Introduction
Human LIGHT (lymphotoxin-related inducible ligand
that competes for glycoprotein D binding to herpesvirus entry
mediator on T cells) is the 14th member of tumor necrosis factor
(TNF) superfamily and is therefore also referred to as TNFSF14
(1). LIGHT is able to bind the
lymphotoxin-β receptor (LTβR), which is expressed on numerous types
of epithelial cancer, and the herpes virus entry mediator (HVEM), a
receptor expressed by T lymphocytes; therefore, LIGHT is
additionally known as HVEM-L (herpesvirus entry mediator-ligand)
(2). LIGHT is a multifunctional
molecule affecting cell proliferation, differentiation and a number
of other biological processes (3).
LIGHT costimulates T-cell amplification effects and enhances the
cell immune reaction to tumors (4),
acting as the most effective tumor immunotherapy factor (5,6).
However, the mechanism by which LIGHT affects cancer cells is
poorly understood.
A previous study by our group showed that LIGHT
promotes HepG2 hepatic carcinoma cell apoptosis through regulating
the expression of Bcl-2 and caspase-8 (7). Furthermore, LIGHT was not detected in
the HCT116 colorectal cancer cell line and the overexpression of
LIGHT transfected into HCT116 cells by plasmid vector inhibited
cell growth. Due to the low transfection efficiency of plasmids, a
stable cell line with LIGHT overexpression may facilitate further
functional studies of LIGHT.
In the present study, LIGHT was overexpressed using
a lentiviral expression vector in the HCT116 colorectal cancer cell
line and its effects on cell biology were investigated, thus
providing a basis for further study of LIGHT functions.
Materials and methods
Cell line and culture
The HCT116 human colon cancer cell line was
purchased from China Centre for Type Culture Collection (Shanghai,
China). The cells were cultured at 37°C in McCoy’s 5α (modified)
medium (Sigma, St. Louis, MO, USA), supplemented with 10% fetal
bovine serum (FBS; Hyclone, Waltham, MA, USA) in a humidified
atmosphere of 5% CO2. The cells were detached using
0.25% trypsin and 0.02% ethylenediaminetetraacetic acid (EDTA).
Animals
Athymic nude male BALBC/c mice, weighing 17–19 g
(4–5 weeks old), were purchased from the Institute of Laboratory
Animal science, Chinese Academy of Medical Science (Beijing,
China). The mice were maintained in specific pathogen-free,
temperature-controlled isolation conditions and fed with sterilized
food and autoclaved water according to the experimental animal
guidelines. The use of animals in the present study complies with
the Guide for the Care and Use of Laboratory Animals. All animal
studies were approved by the Animal Research and Ethical Committee
of Qingdao Medical College (Shandong, China).
Construction of recombinant lentivirus
vector encoding the LIGHT gene
Primers for whole length cDNA of human LIGHT were
designed and synthesized with sequences as follows: Sense,
5′-CGCGGATCCATGGAGGAGAGTGTCG-3′ and antisense,
5′-CTCGTCGACTCACACCATGAAAGCCCC-3′. Briefly, the LIGHT gene was
amplified by HotStar Taq DNA polymerase (Qiagen, Hilden, Germany)
using the pAAV-LIGHT plasmid as a template and then the LIGHT gene
and pLenti vector were cut by SalI and BamHI enzymes.
Following recycling electrophoresis, the LIGHT gene was subcloned
into the pLenti plasmid and the recombined plasmid, pLenti-LIGHT,
was identified by incision with the SalI and BamHI
enzymes and sequencing. Primer synthesis and DNA sequencing were
performed by Shanghai Shangon Co. Ltd. (Shanghai, China). The viral
particles were generated by the cotransfection of 293T cells (ATCC)
via a calcium phosphate-mediated transfection method with
pLenti-LIGHT or pLenti-GFP and three packaging vectors. Three days
after transfection, the cell culture supernatants were harvested
(1,600 × g for 5 min), filtered through 0.45-μm pore size filters
and concentrated 100-fold by ultracentrifugation at 7,000 × g for
16 h. The viral particles were stored in small aliquots at −80°C.
The virus titer was determined by calculating the percentage of
green fluorescent protein (GFP)-positive cells, as observed by
fluorescence microscopy.
Transfection and selection of stable
HCT116 cell lines (HCT116/LIGHT)
For the transfection of the tumor cell lines,
lentiviral vectors harboring LIGHT were constructed and the HCT116
cells were infected. Briefly, the HCT116 cells were cultured in
McCoy’s 5α medium containing 10% FBS and when they reached the
exponential growth phase, 1.0×105 cells per well were
plated in 24 plates. Next, 300 μl complete culture medium,
containing recombinant lentiviruses, control lentiviruses or
McCoy’s 5α medium (all containing 6 μg/ml polybrene; Sigma) was
added into the plates when the cells reached 50–60% confluence. Two
days later, the virus-containing medium was replaced with fresh
complete medium. The expression level of GFP was observed under a
microscope after 3 days. Medium containing blasticidin (6 μg/ml;
Merck KGaA, Darmstadt, Germany) was added every 3 or 4 days to
screen the stable infected cell lines (HCT116/LIGHT or HCT116/GFP,
with clear clone formation) until the uninfected cells were almost
completely removed.
