Introduction
Cholangiocarcinoma is one of the most lethal
malignant tumors (1), as it is
difficult to diagnose in the early stages. Since symptoms develop
later, patients are often diagnosed when the cancer is at a
metastatic stage (2,3). Numerous other malignant tumors are
also difficult to diagnose in their early stages. As a result,
identifying a method of curative therapy for advanced-stage tumors
is urgently required. Invasion and metastasis are significant
factors in the advanced stages of tumors. The inhibition of these
phenomena may enhance the treatment outcome of malignant tumors and
also allow the patient to be treated with a resection or using
chemotherapy, thus inhibiting the appearance of new lesions despite
being at an advanced stage.
Epidermal-mesenchymal transition (EMT) is a process
whereby epidermal cells exhibit reduced intercellular adhesion and
acquire fibroblast-like properties (4). This phenomenon is common to normal
development and carcinogenesis, and is associated with mechanisms
that induce tumor invasion and metastasis (5). By this process, epidermal cells are
converted to cells that display mesenchymal features and become
dedifferentiated and malignant. Biological markers of EMT include a
decrease in the level of epithelial markers, including E-cadherin,
and the expression of mesenchymal markers, including N-cadherin,
vimentin and α-smooth muscle actin (α-SMA) (6–8).
Furthermore, transforming growth factor-β (TGF-β) induces EMT in
tumor cells, activating the TGF-β signaling pathway, which includes
the Smad proteins and also the non-Smad pathways (9,10).
In contrast, certain studies have demonstrated that
paclitaxel (PTX), one of the major anticancer agents that
stabilizes microtubules and arrests the cell cycle in the
G0/G1 and G2/M phases (11,12),
inhibits the invasive ability of breast cancer cell lines when used
in low-doses (13,14). Furthermore, certain studies have
revealed that low-dose PTX inhibits TGF-β/Smad activity in fibrosis
(15,16). Based on these findings, the present
study hypothesized that low-dose PTX may inhibit the induction of
EMT by TGF-β1 in the human cholangiocarcinoma CCKS-1 cell line.
Materials and methods
Reagents
PTX (moleculer weight, 853.91; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and TGF-β1
(Sigma-Aldrich, St. Louis, MO, USA) were used.
Antibodies
Mouse monoclonal antibodies for N-cadherin,
E-cadherin, α-SMA, β-catenin and vimentin were used as primary
antibodies. A rabbit monoclonal antibody was used for N-cadherin
and a goat polyclonal antibody for phosphorylated (p)-Smad2/3
(Santa Cruz Biotechnology, Inc.).
Cell culture
A human ICC cell line, CCKS-1, obtained from the
Department of Human Pathology, Kanazawa University Graduate School
of Medicine (Kanazawa, Ishikawa, Japan) (17,18)
was used. The ICC cell line was maintained at 37°C in a 5%
CO2 incubator and grown in RPMI-1640 medium supplemented
with 2 mM glutamine, 1% fetal bovine serum (FBS; Nichirei
Biosciences, Inc., Tokyo, Japan), 100 U/l penicillin and 100 μg/ml
streptomycin (Invitrogen, Carlsbad, CA, USA).
Cell proliferation assay
The proliferative effect of PTX on the ICC cell
lines was quantified using an MTT colorimetric assay with Cell
Proliferation kit I (Roche, West Sussex, UK), according to the
manufacturer’s instructions. In brief, the CCKS-1 cells
(5×103 cells/well) were grown in 96-well flat-bottom
microtiter plates in 100 μl medium containing 1% FBS and incubated
for 12 h at 37°C in a humidified atmosphere with 5% CO2.
Following the incubation period, the medium was exchanged for a new
medium containing 1% FBS, 5 ng/ml TGF-β1 and/or various
concentrations (1–100 nM) of PTX. The mixture was incubated for 72
h and the resultant absorbance was recorded at 562 nm using a
96-well plate reader (Multiskan GO; Thermo Scientific, Waltham, MA,
USA). The data are represented as the mean ± SD of three
independent experiments and expressed as a percentage of the
untreated control cells.
Cell death assay
The cytotoxic effect of PTX on the ICC cell lines
was quantified by flow cytometry using Pacific Blue™ annexin V and
SYTOX® AADvanced™ dead cell stain (Invitrogen),
according to the manufacturer’s instructions. In brief, the CCKS-1
cells were cultured in a medium containing 10% FBS. Following the
culture, the medium was exchanged for new medium containing 1% FBS
and/or various concentrations of PTX with 5 ng/ml TGF-β1 (control,
5 ng/ml TGF-β1 only; 1 nM PTX + TGF-β1; 2.5 nM PTX + TGF-β1; 5nM
PTX + TGF-β1; and 10 nM PTX + TGF-β1) and incubated for 7 days.
Following the incubation period, the cells, including the floating
cells, were harvested and washed in cold PBS. The cells were
resuspended in 1X annexin binding buffer at ~1×106
cells/ml, preparing a sufficient volume to be able to use 100 μl
per assay. Subsequently, 5 μl Pacific Blue annexin V and 1 μl 500
μM SYTOX AADvanced dead cell stain working solution were added to
each cell suspension and incubated at room temperature for 30 min,
protected from the light. Following the incubation period, 400 μl
1X annexin binding buffer was added to each suspension. During the
analysis, the samples were kept on ice.
