropic effect on intracellular
growth and survival pathways, in particular the inhibition of PKC
(43,44), which is a growth-stimulatory kinase.
Cheng et al(45) reported
that high-dose TAM may potentiate ADM-induced apoptosis of
hepatocellular carcinoma cells and this effect of TAM is associated
with its inhibition on the membrane translocation of PKC-α and is
not mediated by MDR inhibition. Biochemical modulation as a measure
to improve systemic chemotherapy for osteosarcoma requires further
investigation. Appropriate treatment strategies that are formulated
according to the mechanisms of drug resistance and synergistic
cytotoxic effects between TAM and ADM may be guides in the fight
against cancer and thus require investigation. Due to the low
prevalence rate of osteosarcoma, breakthroughs in new therapeutic
approaches based on gene technology may be difficult.
To the best of our knowledge, no studies have been
reported that are concerned with the use endocrine therapy for
treating patients with osteosarcoma and that include an analysis of
the synergistic cytotoxic effect on osteosarcoma cell lines between
TAM and ADM. However, these studies should be pursued, particularly
the molecular mechanism that is complicated by the fact that TAM is
able to exert independent antitumor effects, considering the
relatively poor clinical outcome of osteosarcoma patients with
pulmonary metastases and a poor response to chemotherapy.
In conclusion, ADM, combined with TAM, is able to
enhance the killing of tumor cells, and the addition of TAM may be
a beneficial adjuvant to ADM-based chemotherapy in the treatment of
osteosarcoma. Therefore, it may be worthwhile to consider this
combination regimen for further evaluation in clinical trials. This
information may be used to improve osteosarcoma treatment by
reversing or reducing drug resistance, therefore supporting the
notion that these drug combinations should be further evaluated in
clinical trials.
Acknowledgements
The authors would like to thank The Second Xiangya
Hospital of Central South University for technical assistance
during this study.
References
|
1
|
Smith MA, Ungerleider RS, Horowitz ME and
Simon R: Influence of doxorubicin dose intensity on response and
outcome for patients with osteogenic osteosarcoma and Ewing’s
sarcoma. J Natl Cancer Inst. 83:1460–1470. 1991.PubMed/NCBI
|
|
2
|
Lewis IJ, Nooij MA, Whelan J, et al; MRC
BO06 and EORTC 80931 collaborators; European Osteosarcoma
Intergroup. Improvement in histologic response but not survival in
osteosarcoma patients treated with intensified chemotherapy: a
randomized phase III trial of the European Osteosarcoma Intergroup.
J Natl Cancer Inst. 99:112–128. 2007. View Article : Google Scholar
|
|
3
|
Graf N, Winkler K, Betlemovic M, et al:
Methotrexate pharmacokinetics and prognosis in osteosarcoma. J Clin
Oncol. 12:1443–1451. 1994.PubMed/NCBI
|
|
4
|
Delepine N, Delepine G, Bacci G, et al:
Influence of methotrexate dose intensity on outcome of patients
with high grade osteogenic osteosarcoma. Analysis of the
literature. Cancer. 78:2127–2135. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lewis VO, Raymond K, Mirza AN, Lin P and
Yasko AW: Outcome of postradiation osteosarcoma does not correlate
with chemotherapy response. Clin Orthop Related Res. 450:60–66.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goldsby R, Burke C, Nagarajan R, et al:
Second solid malignancies among children, adolescents, and young
adults diagnosed with malignant bone turmors after 1976: follow up
of a Children’s Oncology Group cohort. Cancer. 113:2597–2604.
2008.PubMed/NCBI
|
|
8
|
Mansky P, Arai A, Stratton P, et al:
Treatment late effects in long-term survivors of pediatric sarcoma.
Pediatr Blood Cancer. 48:192–199. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng A, Kallio A and Härkönen P:
Tamoxifen-induced rapid death of MCF-7 breast cancer cells is
mediated via extracellularly signal-regulated kinase signaling and
can be abrogated by estrogen. Endocrinology. 148:2764–2777. 2007.
View Article : Google Scholar
|
|
10
|
Tan CK, Chow PK, Findlay M, et al: Use of
tamoxifen in hepatocellular carcinoma: a review and paradigm shift.
