Introduction
Hepatocellular carcinoma (HCC) is the most common
type of primary liver cancer and represents the third leading cause
of cancer-related mortality worldwide. HCC is particularly
prevalent in Asia and Africa (1,2). The
majority of HCC cases are detected at advanced stages of the
disease, which precludes the use of curative surgical therapy. The
prognosis of HCC is generally poor and the mortality rate is
similar to the incidence rate. Consequently, detection of
early-stage HCC remains an effective approach to substantially
improve the overall outcome of individuals with HCC (3). The diagnosis of HCC is usually
determined via tumor biomarkers in serum and by instrumental tests,
including hepatic ultrasonography, computed tomography, magnetic
resonance imaging and biopsy. Although a number of studies have
investigated positive cancer biomarkers for HCC, none have been
identified as the optimal choice. At present, α-fetoprotein (AFP)
detection has been widely adopted for the diagnosis of HCC despite
its low sensitivity (4–6). The use of AFP in combination with
additional serum markers, including lens culinaris agglutinin
reactive AFP (7), golgi protein 73
(GP73) (8) and γ-glutamyl
transferase (GGT-II) (9), is likely
to significantly increase the sensitivity compared with AFP
alone.
GP73 is a resident golgi type II transmembrane
protein expressed primarily in human epithelial cells (10). In the normal human liver, GP73 is
expressed in biliary epithelial cells, but detection is negligible
in hepatocytes. However, upregulated expression of GP73 has been
identified in hepatic cells in liver disease (11). Previous studies have also shown
increased serum GP73 levels in patients with chronic liver disease
and, in particular, in HCC patients. This phenomenon may be due to
migration of the GP73 protein to the plasma membrane and diffusion
into the circulation (12,13). Thus, GP73 has been hypothesized to
represent a novel serum marker for HCC. In addition, previous
studies have proposed that GP73 exhibits a diagnostic ability
superior to that of AFP (14,15).
Therefore, it is reasonable to hypothesize that the determination
of GP73, combined with additional significant HCC serum markers,
may enhance diagnostic accuracy.
The aim of the present study was to compare the
expression levels of serum GP73 in control and patient groups of
individuals with liver diseases, including chronic hepatitis, liver
cirrhosis and HCC, to evaluate and investigate the diagnostic value
and accuracy of measuring serum GP73 in combination with AFP and
GGT-II in HCC patients.
Patients and methods
Patient selection
A total of 184 serum samples were obtained from the
Affiliated Hospital of Nantong University (Nantong, China),
including 79 HCC (median age, 53 years old; 51 males and 28
females), 47 liver cirrhosis (median age, 43 years old; 27 males
and 20 females), 30 chronic hepatitis (median age, 57 years old; 23
males and 7 females) and 28 healthy (median age, 50 years old; 14
males and 14 females) individuals. The blood samples of the HCC
patients were collected prior to any interventions, including
surgery or radiochemotherapy. A portal vein thrombosis (PVT) was
identified in 27/79 HCC cases and the tumor diameters were <5
and ≥5 cm in 23 and 56 cases, respectively. This study was approved
by the ethics committee of the Affiliated Hospital of Nantong
University (Nantong, China). Written informed consent was obtained
from the patients.
Time-resolved fluorescence immunological
assay (TRFIA) of serum GP73
Optimal concentrations of the reagents for TRFIA
were determined according to experimental data in a trial-and-error
procedure. Each well was coated with 100 μl mouse monoclonal
antibodies against GP73 (4 mg/l; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) prior to overnight storage at 4°C. The plates
were washed 4 times with 300 μl/well PBS and 0.05% Tween 20,
followed by incubation with 250 μl/well blocking solution (PBS
containing 10% FCS) at 4°C for 48 h. The blocking solution was
poured off and 50 μl serum samples, negative controls and a series
of GP73 standard dilutions were loaded into the appropriate wells
and incubated at 37°C for 4 h. Subsequently, the plates were washed
four times with PBS, and 100 μl goat anti-human polyclonal
antibodies conjugated with biotin (80 μg/l; Santa Cruz
Biotechnology, Inc.) were added to each well. Plates were agitated
on an orbital shaker (Stuart Equipment, Stone, UK) at 1.5 × g,
incubated at room temperature for 2 h and washed 4 times prior to
adding 100 μl europium-labeled streptavidin (500 μg/l;
Perkin-Elmer, Waltham, MA, USA) to each well. Following 1-h
incubation at room temperature, the plates were washed 4 times as
described and 200 μl enhancement solution was added to each well
prior to a 15-min rotating incubation at room temperature in the
dark. The plates were read using a Victor™ X5 automatic
time-resolved fluorescence detector (Perkin-Elmer).