Investigating the optimal administration
interval between PTX and TGF-β
An immunoblotting analysis of p-Smad2/3 was used to
investigate the optimal administration interval between PTX and
TGF-β. Following the culture period, the medium was replaced with
RPMI-1640 medium containing 1% FBS and 2.5 nM PTX. Subsequently, 5
ng/ml TGF-β was administered after PTX using one of the following
intervals; 0, 10, 30 and 120 min. Following 60 min of the TGF-β
reaction time as described, the cells that did not undergo
apoptosis or the dead cells that were floating in the medium were
harvested by trypsinization using 0.25% trypsin-EDTA (Invitrogen),
then washed 3 times with PBS and dissolved in RIPA buffer (Wako
Pure Chemical Industries, Ltd., Osaka, Japan) containing protease
and phosphatase inhibitors (Sigma-Aldrich). The protein
concentration of each sample was measured using a BCA protein assay
kit (Thermo Scientific). The total protein was measured using a
spectrophotometer. The extracted protein was used for the western
blot analysis. In this analysis, 45 μg protein from each sample was
loaded onto 12.5% sodium dodecyl sulfate-polyacrylamide gels
(SDS-PAGE) and the proteins were transferred to a polyvinylidene
difluoride (PVDF) membrane by the semi-dry blotting method using
blotting solution (hydroximethyl; Ez Fast Blot; ATTO Corporation,
Tokyo, Japan). The membrane was washed for 10 min with blocking
solution (0.1% Tween-20; Ez Block; ATTO Corporation), blocked at
room temperature for 30 min again using blocking solution and then
washed with washing solution (0.1% Tween-20; Ez Wash; ATTO
Corporation). The blots were incubated for 8 h at room temperature
with a goat anti-p-Smad2/3 antibody diluted at 1:500 with washing
solution. Subsequent to being washed with a gradient buffer (Ez
Wash), the membranes were incubated with an HRP-conjugated
anti-goat IgG antibody for 1 h at room temperature. The
antibody-antigen complex was detected using the ECL Plus Western
blotting detection system (GE Healthcare UK, Ltd., Buckinghamshire,
UK), according to the supplier’s recommendations.
Immunocytochemistry
The expression of E-cadherin, N-cadherin, vimentin
and β-catenin in the ICC cells was examined immunocytochemically
using their respective primary antibodies. The cells were seeded on
Lab-Tek chamber slides (Nalge Nunc International, Penfield, NY,
USA) with PTX and/or TGF-β (5 ng/ml TGF-β; 1 nM PTX + 5 ng/ml
TGF-β; 2.5 nM PTX + 5ng/ml TGF-β; or 5 nM PTX + 5 ng/ml TGF-β) and
incubated for 7 days at 37°C in a humid atmosphere of 5%
CO2/95% air. Following the incubation period, the waste
solution was discarded and the coverslips with the cells were then
fixed with methanol and acetone 1:1 (v/v) for 10 min.
Immunostaining was performed as described. Briefly,
the slides were blocked with normal goat serum [5% in
phosphate-buffered saline (PBS)] and incubated with each primary
mouse monoclonal antibody, as described previously, for 6 h at room
temperature. The slides were washed in PBS and the immunoreactivity
was visualized by incubating the slides with a goat anti-mouse IgG
antibody conjugated with Alexa Fluor 488 (Invitrogen; 1:400) for 1
h at room temperature. The slides were counterstained with
bis-benzimide (100 ng/ml; Hoechst 33258; Sigma-Aldrich) to
visualize the nuclei. The slides were then examined under a
fluorescence microscope (BZ-9000 Biorevo; Keyence, Osaka, Japan).
In addition, the cadherin switch was examined by double staining. A
primary rabbit monoclonal antibody against N-cadherin was
administered and incubated for 6 h at room temperature following
incubation with the primary mouse monoclonal antibody against
E-cadherin and being washed in PBS. A goat anti-mouse and
anti-rabbit IgG antibody were used at the same time.
Immunoblot analysis
The processes for harvesting and measuring the
protein concentration and for the blotting technique were the same
as described previously. Briefly, each sample used 45 μg protein
and the 4 antibodies for E-cadherin (1:100), N-cadherin (1:100),
vimentin (1:500) and α-SMA (1:500) in the western blot analysis.
The antibodies were used as EMT markers to measure the up or
downregulation of the expression in the CCKS-1 cells that were
incubated in the medium with an added concentration of PTX and/or
TGF-β1 (control, 5 ng/ml TGF-β1; 1 nM PTX + 5 ng/ml TGF-β1; 2.5 nM
PTX + 5 ng/ml TGF-β1; and 5 nM PTX + 5ng/ml TGF-β1) for 8 h at room
temperature.