Determination of the optimal multiplicity
of infection (MOI)
To assess the efficiency of lentiviral transduction
in the human HCT116 cells, the cells were infected with pLenti-GFP
at various MOIs for 24 h. The supernatant was then changed to fresh
complete medium every other day. After 72 h, GFP-expressing cells
were detected by fluorescence microscopy (Olympus; Tokyo,
Japan).
Semi-quantitative reverse
transcription-PCR analysis
The total RNA of HCT116/LIGHT, HCT116/GFP or the
control cells was extracted using RNAiso reagent (Takara, Japan),
and then converted into cDNA using a PrimeScript™ RT reagent kit
(Takara), according to the manufacturer’s instructions. The
specific oligonucleotide primers of the LIGHT (PCR product 128 bp)
and GAPDH (PCR product 151 bp) genes were as follows: Sense,
5′-GTACGGCCCTCAGTGTTTGTG-3′ and antisense,
5′-CCCATCAGCAACAGCAAGAGA-3′; and sense, 5′-CTTAGCACCCCTGGCCAAG-3′
and antisense, 5′-GATGTTCTGGAGAGCCCCG-3′, respectively. The
reaction conditions of pre-denaturation were 95°C for 3 min, 95°C
for 30 sec, 60°C for 30 sec and 72°C for 30 sec, with 22 cycles for
GAPDH and 29 cycles for LIGHT (the cycles were based on PCR
kinetics) and a total reaction volume of 20 μl. Each PCR was
repeated three times. The products were subsequently analyzed using
2% agarose gel electrophoresis. The semiquantitative analysis of
LIGHT and GAPDH mRNA levels was measured using the Syngene Gel
Imaging System and analysis software (Syngene Co., Cambridge,
UK).
Expression of LIGHT protein by ELISA
Cell supernatants were collected after 48 h and the
expression of LIGHT protein was detected by ELISA according to the
manufacturer’s instructions (R&D, Minneapolis, MN, USA). The
kit was capable of detecting LIGHT with a minimal detectable dose
as low as 10 pg/ml. The primary wavelength was 450 nm (optionally
620 nm as the reference wavelength). All the tests were repeated
three times with three wells per group.
Western blot analysis
The HCT116 cells were plated onto type I
collagen-coated 25-cm2 flasks, then treated with
Lenti-GFP or Lenti-LIGHT for 48 h in basal medium containing 10%
FBS. The HCT116/LIGHT, HCT116/GFP or control cells were harvested
with a cell scraper, and stored at −80°C until protein extraction.
The pellets were resuspended with a lysis buffer [50 mM Tris-HCl
(pH 8.0), 1 mM EDTA, 150 mM NaCl, 1% Nonidet P-40, 100 μm
4-amidinophenylmethanesulfonyl fluoride, 1 μg/ml aprotinin, 5 μg/ml
leupeptin, 1 μg/ml pepstatin A and 50 μg/ml antipain] and then
mixed well at 4°C. Following centrifugation, the protein
concentration of each supernatant was determined using the Bio-Rad
Protein Assay (Bio-Rad, Hercules, CA, USA). Samples were subjected
to 10% SDS-PAGE and subsequently transferred to polyvinylidene
difluoride membranes (Millipore, Bedford, MA, USA) in a transfer
buffer. These membranes were blocked in BlockAce (Dainippon
Seiyaku, Japan) overnight at 4°C. Rabbit anti-caspase-3, -Bcl-2 or
-GAPDH antibodies (Abcam, Cambridge, MA, USA) were used as primary
antibodies at a 1:1,000 dilution in 10% BlockAce for 30 min. The
samples were then washed in phosphate-buffered saline containing
0.05% Tween-20 (Bio-Rad). Goat anti-rabbit IgG was used as
secondary antibody at a 1:5,000 dilution for 30 min. An enhanced
chemiluminescence (ECL) detection system (Pierce Biotechnology
Inc., Rockford, IL, USA) was then used to visualize immunoreactive
protein complexes. An autoradiograph was obtained and the protein
levels were measured using a FluorS scanner and Quantity One
software for analysis (Bio-Rad).
Analysis of cell proliferation (8)
The cells (1.0×103/well) were plated and
treated in 96-well plates (three wells per group, total 5 plates)
for 24, 48, 72, 96 and 120 h, respectively. At the indicated times,
the medium was removed and fresh medium containing
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT,
0.5 mg/ml; Sigma) was added to each well. The cells were incubated
at 37°C for 4 h and then the medium was removed and 150 μl
solubilization solution (DMSO) was added and mixed thoroughly.
Absorbance from the plates was read on a Safire II spectrometer
reader (Tecan, Männedorf, Swiss) at 490 nm (8).
Subcutaneous human colorectal cancer cell
xenograft growth and oncogenicity
Balb/c nude mice were maintained in specific
pathogen-free, temperature-controlled isolation conditions, and fed
with sterilized food and autoclaved water. Subsequent to being
washed with serum-free McCoy’s 5α medium, the cells were collected
and 200 μl cell solution (1.0×107 cells in 200 μl PBS,
with a viability of >95%) was injected subcutaneous1y into the
right-side of the backs of the mice. The sizes of the transplanted
tumor xenograft were measured periodically until the long diameter
of tumor xenograft was >1 cm. The tumor xenografts were then
dissected and measured with a vernier caliper. The tumor volumes
were calculated using the following formula: Tumor volume = long
diameter × short diameter2 / 2).