Enzyme-linked immunosorbent assay (ELISA)
detection of serum GP73
Serum GP73 was measured using ELISA kits obtained
from Yifeng Biotechnology Co., Ltd., (Shanghai, China) according to
the manufacturer’s instructions. Briefly, 50 μl samples were loaded
onto ELISA plates precoated with monoclonal anti-GP73 and incubated
at 37°C for 45 min. Subsequent to being washed four times with
washing buffer, 50 μl/well anti-IgG conjugated with biotin was
added and then the samples were incubated at 37°C for 30 min. The
plates were washed again, streptavidin-HRP solution was added and
the samples were incubated at 37°C for 30 min. Color developing
agents A and B (50 μl/well) were added in sequence and incubated in
the dark for 15 min. The reaction was terminated with stop buffer.
Absorbance was measured at 450 nm on a microplate reader. The
concentrations of GP73 were determined by interpolation from the
standard curve.
Determination of serum AFP and
GGT-II
The presence of AFP was determined by
chemiluminescence immunoassay and the reagents were obtained from
Abbott Laboratories (Chicago, IL, USA). Serum GGT-II was determined
using polyacrylamide gel electrophoresis kits (Jiangsu Zongheng
Co., Ltd., Nantong, China), as described previously (16).
Statistical analysis
Stata View 7.0 software package (Stata Corp LP,
College Station, TX, USA) was used for the statistical analysis.
Serum GP73 levels are presented as the median (range) and were
analyzed with a Wilcoxon rank sum test. χ2 or Fisher’s
exact tests were performed for any 2×2 tables. The correlation
between serum GP73 and AFP and GGT-II was determined by Spearman’s
rank correlation. Receiver operating characteristic (ROC) curves
were used to evaluate the diagnostic value of the serum markers.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Distribution of serum GP73 determined by
