Introduction
Colorectal carcinoma (CRC) is one of the most common
cancers with over one million new cases worldwide every year
(1,2). Although surgical resection and
complete removal of the tumor offers the best prognosis for
long-term survival, ~20% of CRC patients present with metastatic
disease at the time of the diagnosis, and surgery may not always
extirpate the recurrence of advanced CRC (3). Therefore, chemotherapy remains one of
the major non-surgical therapeutic approaches for patients with
advanced CRC. Despite the steady progress that has been made in the
field of chemotherapy and targeted therapy, the majority of
patients that undergo chemotherapy experience severe, debilitating
and lethal adverse drug events that considerably outweigh the
benefits (3–5). In addition, the long-term
administration of currently used chemotherapeutic agents usually
generates drug resistance (6).
These problems highlight the urgent requirement for the development
of novel anticancer agents.
Natural products, including traditional Chinese
medicine (TCM), have received great interest as they have
relatively few side-effects and have long been used clinically as a
significant alternative remedy for a variety of cancers (7–14).
Spica Prunellae, the fruit-spikes of the perennial plant,
Prunella vulgaris L., is a medicinal herb that is widely
distributed in Northeast Asia. As a well-known Chinese folk
medicinal herb with pharmacological properties of heat-clearing and
detoxification, Spica Prunellae is traditionally used to treat poor
vision, blood stasis, edema, acute conjunctivitis, lymphatic
tuberculosis, scrofula, acute mastitis, mammary gland hyperplasia,
thyromegaly and hypertension (15).
Furthermore, Spica Prunellae has also been employed as a
significant component in several TCM formulas for the clinical
treatment of several types of cancer, including CRC (16,17).
Although we previously reported that the extract of Spica Prunellae
promotes the apoptosis of human colon carcinoma cells and displays
anti-angiogenic activity in vitro(18,19),
the mode of its anticancer action remains largely unknown. To
further elucidate the mechanism of the tumoricidal activity of
Spica Prunellae, the present study evaluated the effect of the
ethanol extract of Spica Prunellae (EESP) on the proliferation of
human colon carcinoma HT-29 cells and investigated the underlying
molecular mechanisms.
Methods
Materials and reagents
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), penicillin-streptomycin,
trypsin-ethylenediaminetetraacetic acid (EDTA) and TRIzol reagent
were purchased from Invitrogen Corporation (Carlsbad, CA, USA).
SuperScript II reverse transcriptase was provided by Promega
Corporation (Madison, WI, USA). Cyclin D1, cyclin-dependent kinase
4 (CDK4), β-actin antibodies and horseradish peroxidase
(HRP)-conjugated secondary antibodies were obtained from Cell
Signaling Technology (Danvers, MA, USA). All the other chemicals
that were used, unless otherwise stated, were obtained from
Sigma-Aldrich Corporation (St. Louis, MO, USA).
Preparation of EESP
A total of 500 g Spica Prunellae was extracted with
5,000 ml 85% ethanol using a reflux method and filtered. The
ethanol solvent was evaporated on a rotary evaporator (RE-2000;
Shanghai Yarong Biochemical Instrument Factory, Shanghai, China)
and concentrated to a relative density of 1.05. Dried powder EESP
was obtained by spray desiccation using a spray dryer (B-290; Büchi
Labortechnik AG, Flawil, Switzerland). The stock solution of EESP
was prepared by dissolving the EESP powder in 50% dimethyl
sulfoxide (DMSO) to a stock concentration of 500 mg/ml, and the
working concentrations were made by diluting the stock solution in
the cell culture medium. The final concentration of DMSO in the
medium for all the cell experiments was <0.5%.
Cell culture
Human colon carcinoma HT-29 cells were obtained from
the Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
The cells were grown in DMEM containing 10% (v/v) FBS, 100 U/ml
penicillin and 100 μg/ml streptomycin, in a 37°C humidified
incubator with 5% CO2. The cells were subcultured at
80–90% confluency.