J Gastroenterol Hepatol. 15:725–729. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Emst M, Parker MG and Rodan GA: Functional
estrogen receptors in osteoblastic cells demonstrated by
transfection with a reporter gene containing an estrogen response
element. Mol Endocrinol. 5:1597–1606. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carmichael J, DeGraff WG, Gazdar AF, et
al: Evaluation of a tetrazolium-based semiautomated colorimetric
assay: assessment of chemosensitivity testing. Cancer Res.
47:936–942. 1987.PubMed/NCBI
|
|
13
|
Braidman IP, Davennport LK, Carter DH, et
al: Preliminary in situ identification of estrogen target cells in
bone. J Bone Miner Res. 10:74–80. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Walker MJ, Chaudhuri PK, Beattie CW and
Das Gupta TK: Steroid receptors in malignant skeletal tumors.
Cancer. 45:3004–3007. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stedman KE, Moore GE and Morgan RT:
Estrogen receptor proteins in diverse human tumors. Arch Surg.
115:244–248. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen FP, Hsu T, Hu CH, Wang WD, Wang KC
and Teng LF: Expression of estrogen receptors alpha and beta in
human osteoblasts: identification of exon-2 deletion variant of
estrogen receptor beta in postmenopausal women. Chang Gung Med J.
27:107–115. 2004.PubMed/NCBI
|
|
17
|
Dohi O, Hatori M, Suzuki T, et al: Sex
steroid receptors expression and hormone-induced cell proliferation
in human osteosarcoma. Cancer Sci. 99:518–523. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Monroe DG, Secreto FJ, Subramaniam M, Getz
BJ, Khosla S and Spelsberg TC: Estrogen receptor alpha and beta
heterodimers exert unique effects on estrogen- and
tamoxifen-dependent gene expression in human U2OS osteosarcoma
cells. Mol Endocrinol. 19:1555–1568. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Solakidi S, Psarra AM and Sekeris CE:
Differential subcellular distribution of estrogen receptor
isoforms: localization of ERalpha in the nucleoli and ERbeta in the
mitochondria of human osteosarcoma SaOS-2 and hepatocarcinoma HepG2
cell lines. Biochim Biophys Acta. 1745:382–392. 2005. View Article : Google Scholar
|
|
20
|
Luo XH and Liao EY: Effects of estriol on
the proliferation and differentiation of human osteoblastics MG63
cells. Endocr Res. 29:343–351. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Berman E, Adams M, Duiguo-Osterndorf R,
Godfrey L, Clarkson B and Andreeff M: Effect of tamoxifen on cell
lines displaying the multidrug-resistant phenotype. Blood.
77:818–825. 1991.PubMed/NCBI
|
|
22
|
Kang Y and Perry RR: Modulatory effects of
tamoxifen and recombinant human alpha-interferon on doxorubicin
resistance. Cancer Res. 53:3040–3045. 1993.PubMed/NCBI
|
|
23
|
Kirk J, Houlbrook S, Stuart NSA, Stratford
IJ, Harris AL and Carmichael J: Differential modulation of
doxorubicin toxicity to multidrug and intrinsically drug resistant
cell lines by anti-oestrogens and their major metabolites. Br J
Cancer. 67:1189–1195. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
O’Brian CA, Liskamp RM, Solomon DH and
Weinstein IB: Inhibition of protein kinase C by tamoxifen. Cancer
Res. 45:2462–2465. 1985.PubMed/NCBI
|
|
25
|
Lam HY: Tamoxifen is a calmodulin
antagonist in the activation of cAMP phosphodiesterase. Biochem
Biophys Res Commun. 118:27–32. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huynh TH, Tetenes E, Wallace L and Pollak
M: In vivo inhibition of insulin-like growth factor I gene
expression by tamoxifen. Cancer Res. 53:1727–1730. 1993.PubMed/NCBI
|
|
27
|
Noguchi S, Motomura K, Inaji H, Imalka S
and Koyama H: Down-regulation of transforming growth factor-alpha
by tamoxifen in human breast cancer. Cancer. 72:131–136. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Butta A, MacLennan K, Flanders KC, Sacks
NP, Smith I, McKinna A, Dowsett M, Wakefield LM, Sporn MB, Baum M,
et al: Induction of transforming growth factor beta 1 in human
breast cancer in vivo following tamoxifen treatment. Cancer Res.