TRFIA or ELISA
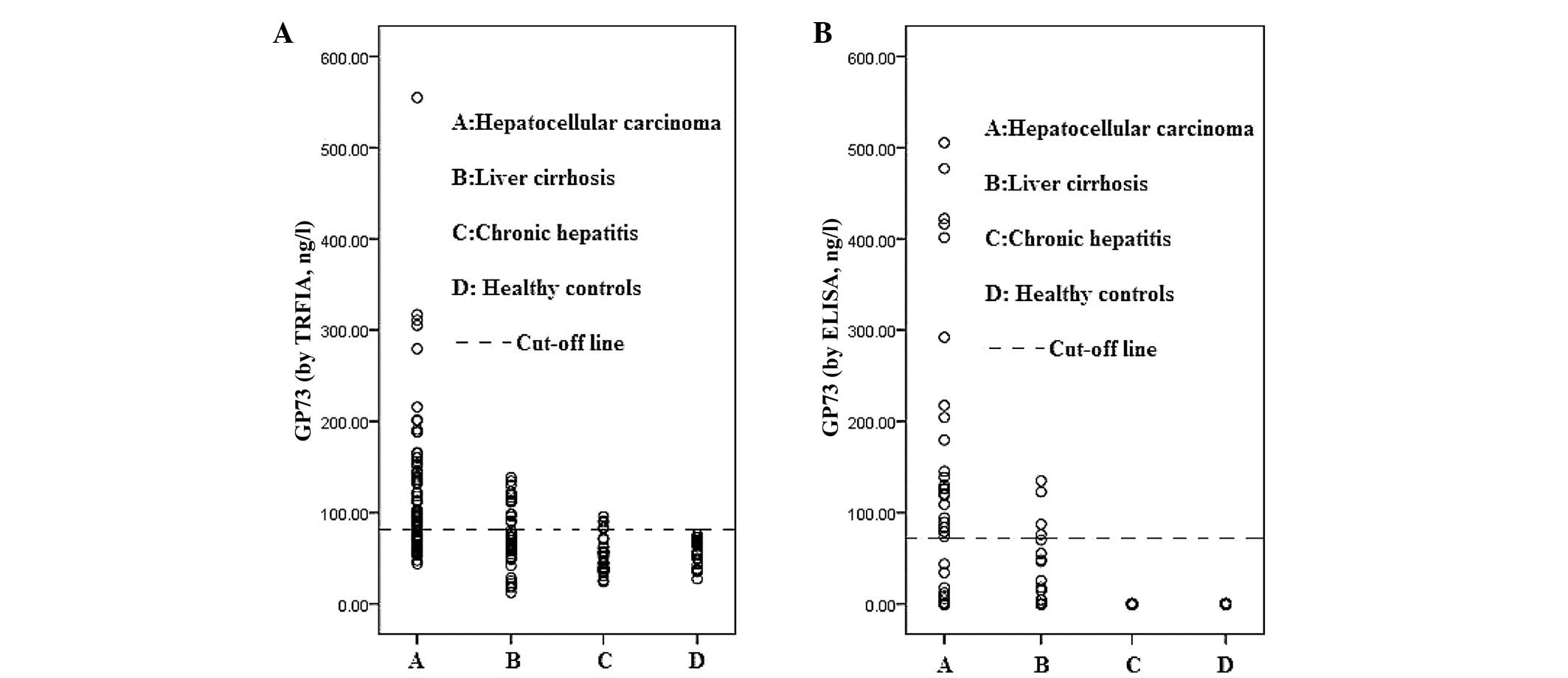
Each blood sample was assessed for the GP73 levels
using TRFIA and ELISA. The median (range) values of the GP73 levels
in the HCC, liver cirrhosis, chronic hepatitis and healthy controls
were 95.5 (43.9–554.8 ng/l), 69.3 (12.4–138.5 ng/l), 63.2
(27.2–95.6 ng/l) and 50.4 (24.1–75.8 ng/l), respectively, as
determined by TRFIA. The serum GP73 levels in the HCC samples were
markedly higher when compared with the benign liver disease and
healthy control samples. The median levels of GP73, as determined
by ELISA, were 0 ng/l for all groups, indicating that the
sensitivity of ELISA for serum GP73 was considerably lower when
compared with that of TRFIA. However, ELISA also identified higher
levels of GP73 in the HCC patients compared with the additional
groups (Table I; Fig. 1).
 | Table ISerum GP73 levels, as detected by
TRFIA and ELISA. |
Table I
Serum GP73 levels, as detected by
TRFIA and ELISA.
| | TRFIA (ng/l) | ELISA (ng/l) |
|---|
| |
|
|
|---|
| Subgroups | n | Median (range) | Z | P-value | Median (range) | Z | P-value |
|---|
| HCC | 79 | 95.5
(43.9–554.8) | | | 0 (0–505.4) | | |
| Liver cirrhosis | 47 | 69.3
(12.4–138.5) | 4.152 | 0.000 | 0 (0–134.9) | 1.978 | 0.045 |
| Chronic
hepatitis | 30 | 63.2 (27.2–95.6) | 6.085 | 0.000 | 0 (0–0.1) | 3.578 | 0.000 |
| Healthy controls | 28 | 50.4 (24.1–75.8) | 6.255 | 0.000 | 0 (0–0.0) | 3.921 | 0.000 |
In addition, ROC curves were plotted to determine
the optimal cut-off values to identify the sensitivity and
specificity of serum GP73 in the patients with HCC. Accuracy was
measured by the area under the ROC (AUROC) curve. The AUROC curve
by TRFIA for GP73 was 0.814 (95% CI, 0.753–0.874) and the
sensitivity and specificity, with cut-off values of 78.1 ng/l, were
73.4 and 79.0%, respectively (Fig.