Statistical analysis
All data were shown as the mean ± SD unless
otherwise mentioned. Statistical analyses were performed using SPSS
11.5 (SPSS Inc., Chicago, IL, USA). Differences were assessed
between the two groups using a t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Identification of recombinant lentiviral
vectors and GFP visualization
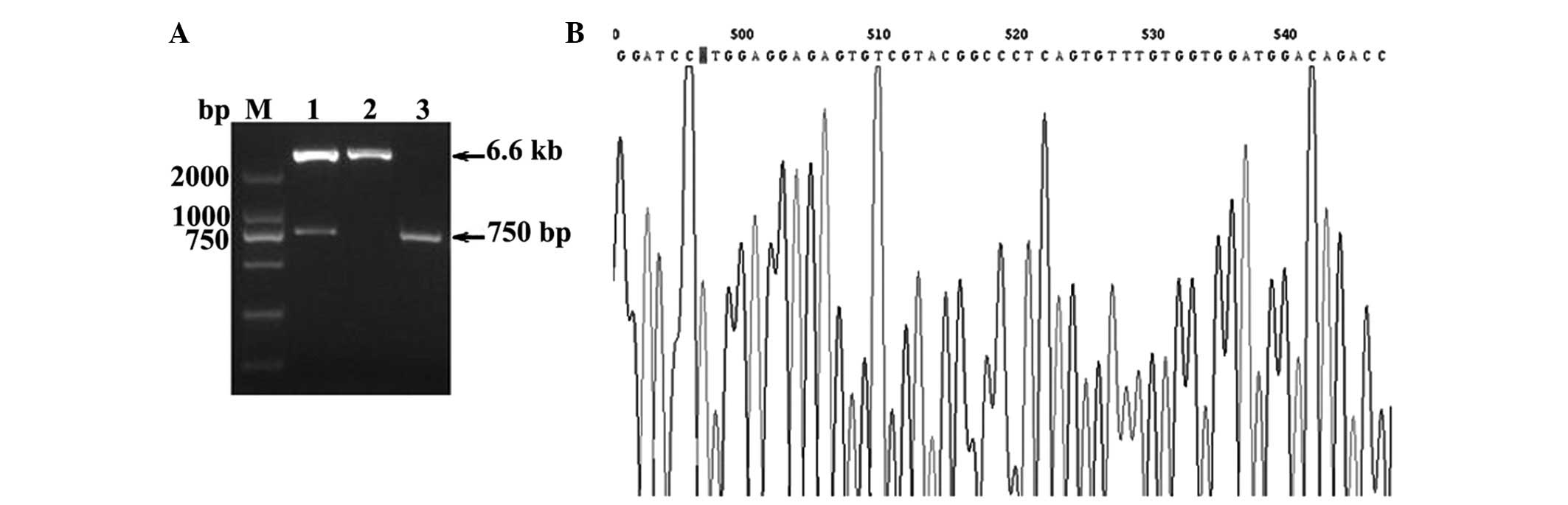
The 1.5% agarose gel electrophoresis showed a LIGHT
gene product, recombinant plasmid and pLenti plasmid (plasmids were
incised by SalI and BamHI), as shown in Fig. 1. The PCR product of the LIGHT gene
was ~750 bp and the recombinant plasmid exhibited 6.6 kb and 750 bp
products after enzyme incision. The sequence of the LIGHT gene was
demonstrated to be the same compared with the sequence from GenBank
(Fig. 1).