Results
Determining the optimal PTX
concentration
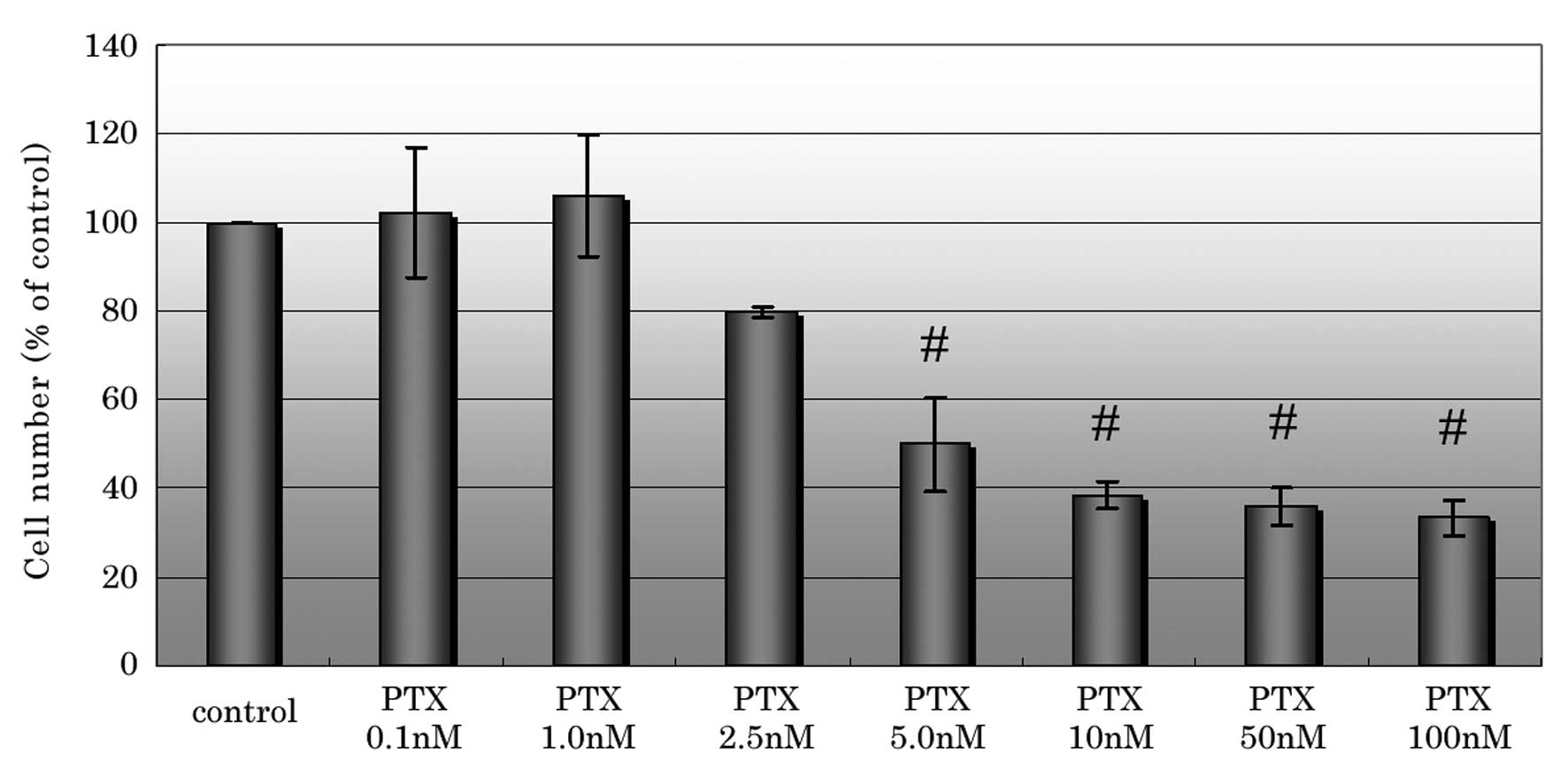
In the cell proliferation assay, PTX inhibited cell
proliferation at concentrations of ≥2.5 nM and significantly
inhibited cell proliferation at concentrations of ≥5 nM compared
with the control (Fig 1). The
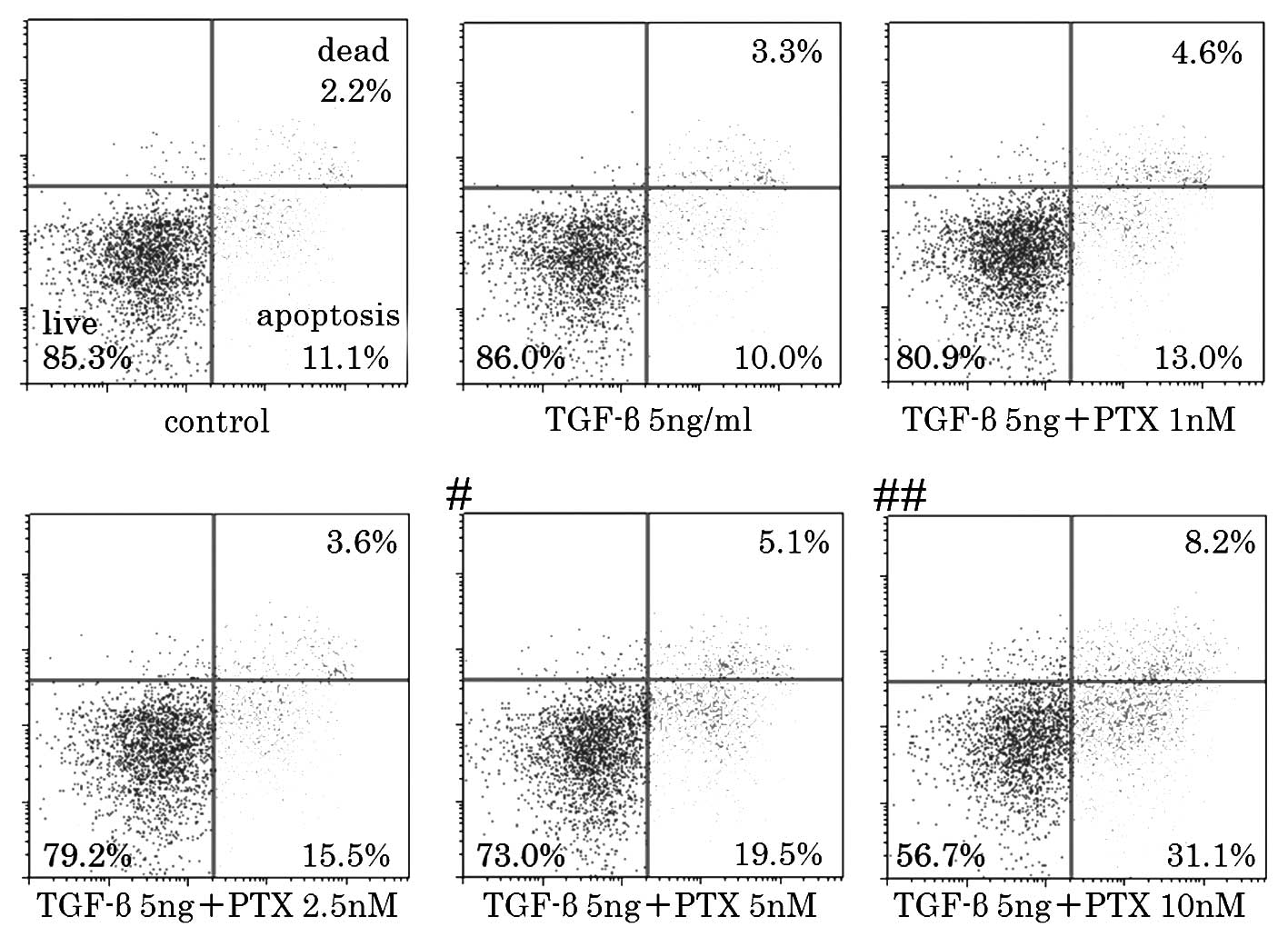
percentage of the dead and apoptotic cells markedly increased when
using a concentration of 5 nM or higher in the cell death assay