Cell viability evaluation
Cell viability was assessed using a
3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
colorimetric assay. The HT-29 cells were seeded into 96-well plates
at a density of 1×104 cells/well in 0.1 ml medium. The
cells were treated with various concentrations of EESP for
different periods of time. At the end of the treatment, 100 μl MTT
[0.5 mg/ml in phosphate-buffered saline (PBS)] was added to each
well and the samples were incubated for an additional 4 h at 37°C.
The purple-blue MTT formazan precipitate was dissolved in 100 μl
DMSO. The absorbance was measured at 570 nm using an ELISA reader
(ELX800; BioTek, Winooski, VT, USA).
Colony formation
The HT-29 cells were seeded into 6-well plates at a
density of 2×105 cells/well in 2 ml medium. Following
the treatment with various concentrations of EESP for 24 h, the
cells were harvested and diluted in fresh medium in the absence of
EESP and then reseeded into 6-well plates at a density of
1×103 cells/well. Following an eight-day incubation
period in a 37°C humidified incubator with 5% CO2, the
formed colonies were fixed with 10% formaldehyde, stained with
0.01% crystal violet and counted. Cell survival was calculated by
normalizing the survival of the control cells as 100%.
Cell cycle analysis
The cell cycle analysis was performed by flow
cytometry using a fluorescence-activated cell sorting (FACS)
caliber (Becton Dickinson, San Jose, CA, USA) and propidium iodide
(PI) staining. Subsequent to being treated with various
concentrations of EESP for 24 h, the HT-29 cells were harvested and
adjusted to a concentration of 1×106 cells/ml, then
fixed in 70% ethanol at 4°C overnight. The fixed cells were washed
twice with cold PBS and then incubated for 30 min with RNase (8
μg/ml) and PI (10 μg/ml). The fluorescent signal was detected
through the FL2 channel and the proportion of DNA that was present
in the various phases was analyzed using ModfitLT Version 3.0
(Verity Software House, Topsham, ME, USA).
RNA extraction and RT-PCR analysis
The HT-29 cells were seeded into 6-well plates at a
density of 2×105 cells/well in 2 ml medium. The cells
were treated with various concentrations of EESP for 24 h. Total
RNA was isolated using TRIzol reagent. Oligo(dT)-primed RNA (1 μg)
was reverse transcribed with SuperScript II reverse transcriptase
according to the manufacturer's instructions. The obtained cDNA was
used to determine the amount of CDK4, cyclin D1 and glyceraldehyde
3-phosphate dehydrogenase (GAPDH) mRNA using PCR with Taq DNA
polymerase (Fermentas, Waltham, MA, USA). GAPDH was used as an
internal control. The primers that were used for amplification of
the CDK4, cyclin D1 and GAPDH transcripts were as follows: CDK4
forward, 5′-CAT GTA GAC CAG GAC CTA AGC-3′ and reverse, 5′-AAC TGG
CGC ATC AGA TCC TAG-3′; cyclin D1 forward, 5′-TGG ATG CTG GAG GTC
TGC GAG GAA-3′ and reverse, 5′-GGC TTC GAT CTG CTC CTG GCA GGC-3′;
and GAPDH forward, 5′-GT CAT CCA TGA CAA CTT TGG-3′ and reverse,
5′-GA GCT TGA CAA AGT GGT CGT-3′.
Western blotting
The HT-29 cells were seeded into 25-cm2
flasks at a density of 2×105 cells/well in 5 ml medium.
The cells were treated with various concentrations of EESP for 24 h
and then lysed with mammalian cell lysis buffer containing protease
and phosphatase inhibitor cocktails. The lysates were resolved in
12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and electroblotted. The polyvinylidene difluoride (PVDF)
membranes were blocked with 5% skimmed milk and probed with primary
antibodies against cyclin D1 (monoclonal, mouse), CDK4 (monoclonal,
mouse) and β-actin (polyclonal, rabbit) at 1:1,000 dilution
overnight at 4°C and then with the appropriate HRP-conjugated
secondary antibody followed by enhanced chemiluminescence
detection.