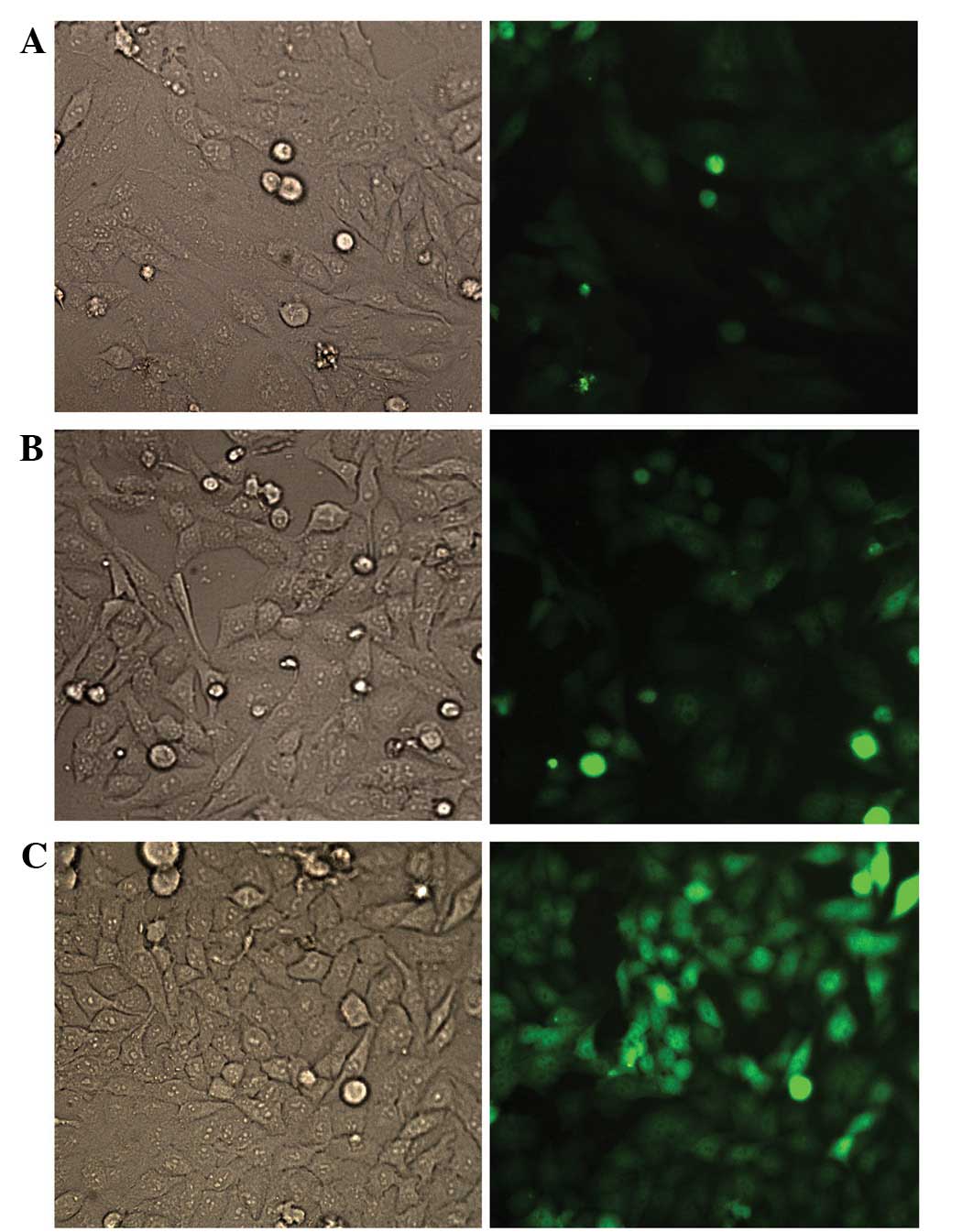
The HCT116 cells exhibited a high lentiviral
transduction efficiency at a MOI of 5 at 72 h. The visualized GFP
results showed that the number of GFP-expressing cells increased in
an MOI-dependent manner (Fig. 2).
As an MOI of 5 was shown to be optimal for cell infection, an MOI
of 5 was selected for subsequent studies. In total, ~90–95% of
cells in the HCT116/LIGHT and HCT116/GFP cell lines infected with
lentivirus expressed significant fluorescent signals.
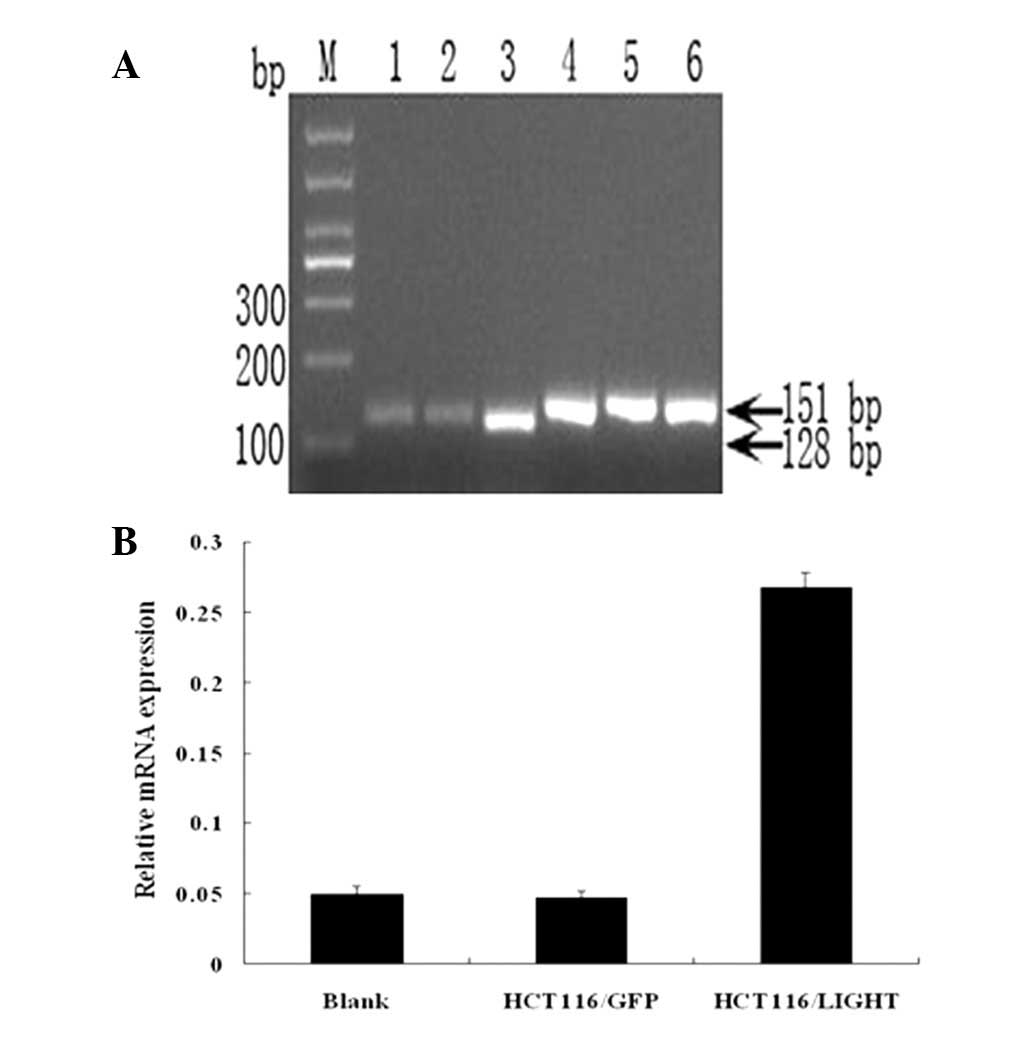
mRNA levels of LIGHT in the cells stably
transfected with LIGHT
The number of PCR cycles required for LIGHT and
GAPDH was 29 and 22, respectively, as determined by the
amplification dynamic experiments (data not shown). The lengths of
the PCR products were 128 bp (LIGHT) and 151 bp (GAPDH),
respectively. The PCR product electrophoresis and semiquantitative
analysis showed that the relative mRNA level of LIGHT in the
HCT116/LIGHT cell line was significantly higher than that of the
blank control (~5.42 times more than that of the blank control) and
HCT116/GFP cells (Fig. 3).
Overexpression of LIGHT protein
The A450 values in the HCT116/GFP and