2). The AUROC curve by ELISA for GP73 was 0.643 (95% CI,
0.559–0.725) and the sensitivity and specificity, with cut-off
values of 71.7 ng/l, were 30.4 and 96.2%, respectively (Table II; Fig.
2).
 | Table IIDiagnostic sensitivities of GP73
(ng/l), as detected by TRFIA and ELISA. |
Table II
Diagnostic sensitivities of GP73
(ng/l), as detected by TRFIA and ELISA.
| Subgroups | Total, n | TRFIAb | ELISAc |
|---|
|
|
|---|
| n | % | n | % |
|---|
| HCC | 79 | 58 | 73.4a | 25 | 31.6a |
| Liver cirrhosis | 47 | 16 | 34.0 | 4 | 8.5 |
| Chronic
hepatitis | 30 | 5 | 16.7 | 0 | 0.0 |
| Healthy controls | 28 | 0 | 0.0 | 0 | 0.0 |
The correlation between the serum GP73 levels and a
number of tumor grading parameters was also evaluated, and the
statistical analyses identified no correlations between the serum
GP73 levels and the tumor size or PVT tumors (P>0.05; Table III).
 | Table IIICorrelation between GP73 levels and
tumor size and PVT in 79 HCC patients, as detected by TRFIA. |
Table III
Correlation between GP73 levels and
tumor size and PVT in 79 HCC patients, as detected by TRFIA.
| Groups | n | GP73 positive, n | GP73 positive rate,
% |
|---|
| Tumor size, cm |
| <5 | 23 | 14 | 60.9a |
| ≥5 | 56 | 44 | 78.6 |
| PVT |
| + | 27 | 23 | 85.2b |
| − | 52 | 35 | 67.3 |
Serum AFP and GGT-II in HCC
The serum AFP level was significantly higher in the
HCC group compared with the additional groups (P<0.05). The
median serum AFP level in HCC was 270.00 ng/ml, which was higher
compared with the liver cirrhosis (7.26 ng/ml), chronic hepatitis
(13.80 ng/ml) and healthy control (2.33 ng/ml; Table IV) samples. According to the ROC
curves, the sensitivity and specificity of GP73, with cut-off
values of 47.8 ng/ml, were 55.6 and 86.7%, respectively (Table IV; Fig.
3). The sensitivity of the serum GGT-II levels for the
diagnosis of HCC was also evaluated and was 68.4% in the HCC
patients (Table IV).
 | Table IVSerum AFP and GGT-II levels in
various hepatic diseases and healthy controls. |
Table IV
Serum AFP and GGT-II levels in
various hepatic diseases and healthy controls.
| AFP (ng/ml),
CLEIA | |
|---|
|
| |
|---|
| Subgroups | n | Median (range) | Z | P-value | ≥47.8, n (%) | GGT-II positive, n
(%) |
|---|
| HCC | 79 | 270.00
(0.65–10,001.00) | | | 44 (55.6)a | 54 (68.4)a |
| Liver
cirrhosis | 47 | 7.26
(0.38–860.68) | 4.723 | 0.000 | 12 (25.5) | 3 (6.4) |
| Chronic
hepatitis | 30 | 13.80
(0.28–237.31) | 4.839 | 0.000 | 2 (6.7) | 0 (0.0) |
| Healthy
controls | 28 | 2.33
(0.21–9.80) | 5.898 | 0.000 | 0 (0.0) | 0 (0.0) |
Serum GP73 levels are complementary to
the serum levels of AFP and GGT-II
Spearman’s rank correlation test was performed on
the serum GP73, AFP and GGT-II levels. No correlations were
identified between the serum GP73 levels and AFP (r=0.0920;
P=0.5384) or GGT-II (r=0.1321; P=0.3763), indicating that the three
serum markers may have complementary roles in the diagnosis of HCC.