Statistical analysis
All the data are presented as the mean of three
determinations. The data were analyzed using the SPSS package for
Windows (version 11.5; SPSS, Inc., Chicago, IL, USA). The
statistical analysis of the data was performed with an ANOVA.
P<0.05 was considered to indicate a statistically significant
difference.
Results
EESP inhibits HT-29 cell
proliferation
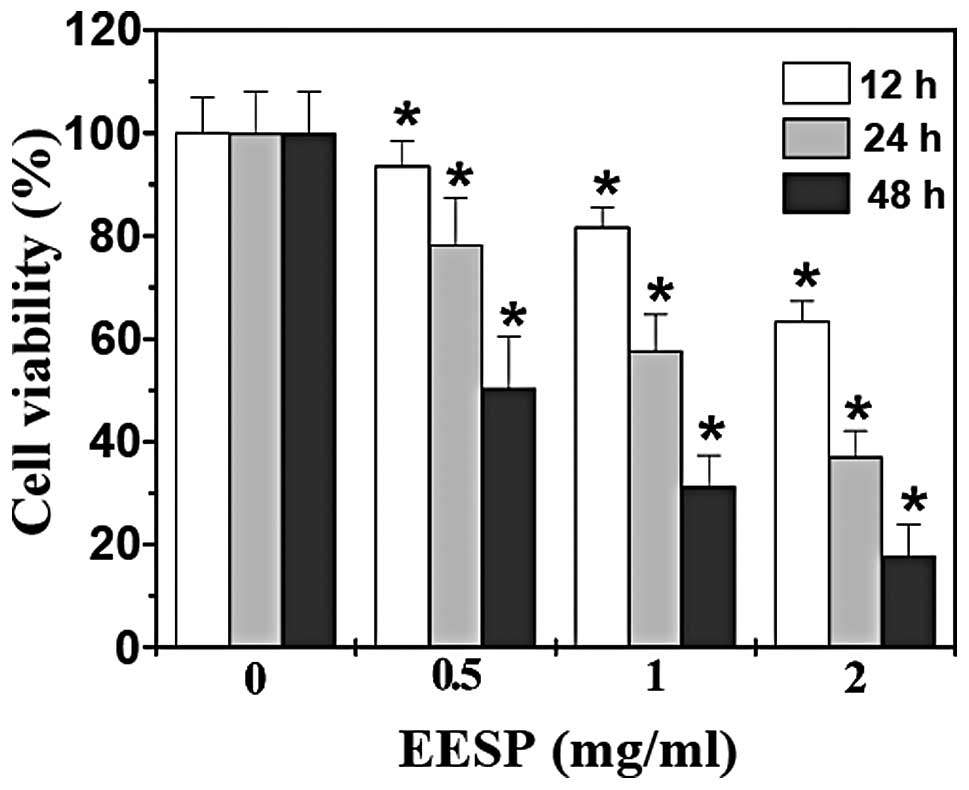
HT-29 cell viability was examined using an MTT assay
to compare the relative number of cells in EESP-treated monolayers
with untreated controls. As shown in Fig. 1, treatment with 0.5–2.0 mg/ml EESP