blank control groups showed no difference with the use of McCoy’s
5α complete medium, which indicated that endogenous LIGHT in the
HCT116 cells was not expressed or was weakly expressed (<10
pg/ml). The protein levels of LIGHT in the HCT116/LIGHT cells were
significantly increased compared with the HCT116/GFP and blank
control groups (3.16±0.15 vs. 0 ng/ml).
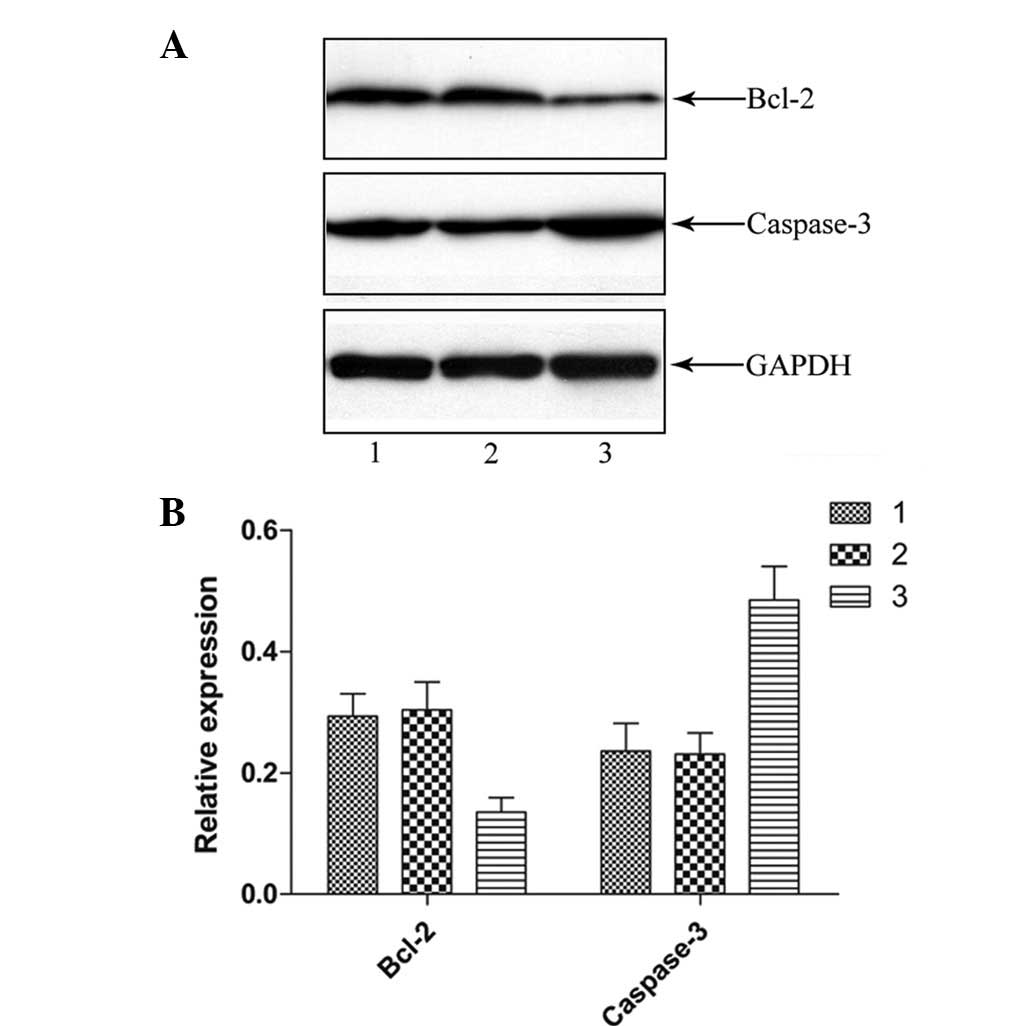
LIGHT activates caspase-3 and inhibits
Bcl-2 activation in HCT116 cells
Several caspases play significant roles in TNF
family-mediated apoptosis. To evaluate the anti-apoptotic effect of
LIGHT on the HCT116 cells, a western blot analysis was performed to
investigate the processing of caspase-3 using cell lysates from the
HCT116 cells pretreated with or without LIGHT. At 48 h after
Lenti-GFP or Lenti-LIGHT incubation, the cleavage product of
caspase-3 was clearly detected, indicating that caspase-3
activation had occurred by that time (Fig. 4). Furthermore, lower levels of Bcl-2
remained as compared with the unpretreated control. There was no
difference in the quantity of cleavage products or Bcl-2 observed
in the reactions with Lenti-GFP and the untreated control.