(Fig. 2). Based on these results,
the cytotoxic concentration of PTX for CCKS-1 was estimated to be
2.5–5nM. Therefore, the concentrations that were used in the
subsequent experiments were 1, 2.5 and 5 nM PTX. A concentration of
5 nM PTX was included in order to observe whether EMT is inhibited
at a cytotoxic concentration.
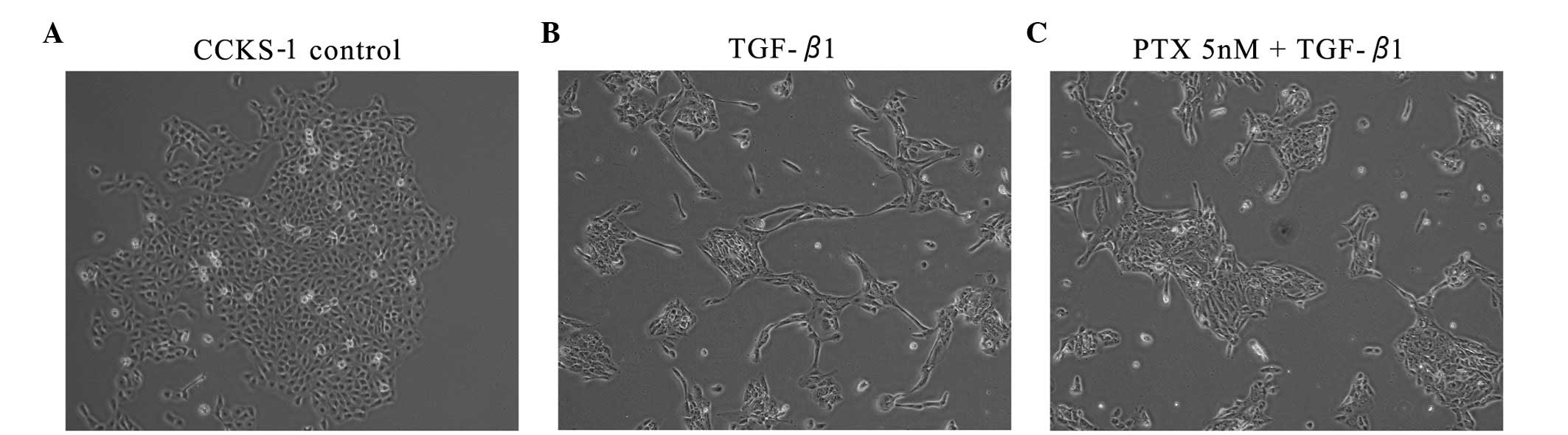
Morphological investigation
The untreated CCKS-1 cells displayed a
cobblestone-like morphology and cell-to-cell adhesion was intact.