52:4261–4264. 1992.PubMed/NCBI
|
|
29
|
Baral E, Nagy E and Berczi I: Modulation
of natural killer cell-mediated cytotoxicity by tamoxiefn and
estradiol. Cancer. 75:591–599. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Couldwell WT, Hinton DR, He S, Chen TC,
Sebat I, Weiss MH and Law RE: Protein kinase C inhibitors induce
apoptosis in human malignant glioma cell lines. FEBS Lett.
345:43–46. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gelmann EP: Tamoxifen induction of
apoptosis in estrogen receptor-negative cancers: New tricks for an
old dog. J Natl Cancer Inst. 88:224–226. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Clarke R, van den Berg HW and Murphy RF:
Reduction of the membrane fluidity of human breast cancer cells by
tamoxifen and 17 beta-estradiol. J Natl Cancer Inst. 82:1702–1705.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Del Prete SA, Maurer LH, O’Donnel J,
Forcier RJ and LeMarbre P: Combination chemotherapy with cisplatin,
carmustine, dacarbazine, and tamoxifen in metastatic melanoma.
Cancer Treat Rep. 68:1403–1405. 1984.PubMed/NCBI
|
|
34
|
Cocconi G, Bella M, Calabresi F, Tonato M,
Canaletti R, Boni C, Buzzi F, Ceci G, Corgna E, Costa P, et al:
Treatment of metastatic malignant melanoma with dacarbazine plus
tamoxifen. N Engl J Med. 327:516–523. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vertosick FT Jr, Selker RG, Pollack IF and
Arena V: The treatment of intracranial malignant glioma using
orally administered tamoxifen therapy: preliminary results in a
series of ‘failed’ patients. Neurosurgery. 30:897–903.
1992.PubMed/NCBI
|
|
36
|
Narasimhan P: Tamoxifen in the treatment
of refractory lymphoma. N Engl J Med. 311:1258–1259. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ferguson PJ, Brisson AR, Koropatnick J and
Vincent MD: Enhancement of cytotoxicity of natural product drugs
against multidrug resistant variant cell lines of human head and
neck squamous cell carcinoma and breast carcinoma by tesmilifene.
Cancer Lett. 274:279–289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Singh MN, Martin-Hirsch PL and Martin FL:
The multiple applications of tamoxifen: an example pointing to SERM
modulation being the aspirin of the 21st century. Med Sci Monit.
14:RA144–RA148. 2008.PubMed/NCBI
|
|
39
|
Kang Y, Cortina R and Perry RR: Role of
c-myc in tamoxifen induced apoptosis in estrogen-independent breast
cancer cells. J Natl Cancer Inst. 88:279–284. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Incles CM, Schultes CM, Kelland LR and
Neidle S: Acquired cellular resistance to flavopiridol in a human
colon carcinoma cell line involves up-regulation of the telomerase
catalytic subunit and telomere elongation. Sensitivity of resistant
cells to combination treatment with a telomerase inhibitor 1. Mol
Pharmacol. 64:1101–1108. 2003. View Article : Google Scholar
|
|
41
|
Cory S and Adams JM: Killing cancer cells
by flipping the Bcl-2/Bax switch. Cancer Cell. 8:5–6. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Korsmeyer SJ, Shutter JR, Veis DJ, et al:
Bcl-2/Bax: a rheostat that relgulates an anti-oxidant pathway and
cell death. Semin Cancer Biol. 4:327–332. 1993.PubMed/NCBI
|
|
43
|
Agostinis P, Vantieghem A, Merlevede W and
de Witte PA: Hypericin in cancer treatment: more light on the way.
Int J Biochem Cell Biol. 34:221–241. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mandlekar S and Kong AN: Mechanism of
tamoxifen-induced apoptosis. Apoptosis. 6:469–477. 2001. View Article : Google Scholar
|
|
45
|
Cheng AL, Chuang SE, Fine RL, Yeh KH, Liao
CM, Lay JD and Chen DS: Inhibition of the membrane translocation
and activation of protein kinase C, and potentiation of
doxorubicin-induced apoptosis of hepatocellular carcinoma cells by
tamoxifen. Biochem Pharmacol. 55:523–531. 1998. View Article : Google Scholar
|