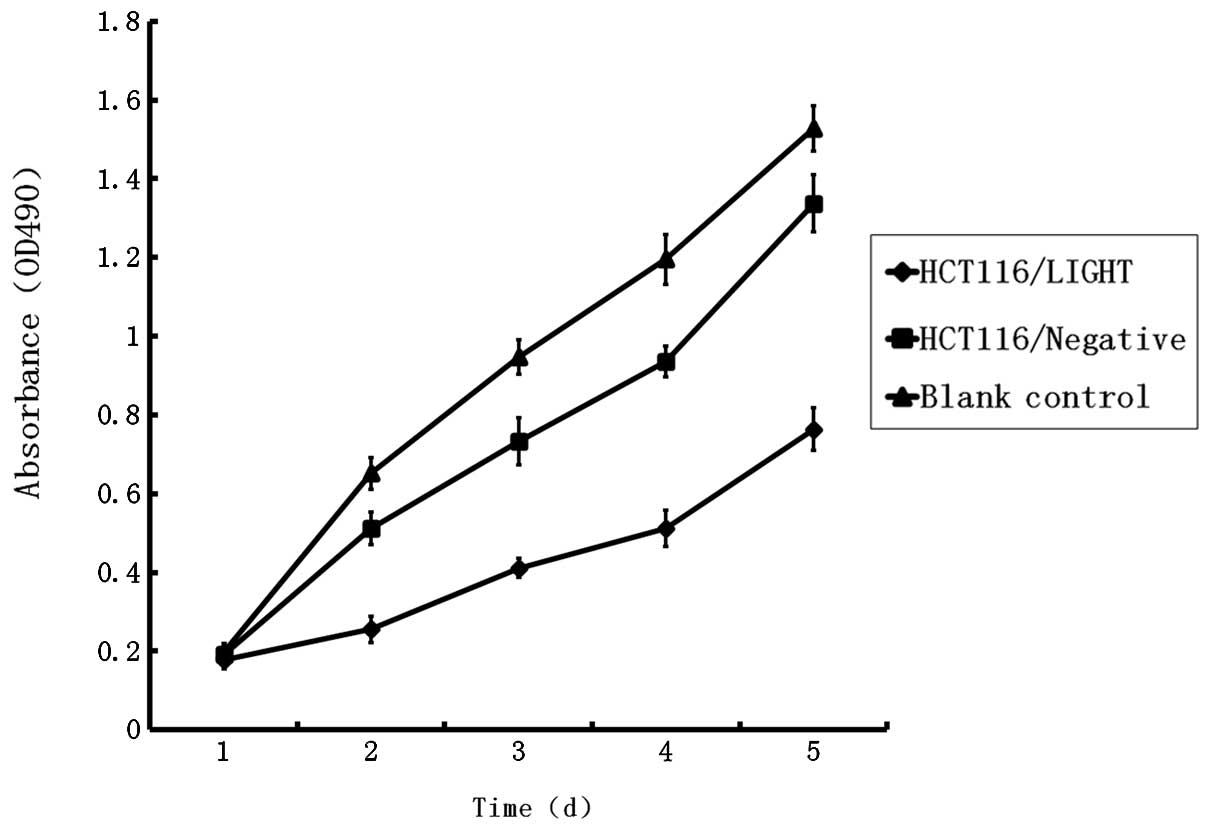
Effect of LIGHT on HCT116 cell
proliferation
Growth curves showed a slight deceleration of cell
growth in the HCT116/GFP group compared with the blank control
group. There was significant growth inhibition in the HCT116/LIGHT
group compared with other groups, which indicated that the
expression of LIGHT caused inhibition of cell growth (Fig. 5).
Effect of LIGHT on oncogenicity in nude
mice
Eight days after subcutaneous cell injection, the
tumor xenografts were successfully inoculated and the volumes of
the tumor xenografts became enlarged with the progression of time.