for 12, 24 or 48 h, respectively, reduced cell viability by
6.5–49.6, 18.4–68.7 or 36.7–82.2% compared with the untreated
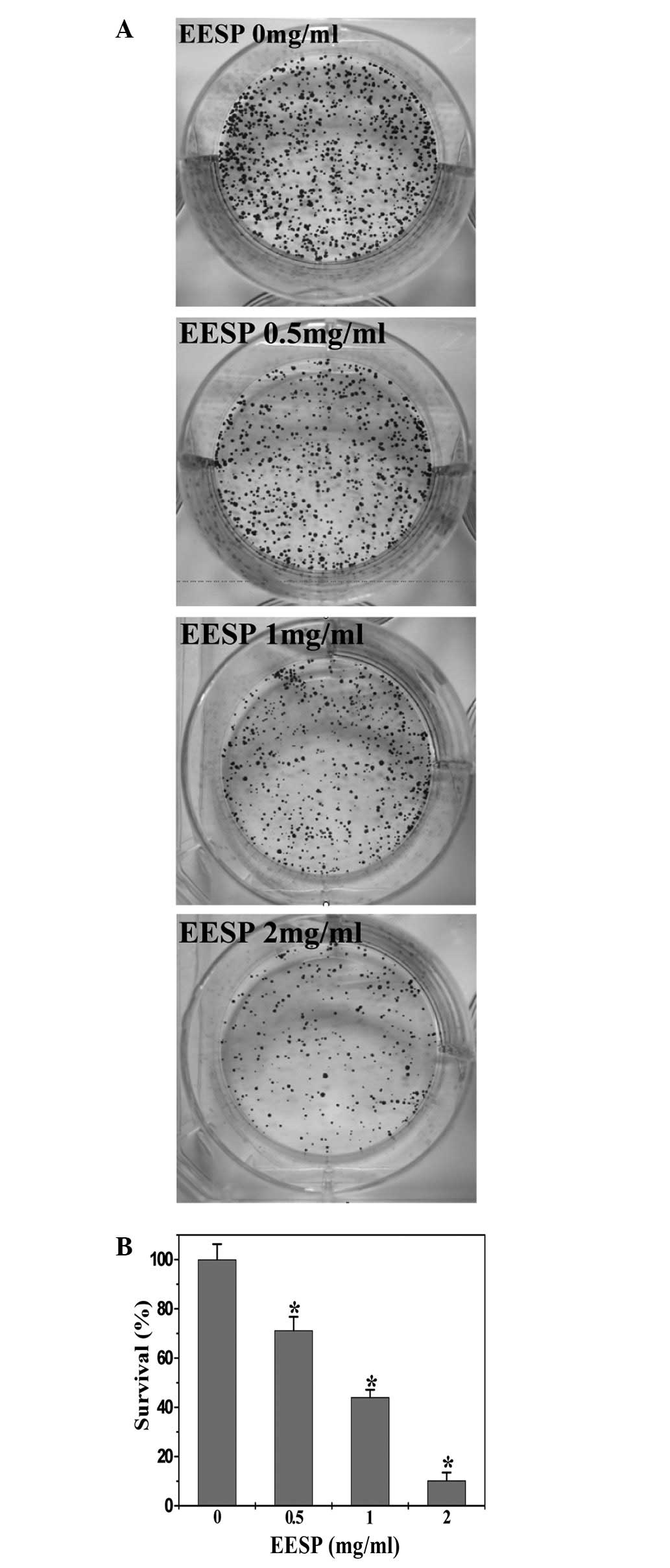
control cells (P<0.05). To further verify these results, the
effect of EESP on HT-29 cell survival was examined using a colony
formation assay. As shown in Fig.
2, EESP treatment dose-dependently reduced the cell survival
rate by 28.8–89.8% compared with the untreated control cells
(P<0.05). Collectively, these data indicate that EESP inhibited
HT-29 cell growth and proliferation in a dose- and time-dependent
manner.