However, the TGF-β1-treated CCKS-1 cells developed a spindle-shaped
morphology, the cell-to-cell adhesions became weak and the cells
were scattered. The cells that were treated with low-dose PTX were
assembled closely, although the morphology did not completely
resemble the cobblestone-like appearance of the control cells. The
morphological changes were concentration-dependent (Fig. 3).
Immunofluorescence investigation
In the investigation of the cadherin switch and
vimentin expression, the untreated CCKS-1 cells predominantly
expressed E-cadherin on the cell membrane and weakly expressed
vimentin. The TGF-β1-treated CCKS-1 cells expressed N-cadherin and
vimentin strongly. However, the expression of E-cadherin in the
low-dose PTX-treated CCKS-1 cells was stronger than the expression
of N-cadherin, and the vimentin expression was weak, which was
consistent with the control. Similarly, the untreated CCKS-1 cells
expressed β-catenin on the cell membrane and in the cytoplasm. In
the TGF-β1-treated CCKS-1 cells, β-catenin expression shifted
partially to the nucleus (arrows), but the administration of
low-dose PTX inhibited this movement (Fig. 4).
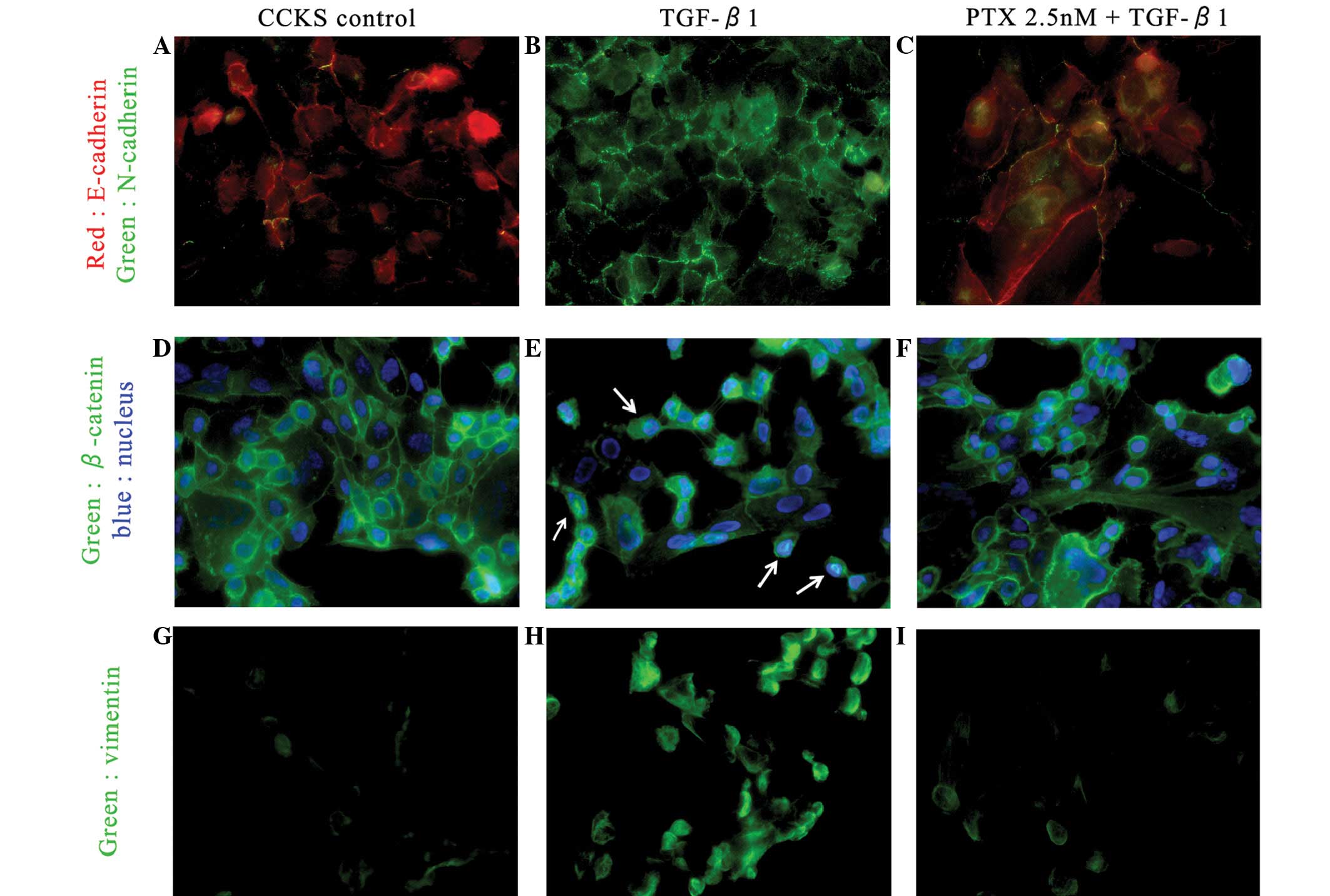 | Figure 4Immunofluorescence investigations of
the cadherin switch (red, E-cadherin; green, N-cadherin). (A)
Untreated CCKS-1 cells strongly express E-cadherin on the cell
membrane, but the administration of 5ng/ml TGF-β1 leads to the
cadherin switch. (B) CCKS-1 cells strongly express N-cadherin. (C)
CCKS-1 cells treated with low-dose PTX express E-cadherin more
strongly than N-cadherin (5ng/ml TGF-β1 + 2.5nM PTX).