The tumor volume in the HCT116/LIGHT group was smaller than in the
HCT116/GFP and blank control groups (3.08 times in the blank
control group and 2.80 times in the HCT116/GFP group as compared
with that in the HCT116/LIGHT group; Fig. 6).
Discussion
Colorectal cancer is a type of malignant tumor with
a high clinical incidence (9).
Despite surgical resection, systemic chemotherapy and radiotherapy,
tumor recurrence or metastasis frequently occurs due to the
incomplete elimination of tumor cells and the inadvertent
impairment of normal tissue and cells. The immunotherapy of tumors
may stimulate and reinforce the immune system of the body and thus
control and/or kill tumor cells. Immune responses markedly affect
whether an infection is cleared or will persist to pose a risk for
the development of cancer. The cytokine therapy of tumors may
modulate or reinforce one and/or multiple immune cell functions
after cytokine injection (10,11).
Cancer immunotherapies employing the tumor necrosis factor
superfamily (TNFSF) molecules exhibit antitumor effects through two
predominant mechanisms, the direct killing of tumor cells and
indirect killing by activating antitumor immunity. The former
mechanism is limited to tumors that express the appropriate tumor
necrosis factor receptor superfamily (TNFRSF) molecules, while the
latter works irrespective of tumor type, so it may have broad
applicability as a cancer therapy (12).
LIGHT is a lymphotoxin analogue of glycoprotein D
binding to HVEM on T cells, which may be expressed on activated T
cells and premature dendritic cells. LIGHT interacts with two
distinct cell-membrane receptors, HVEM and LTβR, and one decoy
receptor, TR6/DcR3 (1,13,14).
LIGHT may costimulate T cells and induce cell apoptosis (15), and has three different receptors
with various biological functions. A number of studies have
confirmed that the effect of the inhibition of LIGHT on tumor cell
growth and the induction of apoptosis is correlated with the
expression of receptors on tumor cells. LIGHT may inhibit the
growth of MDA-MB-231 breast cancer cells, HT-29 colorectal cancer
cells and A375 melanoma cells through its secretion and solubility
pattern (6,16). LIGHT may also induce the apoptosis
of tumor cells expressing LIGHT receptors (6) and synergistically induce tumor cell
apoptosis with IFN-γ (7,17). Furthermore, LIGHT may induce the
expression of Mig and IP-10, chemotactic factors in antitumor
angiogenesis, inhibit tumor angiogenesis and act with natural
killer (NK) cells (18) and
accelerate antitumor T-cell immunity, which may result in delayed
growth or the spontaneous regression of tumors (4), all indicating that LIGHT may be an
significant antitumor factor.
The ideal viral vector should provide efficient gene
transfers, stable long-term gene expression and good biological
safety. The lentivirus systems used in the present study are the
third generation of lentiviral vectors and HIV-based expression
vectors, which offer unique versatility and robustness as vehicles
for gene delivery (19). The
lentivirus vectors offer significant advantages over retroviral
vectors in the process of gene delivery to target cells (20,21),
they are genetically altered from wild-type HIV so as to increase
their biosafety and they may transduce a wide range of cell types
and integrate into the host genome in dividing and post-mitotic
cells, resulting in long-term expression of the transgene in
vitro and in vivo(22).
In the present study, a lentivirus coexpressing GFP
and LIGHT genes was first constructed. GFP was used as a reporter
gene to monitor the expression of LIGHT. LIGHT was overexpressed in
the HCT116 colorectal cancer cell line using pLenti-LIGHT and then
the stable and overexpressed LIGHT mRNA and protein cell lines
(HCT116/LIGHT) were screened. The results indicated that the mRNA
and protein levels of LIGHT in the HCT116/LIGHT cells were higher
than the levels in the HCT116 cells, while the overexpression of
LIGHT clearly inhibited the growth of the HCT116 cells and the
oncogenicity of the cells in the nude mice. Furthermore, the
present study demonstrated that LIGHT may inhibit the cellular
proliferative capacity of the HCT116 cells via the upregulation of
caspase-3 and the downregulation of Bcl-2.
The effective immunotherapy of tumors not only
inhibits or eliminates the local tumor burden, it also induces
certain types of protective immunity in the body and thus may
prevent further recurrence of tumor cells. Therefore, LIGHT may
serve as a promising tumor immunotherapy factor and its mechanism
of action consequently requires further investigation.
Acknowledgements
This study was supported by grants from the Shandong
Natural Science Fund (Y2008C48) and the Shandong Education
Department Fund Project (J11LF05).
References
|
1
|
Misawa K, Nosaka T, Kojima T, Hirai M and
Kitamura T: Molecular cloning and characterization of a mouse
homolog of human TNFSF14, a member of the TNF superfamily.
Cytogenet Cell Genet. 89:89–91. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang JM, Deng X, Gong W and Su S:
Chemokines and their role in tumor growth and metastasis. J Immunol
Methods. 220:1–17. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mauri DN, Ebner R, Montgomery RI, Kochel
KD, Cheung TC, Yu GL, Ruben S, Murphy M, Eisenberg RJ, Cohen GH,
Spear PG and Ware CF: LIGHT, a new member of the TNF superfamily,
and lymphotoxin alpha are ligands for herpesvirus entry mediator.