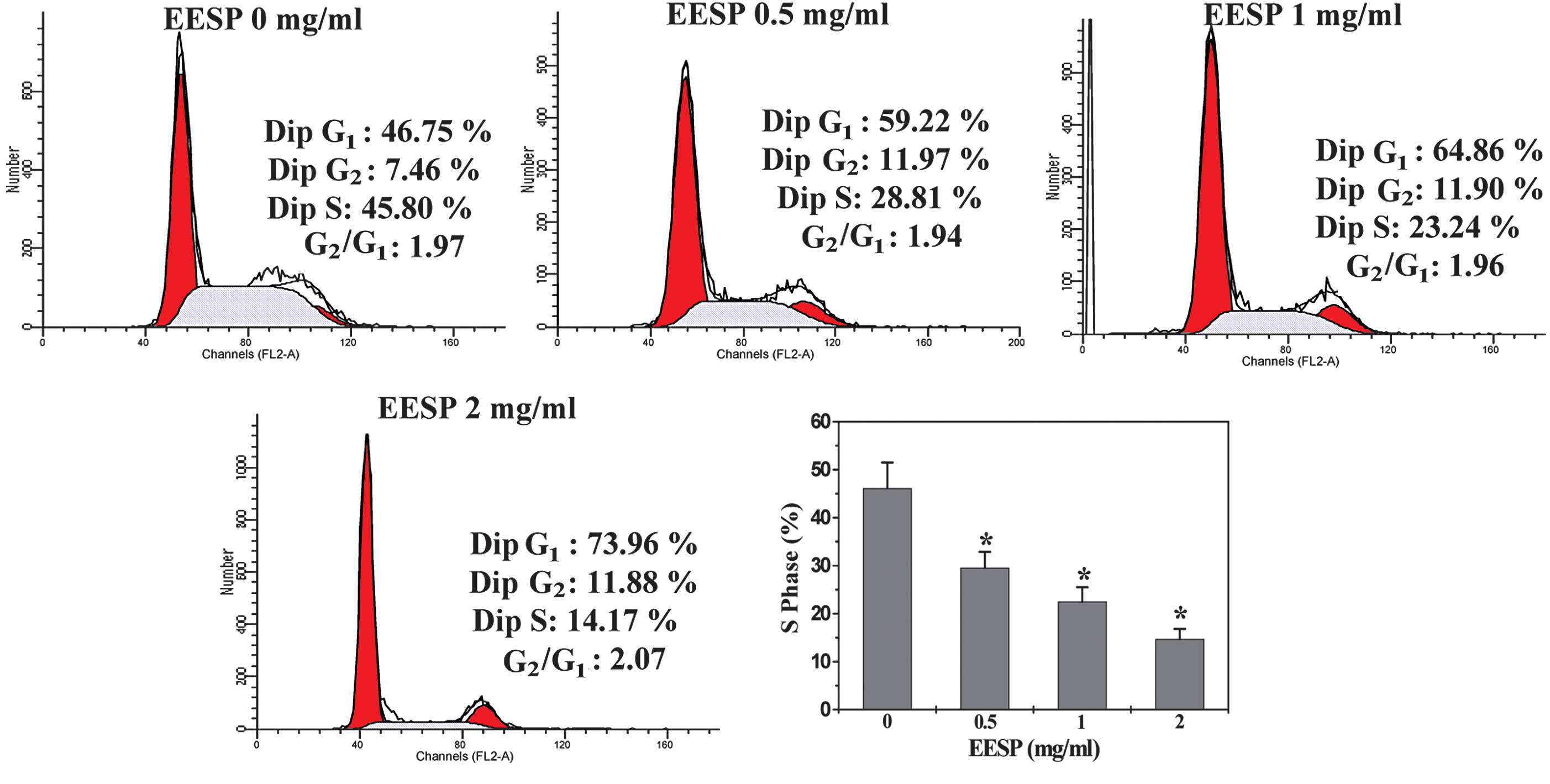
EESP prevents the G1/S
progression of HT-29 cells
To elucidate the mechanism of the anti-proliferative
activity of EESP, its effect on cell cycle progression was examined
in HT-29 cells using FACS analysis with PI staining. As shown in
Fig. 3, the percentage proportion
of S-phase cells following treatment with 0, 0.5, 1 and 2 mg/ml
EESP was 46.1±5.3, 29.5±3.3, 22.5±3.0 and 14.7±2.1%, respectively
(P<0.05), indicating that the inhibitory effect of EESP on HT-29
cell proliferation was mediated by G1/S cell cycle
arrest.