Immunofluorescence investigations of β-catenin (green, β-catenin;
blue, nuclei). (D) Untreated CCKS-1 cells express β-catenin on the
cell membrane and cytoplasm. (E) TGF-β1-treated CCKS-1 cells
express β-catenin in the nucleus (arrow; 5ng/ml TGF-β1), but (F)
low-dose PTX inhibited those changes (5 ng/ml TGF-β1 + 2.5nM PTX).
Immunofluorescence investigations of vimentin. (G) Untreated CCKS-1
cells weakly express vimentin, but (H) TGF-β1-treated CCKS-1 cells
express vimentin strongly (5ng/ml TGF-β1). (I) Low-dose PTX
inhibits the expression of vimentin, similar to the untreated cells
(5ng/ml TGF-β1 + 5 nM PTX). TGF-β1, transforming growth factor-β1;
PTX, paclitaxel. |
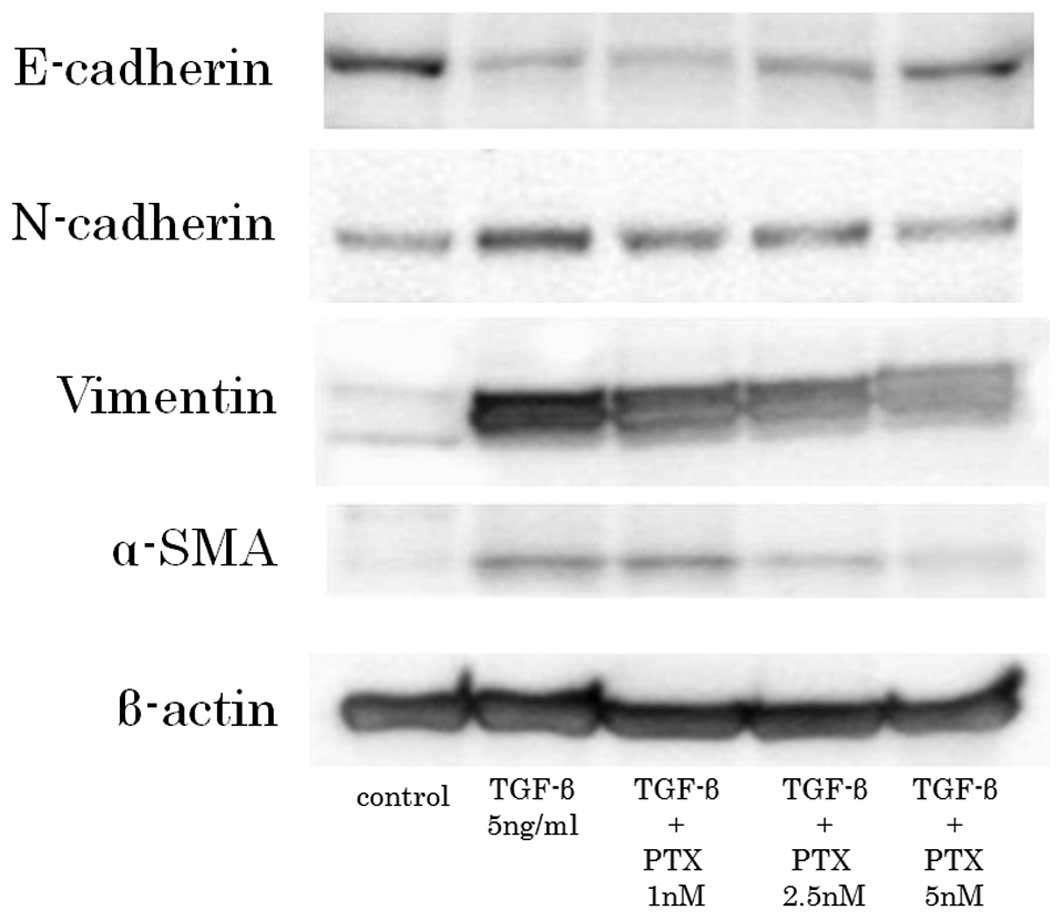
Immunoblot investigation
The untreated CCKS-1 cells strongly expressed
E-cadherin and weakly expressed the mesenchymal markers. In
contrast, the TGF-β1-treated CCKS-1 cells expressed the mesenchymal
markers strongly and E-cadherin weakly. However, the low-dose PTX
weakened the expression of the mesenchymal markers and enhanced
E-cadherin expression in a concentration dependent manner (Fig. 5).