Immunity. 8:21–30. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tamada K, Shimozaki K, Chapoval AI, Zhu G,
Sica G, Files D, Boone T, Hsu H, Fu YX, Nagata S, Ni J and Chen L:
Modulation of T-cell-mediated immunity in tumor and
graft-versushost disease models through the LIGHT co-stimulatory
pathway. Nat Med. 6:283–289. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harrop JA, McDonnell PC, Brigham BM, Lyn
SD, Minton J, Tan KB, Dede K, Spampanato J, Silverman C, Hensley P,
Diprinzio R, Emery JG, Deen K, Eichman C, Chabot FM, Truneh A and
Young PR: Herpesvirus entry mediator ligand(HVEM-L), a novel ligand
for HVEM/TR2, stimulates proliferation of T cells and inhibits HT29
cell growth. J Biol Chem. 273:27548–27556. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhai Y, Guo R, Hsu TL, Yu GL, Ni J, Kwon
BS, Jiang GW, Lu J, Tan J, Ugustus M, Carter K, Rojas L, Zhu F,
Lincoln C, Endress G, Xing L, Wang S, Oh KO, Gentz R, Ruben S,
Lippman ME, Hsieh SL and Yang D: LIGHT, a novel ligand for
lymphotoxin beta receptor and TR2/HVEM induces apoptosis and
suppresses in vivo tumor formation via gene transfer. J Clin
Invest. 102:1142–1151. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang ZH, Wu LQ, Han B, Lu Y, Lu ZH, Liu
XP, Yang K, Sui AH, Bi CY and Li JP: Expression of Fas/FasL and the
apoptosis of HepG2 cells transfected with LIGHT and IFN-γ. Chin J
Curr Adv Gen Surg. 11:301–304. 2008.
|
|
8
|
Liu XP, Wang HB, Yang K, Sui AH, Shi Q and
Qu S: Inhibitory effects of adenovirus mediated tandem expression
of RhoA and RhoC shRNAs in HCT116 cells. J Exp Clin Cancer Res.
28:522009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jemal A, Ward E, Hao Y and Thun M: Trends
in the leading causes of death in the United States, 1970–2002.
JAMA. 294:1255–1259. 2005.
|
|
10
|
Kim D, Gambhira R, Karanam B, Monie A,
Hung CF, Roden R and Wu TC: Generation and characterization of a
preventive and therapeutic HPV DNA vaccine. Vaccine. 26:351–360.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Santin AD, Bellone S, Palmieri M, Zanolini
A, Ravaggi A, Siegel ER, Roman JJ, Pecorelli S and Cannon MJ: Human
papillomavirus type 16 and 18 E7-pulsed dendritic cell vaccination
of stage IB or IIA cervical cancer patients: a phase I
escalating-dose trial. J Virol. 82:1968–1979. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tamada K and Chen L: Renewed interest in
cancer immunotherapy with the tumor necrosis factor superfamily
molecules. Cancer Immunol Immunother. 55:355–362. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tamada K, Shimozaki K, Chapoval AI, Zhai
Y, Su J, Chen SF, Hsieh SL, Nagata S, Ni J and Chen L: LIGHT, a
TNF-like molecule, costimulates T cell proliferation and is
required for dendritic cell-mediated allogeneic T cell response. J
Immunol. 164:4105–4110. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu KY, Kwon B, Ni J, Zhai Y, Ebner R and
Kwon BS: A newly identified member of tumor necrosis factor
receptor superfamily (TR6) suppresses LIGHT-mediated apoptosis. J
Biol Chem. 274:13733–13736. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zou GM and Hu WY: LIGHT regulates CD86
expression on dendritic cells through NF-κB, but not JNK/AP-1
signal transduction pathway. J Cell Physiol. 205:437–443. 2005.
|
|
16
|
Rooney IA, Butrovich KD, Glass AA,
Borboroglu S, Benedict CA, Whitbeck JC, Cohen GH, Eisenberg RJ and
Ware CF: The lymphotoxin-beta receptor is necessary and sufficient
for LIGHT-mediated apoptosis of tumor cells. J Biol Chem.
275:14307–14315. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen MC, Hsu TL, Luh TY and Hsieh SL:
Overexpression of bcl-2 enhances LIGHT and
interferon-gamma-mediated apoptosis in Hep3BT2 cells. J Biol Chem.
275:38794–38801. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu p, Lee Y, Liu W, Chin RK, Wang J, Wang
Y, Schietinger A, Philip M, Schreober H and Fu YX: Priming of naive
T cells inside tumors leads to eradication of established tumors.
Nat Immunol. 5:141–149. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tiscornia G, Singer O and Verma IM:
Production and purification of lentiviral vectors. Nat Protoc.
1:241–245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wiznerowicz M and Trono D: Harnessing HIV
for therapy, basic research and biotechnology. Trends Biotechnol.
23:42–47. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lever AM, Strappe PM and Zhao J:
Lentiviral vectors. J Biomed Sci. 11:439–449. 2004. View Article : Google Scholar
|
|
22
|
Buchschacher GL Jr and Wong-Staal F:
Development of lentiviral vectors for gene therapy for human
diseases. Blood. 95:2499–2504. 2000.PubMed/NCBI
|