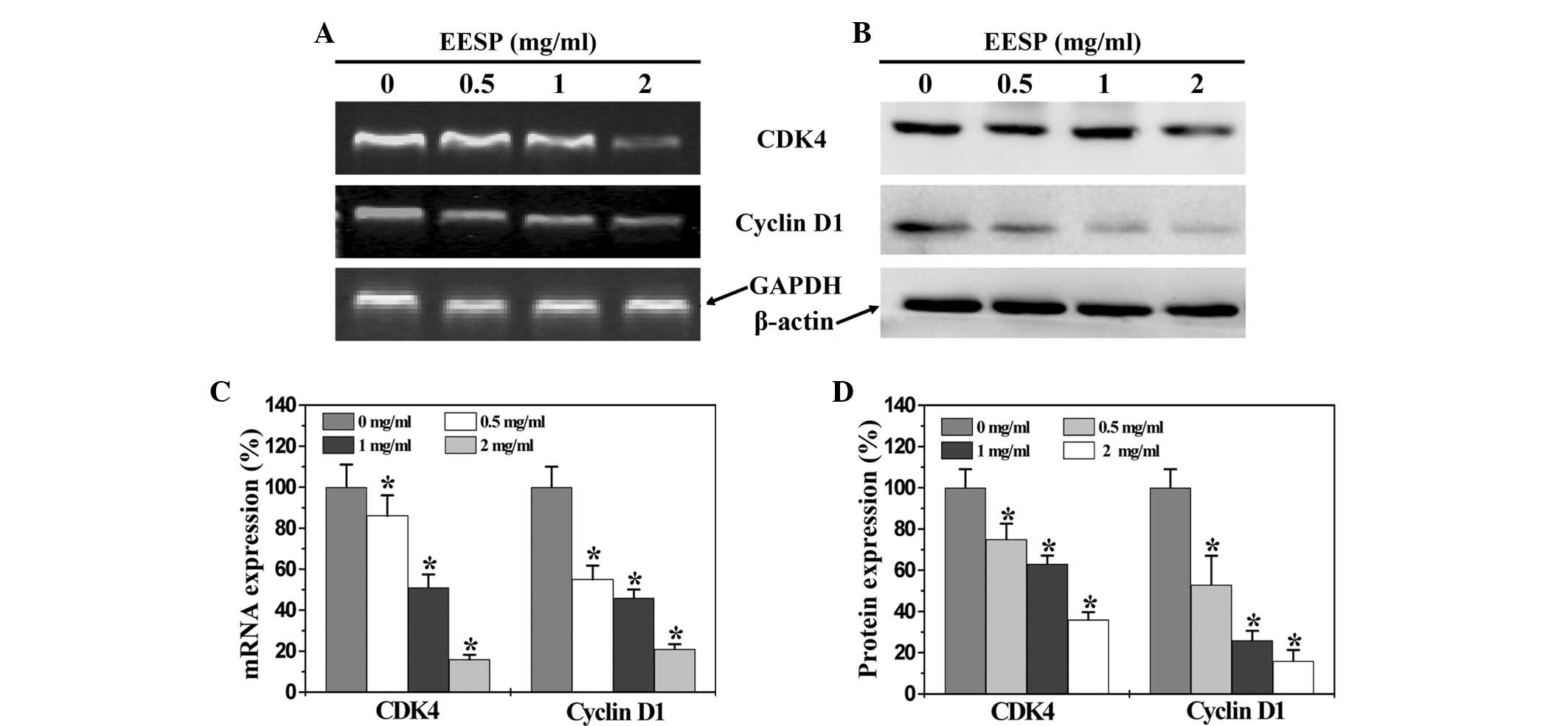
EESP inhibits the expression of cyclin D1
and CDK4 in HT-29 cells
To further explore the mechanism by which EESP
inhibited cell proliferation and G1/S transition, RT-PCR
and western blot analysis were performed to respectively examine
the mRNA and protein expression of cyclin D1 and CDK4 in the HT-29
cells. As shown in Fig. 4A and C,
EESP treatment significantly and dose-dependently reduced the mRNA
expression of pro-proliferative cyclin D1 and CDK4 in the HT-29
cells. The results of the western blot analysis revealed that the
protein expression patterns of cyclin D1 and CDK4 were similar to
their respective mRNA levels (Fig 4B
and D).