Discussion
EMT is a concept by Hay et al that is
observed during the mesenchymal transition of primitive epidermal
cells during gastrulation (19). In
subsequent studies, EMT has been classified into three subtypes
(20). Type 1 EMT is involved
during developmental stages, including gastrulation, the migration
of neural crest cells from neuroepithelial cells and the formation
of endocardial cushion tissue from cardiac endothelial cells. Type
2 EMT involves the transition of epidermal cells to tissue
fibroblasts, which participate in wound healing, regeneration and
fibrosis in adult tissues. Type 3 EMT involves the metastatic or
invasive process of carcinoma. However, while these three processes
are different, there are certain aspects in common in the induction
of the EMT mechanism. TGF-β is a well-known factor to induce EMT
(9,10). Although the detailed explanation
with regard to the association between TGF-β signaling and EMT is
skipped, the Smad pathway is significant to the signaling process.
In brief, p-Smad2/3 forms heteromeric complexes with Smad4, which
translocate into the nucleus and act as transcriptional regulators
of target genes by interacting with other transcription factors and
transcriptional regulators.
Recently, several anti-EMT agents have been reported
in in vitro analyses, including vorinostat (21), panobinostat (22), valproic acid (23) and PTX. As discussed previously,
low-dose PTX has been shown to inhibit fibrosis and the invasive
ability of cancer cells in several cell lines. A low dose of PTX is
considered to suppress the phosphorylation of Smad2/3. Although it
is well known that PTX behaves as an anticancer agent by
stabilizing microtubules, it is also been identified that
microtubules and Smad 2/3 are closely connected (24). Taken together, this data indicates
that PTX acquires anticancer abilities by regulating Smad 2/3,
which is closely connected with the progression of all types of
EMT. PTX, through the regulation of Smad 2/3, has the potential to
inhibit fibrosis and the invasive abilities of cancer.
The present study was performed based on these
aforementioned phenomena. PTX was observed to potentially inhibit
EMT in CCKS-1 cells, as shown by the aspects of their morphology
and the inhibition of the expression of mesenchymal markers on
immunofluorescence and immunoblot investigations. However, it is
noteworthy that PTX inhibited the expression of mesenchymal markers
in a concentration-dependent manner in the immunoblotting
investigation. The optimal PTX concentration was estimated at 5 nM
PTX, as this was the cytotoxic concentration for the CCKS-1 cells.
Accordingly, PTX may potentially inhibit EMT in low-dose and
normal-dose concentrations for viable CCKS-1 cells. Using 10 nM
concentrations of PTX to examine this is difficult, since few cells
are able to survive in strongly cytotoxic concentrations,
therefore, further study is required. Furthermore, the present
study also indicates further investigation is warranted into the
role of the pathways that include Smad2/3 in EMT.
As biliary tract cancers, including
cholangiocarcinoma, are difficult to diagnose in their early stage,
there are numerous cases that are treated with chemotherapy. While
one of the major chemotherapeutic regimens for biliary tract
cancers is cisplatin with gemcitabine, as established by Valle
et al(25), the median
overall survival time is only 11.7 months. Other regimens based on
5-fluorouracil (FU), one of the major drugs that is used in the
treatment of hepatobiliary-pancreatic cancers, were not shown to
contribute to survival and quality of life (26,27).
Furthermore, it has been reported that anti-cancer
treatments, including chemotherapy, radiation therapy and
radiofrequency ablation, may induce EMT in cancer cells (28–30).
Based on these studies, major anticancer treatments using anti-EMT
treatments, including low-dose PTX, may be truly effective
treatment approaches. In a previous study, we experienced a case of
successful treatment for unresectable gallbradder cancer with
low-dose PTX following the failure of gemcitabine and oral S-1
(31), which is an oral prodrug for
5-FU that is widely used in Japan (32).
In conclusion, though the possibility of inhibiting
EMT using PTX for biliary tract cancers in clinical practice is
unclear, the potential of PTX warrants further investigation.
References
|
1
|
Farley DR, Weaver AL and Nagorney DM:
‘Natural history’ of unresected cholangiocarcinoma: patient outcome
after noncurative intervention. Mayo Clin Proc. 70:425–429.
1995.
|
|
2
|
Malhi H and Gores GJ: Review article: the
modern diagnosis and therapy of cholangiocarcinoma. Aliment
Pharmacol Ther. 23:1287–1296. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee GW, Kang JH, Kim HG, Lee JS, Lee JS
and Jang JS: Combination chemotherapy with gemcitabine and
cisplatin as first-line treatment for immunohistochemically proven
cholangiocarcinoma. Am J Clin Oncol. 29:127–131. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Greenburg G and Hay ED: Epithelia
suspended in collagen gels can lose polarity and express
characteristics of migrating mesenchymal cells. J Cell Biol.
95:333–339. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: new insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nieto MA: The snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miyazono K, Ehata S and Koinuma D:
Tumor-promoting functions of transforming growth factor-β in
progression of cancer. Ups J Med Sci. 117:143–152. 2012.
|
|
10
|
Wendt MK, Allington TM and Schiemann WP:
Mechanisms of the epithelial-mesenchymal transition by TGF-beta.
Future Oncol. 5:1145–1168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Donaldson KL, Goolsby GL, Kiener PA and
Wahl AF: Activation of p34cdc2 coincident with taxol-induced
apoptosis. Cell Growth Differ. 5:1041–1050. 1994.PubMed/NCBI
|
|
12
|
Schiff PB, Fant J and Horwitz SB:
Promotion of microtubule assembly in vitro by taxol. Nature.
277:665–667. 1979. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tran TA, Gillet L, Roger S, Besson P,
White E and Le Guennec JY: Non-anti-mitotic concentrations of taxol
reduce breast cancer cell invasiveness. Biochem Biophys Res Commun.
379:304–308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang C, Zhang H, Huang W, Lin Q and Wei H:
Effect of combined use of PDTC and paclitaxel on proliferation and
invasion of human breast cancer cell line MCF-7. Sheng Wu Yi Xue
Gong Cheng Xue Za Zhi. 27:1105–1109. 2010.(In Chinese).
|
|
15
|
Zhou J, Zhong DW, Wang QW, Miao XY and Xu
XD: Paclitaxel ameliorates fibrosis in hepatic stellate cells via
inhibition of TGF-beta/Smad activity. World J Gastroenterol.
16:3330–3334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang D, Sun L, Xian W, et al: Low-dose
paclitaxel ameliorates renal fibrosis in rat UUO model by
inhibition of TGF-beta/Smad activity. Lab Invest. 90:436–447. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sugawara H, Yasoshima M, Katayanagi K,
Kono N, Watanabe Y, Harada K and Nakanuma Y: Relationship between
interleukin-6 and proliferation and differentiation in
cholangiocarcinoma. Histopathology. 33:145–153. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okamoto K, Tajima H, Ohta T, et al:
Angiotensin II induces tumor progression and fibrosis in
intrahepatic cholangiocarcinoma through an interaction with hepatic
stellate cells. Int J Oncol. 37:1251–1259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hay ED: The mesenchymal cell, its role in
the embryo, and the remarkable signaling mechanisms that create it.
Dev Dyn. 233:706–720. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bruzzese F, Leone A, Rocco M, et al: HDAC
inhibitor vorinostat enhances the antitumor effect of gefitinib in
squamous cell carcinoma of head and neck by modulating ErbB
receptor expression and reverting EMT. J Cell Physiol.
226:2378–2390. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
DI Fazio P, Montalbano R, Quint K, et al:
The pan-deacetylase inhibitor panobinostat modulates the expression
of epithelial-mesenchymal transition markers in hepatocellular
carcinoma models. Oncol Lett. 5:127–134. 2013.
|
|
23
|
Noh H, Oh EY, Seo JY, Yu MR, Kim YO, Ha H
and Lee HB: Histone deacetylase-2 is a key regulator of diabetes-
and transforming growth factor-beta1-induced renal injury. Am J
Physiol Renal Physiol. 297:F729–F739. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dong C, Li Z, Alvarez R Jr, Feng XH and
Goldschmidt-Clermont PJ: Microtubule binding to Smads may regulate
TGF beta activity. Mol Cell. 5:27–34. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Valle J, Wasan H, Palmer DH, et al; ABC-02
Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine
for biliary tract cancer. N Engl Med. 362:1273–1281. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ellis PA, Norman A, Hill A, O’Brien ME,
Nicolson M, Hickish T and Cunningham D: Epirubicin, cisplatin and
infusional 5-fluorouracil (5-FU) (ECF) in hepatobiliary tumours.
Eur J Cancer. 31A:1594–1598. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Patt YZ, Jones DV Jr, Hoque A, et al:
Phase II trial of intravenous flourouracil and subcutaneous
interferon alfa-2b for biliary tract cancer. J Clin Oncol.
14:2311–2315. 1996.PubMed/NCBI
|
|
28
|
Yang AD, Fan F, Camp ER, et al: Chronic
oxaliplatin resistance induces epithelial-to-mesenchymal transition
in colorectal cancer cell lines. Clin Cancer Res. 12:4147–4153.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsukamoto H, Shibata K, Kajiyama H,
Terauchi M, Nawa A and Kikkawa F: Irradiation-induced
epithelial-mesenchymal transition (EMT) related to invasive
potential in endometrial carcinoma cells. Gynecol Oncol.
107:500–504. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tajima H, Ohta T, Shoji Y, et al:
Expression of epithelial-mesenchymal transition markers in locally
recurrent hepatocellular carcinoma after radiofrequency ablation.
Exp Therap Med. 1:347–350. 2010. View Article : Google Scholar
|
|
31
|
Tajima H, Ohta T, Shinbashi H, Hirose A,
et al: Successful treatment of unresectable gallbladder cancer with
low-dose paclitaxel as palliative chemotherapy after failure of
gemcitabine and oral S-1: A case report. Oncol Lett. 4:1281–1284.
2012.PubMed/NCBI
|
|
32
|
Tajima H, Ohta T, Kitagawa H, et al: Pilot
study of neoadjuvant chemotherapy with gemcitabine and oral S-1 for
resectable pancreatic cancer. Exp Ther Med. 3:787–792.
2012.PubMed/NCBI
|