Discussion
Due to drug resistance and the adverse effects of
the majority of currently used cancer chemotherapies, natural
products receive great interest since they have relatively fewer
side-effects and have been used clinically for thousands of years
as important alternative remedies for a variety of diseases,
including cancer (7–14). One promising medicinal plant is
Spica Prunellae, which is widely distributed in Northeast Asia. As
a well-known traditional Chinese folk-medicine, it is traditionally
used to treat poor vision, blood stasis, edema, acute
conjunctivitis, lymphatic tuberculosis, scrofula, acute mastitis,
mammary gland hyperplasia, thyromegaly and hypertention (15). In addition, Spica Prunellae has long
been employed for the clinical treatment of several types of cancer
(16,17). Although we previously reported that
Spica Prunellae promotes cancer cell apoptosis and inhibits tumor
angiogenesis (18,19), the precise mechanism of its
potential tumoricidal activity remains largely unclear. Therefore,
prior to the development of Spica Prunellae as an anticancer agent,
the mode of its anti-tumor action should be further elucidated.
Cancer cells are characterized by an uncontrolled
increase in cell proliferation (20). The presents study therefore
investigated the effect of Spica Prunellae on the proliferation of
human colon carcinoma HT-29 cells. By using MTT and colony
formation analyses, it was demonstrated that EESP dose- and
time-dependently inhibited the proliferation of the HT-29 cells.
Eukaryotic cell proliferation is primarily regulated by the cell
cycle, which consists of four periods: The S phase (DNA synthesis
phase), M phase (mitosis), G1 phase and G2
phase. G1/S transition is one of the two main
checkpoints of the cell cycle (21), which is responsible for the
initiation and completion of DNA replication. Using FACS analysis
with PI staining the present study observed that the percentage
proportion of S-phase cells was reduced by EESP treatment in a
dose-dependent manner, indicating that the inhibitory effect of
EESP on HT-29 cell proliferation was mediated by G1/S
cell cycle arrest. G1/S progression is strongly
regulated by cyclin D1, which exerts its function by forming an
active complex with its major catalytic partners, including CDK4
(22–24). An unchecked or hyperactivated cyclin
D1/CDK4 complex often leads to uncontrolled cell division and
malignancy (25). By performing
RT-PCR and western blot analyses, the present study identified that
EESP treatment suppressed the expression of pro-proliferative
cyclin D1 and CDK4 in the HT-29 cells at the transcriptional and
translational levels.
In conclusion, the present study demonstrated for
the first time that Spica Prunellae inhibits the proliferation of
cancer cells through G1/S cell cycle arrest, which may
be one of the mechanisms through which Spica Prunellae exerts its
antitumor activity.
Acknowledgements
This study was sponsored by the National Natural
Science Foundations of China (grant nos. 81073097 and
81202790).
Abbreviations:
|
CRC
|
colorectal cancer
|
|
TCM
|
traditional Chinese medicine
|
|
EESP
|
ethanol extract of Spica Prunellae
|
|
CDK4
|
cyclin dependent kinase 4
|
|
MTT
|
3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
DMSO
|
dimethyl sulfoxide
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Markowitz SD and Bertagnolli MM: Molecular
origins of cancer: Molecular basis of colorectal cancer. N Engl J
Med. 361:2449–2460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang WQ, Fu FF, Li YX, Wang WB, Wang HH,
Jiang HP and Teng LS: Molecular biomarkers of colorectal cancer:
prognostic and predictive tools for clinical practice. J Zhejiang
Univ Sci B. 13:663–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar
|
|
5
|
Lippman SM: The dilemma and promise of
cancer chemoprevention. Nat Clin Pract Oncol. 10:5232006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Longley DB, Allen WL and Johnston PG: Drug
resistance, predictive markers and pharmacogenomics in colorectal
cancer. Biochim Biophys Acta. 1766:184–196. 2006.PubMed/NCBI
|
|
7
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jia L: Cancer complementary and
alternative medicine research at the US National Cancer Institute.
Chin J Integr Med. 18:325–332. 2012. View Article : Google Scholar
|
|
9
|
Carmady B and Smith CA: Use of Chinese
medicine by cancer patients: a review of surveys. Chin Med.
9:222011. View Article : Google Scholar
|
|
10
|
Liu J, Li X, Liu J, Ma L, Li X and Fønnebø
V: Traditional Chinese medicine in cancer care: a review of case
reports published in Chinese literature. Forsch Komplementmed.
18:257–263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang GY, Li X, Li XL, Wang L, Li J, Song
X, Chen J, Guo Y, Sun X, Wang S, Zhang Z, Zhou X and Liu J:
Traditional Chinese medicine in cancer care: a review of case
series published in the chinese literature. Evid Based Complement
Alternat. 2012:1–8. 2012. View Article : Google Scholar
|
|
12
|
Newman DJ, Cragg GM and Snader KM: The
influence of natural products upon drug discovery. Nat Prod Rep.
17:215–234. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Taixiang W, Munro AJ and Guanjian L:
Chinese medical herbs for chemotherapy side effects in colorectal
cancer patients. Cochrane Database of Systematic Reviews. (1):
CD004540 View Article : Google Scholar
|
|
14
|
Zhang M, Liu X, Li J, He L and Tripathy D:
Chinese medicinal herbs to treat the side-effects of chemotherapy
in breast cancer patients. Cochrane Database of Systematic Reviews.
(2): CD004921 View Article : Google Scholar
|
|
15
|
Chinese Pharmacopeia Commission.
Pharmacopoeia of the People's Republic of China Chinese. Medical
Science and Technology Press; Beijing: 263. 2010
|
|
16
|
Cao Z, Lin W, Huang Z, Chen X, Zhao J,
Zheng L, Ye H, Liu Z, Liao L and Du J: Ethyl acetate extraction
from a Chinese herbal formula, Jiedu Xiaozheng Yin, inhibits the
proliferation of hepatocellular carcinoma cells via induction of
G0/G1 phase arrest in vivo and in vitro. Int J Oncol. 42:202–210.
2013.PubMed/NCBI
|
|
17
|
Cao Z, Lin W, Huang Z, Chen X, Zhao J,
Zheng L, Ye H, Liu Z, Liao L and Du J: Jiedu Xiaozheng Yin, a
Chinese herbal formula, inhibits tumor angiogenesis via
downregulation of VEGF-A and VEGFR-2 expression in vivo and in
vitro. Oncol Rep. 29:1080–1086. 2013.PubMed/NCBI
|
|
18
|
Zheng LP, Chen YQ, Lin W, Zhuang QC, Chen
XZ, Xu W, Liu XX, Peng J and Sferra TJ: Spica Prunellae extract
promotes mitochondrion-dependent apoptosis in a human colon
carcinoma cell line. Afr J Phar Pharmacol. 5:327–335. 2011.
View Article : Google Scholar
|
|
19
|
Lin W, Zheng LP, Zhao JY, Zhuang QC, Hong
ZF, Xu W, Chen YQ, Sferra TJ and Peng J: Anti-angiogenic effect of
Spica Prunellae extract in vivo and in vitro. Afr J Phar Pharmacol.
24:2647–2654. 2011.
|
|
20
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nurse P: Ordering S phase and M phase in
the cell cycle. Cell. 79:547–550. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morgan DO: Principles of CDK regulation.
Nature. 374:131–134. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Y, Robles AI, Martinez LA, Liu F,
Gimenez-Conti IB and Conti CJ: Expression of G1 cyclins,
cyclin-dependent kinases, and cyclin-dependent kinase inhibitors in
androgen-induced prostate proliferation in castrated rats. Cell
Growth Differ. 7:1571–1578. 1996.PubMed/NCBI
|
|
24
|
Graña X and Reddy EP: Cell cycle control
in mammalian cells: role of cyclins, cyclin dependent kinases
(CDKs), growth suppressor genes and cyclin-dependent kinase
inhibitors (CKIs). Oncogene. 11:211–219. 1995.PubMed/NCBI
|
|
25
|
Zafonte BT, Hulit J, Amanatullah DF,
Albanese C, Wang C, Rosen E, Reutens A, Sparano JA, Lisanti MP and
Pestell RG: Cell-cycle dysregulation in breast cancer: breast
cancer therapies targeting the cell cycle. Front Biosci.
5:D938–D961. 2000. View
Article : Google Scholar : PubMed/NCBI
|