The sensitivities of GP73, AFP and GGT-II for the diagnosis of HCC
were 73.4, 55.6 and 68.4%, respectively, however, the combination
of the three markers may increase the sensitivity to 96.3%
(Table V).
 | Table VComplementary values of GP73, AFP and
GGT-II for HCC diagnosis. |
Table V
Complementary values of GP73, AFP and
GGT-II for HCC diagnosis.
| Sensitivity, % | Specificity, % | Accuracy, % |
|---|
| GP73
(TRFIA)-positive | 73.4
(58/79)a | 80.0 (84/105) | 77.2 (142/184) |
| AFP-positive | 55.6
(44/79)a | 86.7 (91/105) | 73.4 (135/184) |
|
GGT-II-positive | 68.4
(54/79)a | 97.1 (102/105) | 84.8 (156/184) |
| a+b | 88.6 (70/79) | 69.5 (73/105) | 77.7 (143/184) |
| a+c | 91.1 (72/79) | 77.1 (81/105) | 83.2 (153/184) |
| b+c | 86.1 (68/79) | 84.8 (89/105) | 85.3 (157/184) |
| a+b+c | 96.2 (76/79) | 67.6 (71/105) | 79.9 (147/184) |
Discussion
The present study demonstrated that patients with
HCC exhibit markedly higher levels of GP73 in the serum compared
with patients with chronic hepatitis, liver cirrhosis and healthy
controls. No correlations were identified among serum GP73 levels
and the additional parameters, including tumor size and grading. In
addition, no correlations were identified between the serum levels
of AFP, GGT-II and GP73. The detection of serum GP73 in combination
with classic HCC tumor markers, AFP and GGT-II, may improve the
diagnostic power for HCC. We hypothesized that GP73 may represent a
serum marker for HCC.
The early diagnosis and treatment of HCC is vital
for improving the overall survival rate of patients (17). Routine surveillance is generally
recommended worldwide for patients at risk of developing HCC,
including individuals with chronic hepatitis and liver cirrhosis.
At present, serum AFP and abdominal ultrasonography are the most
common tools adopted for the diagnosis and monitoring of HCC in
clinical practice. However, the clinical value of AFP, also
referred to as the ‘gold-standard’ biomarker of HCC, remains
controversial, as elevated AFP levels are often detected in
patients with benign liver disease and additional malignancies, but
not in a large proportion of early-stage HCCs (18). The clinical value of AFP has
previously been challenged due to its low sensitivity and
specificity (4–6).
A hepatoma-specific band of serum GGT-II has been
determined to be an effective tumor marker complementary to AFP for
the diagnosis of HCC. In 1985, following 10 years of follow-up, Xu
et al reported GGT-II-positive expression in 90% (81/90) of
HCC cases and no expression in the majority of patients with acute
and chronic viral hepatitis, extrahepatic tumors, pregnant women
and healthy controls (19). In
addition, GGT-II-positive expression was identified in 74.0%
(89/120) of HCC (sensitivity), 17.78% (16/90) of cirrhosis and
43.8% (14/32) of small HCC cases, indicating that GCT-II may
represent an effective tumor marker for the diagnosis of HCC
(9).
Consistent with the present results, no correlation
has been reported between the levels of serum expression and AFP
and GGT-II in previous studies (20). It has been indicated that the
simultaneous determination of AFP and GGT-II may improve diagnostic
accuracy for HCC (9). Previously,
additional serum biomarkers, including DCP, AFU and GPC3, have been
widely investigated, however, none have been identified as optimal
for the early detection of HCC. A number of clinical and
experimental studies have shown the significance of serum GP73
expression in liver diseases, particularly in HCC. In 2005, Block
et al reported that increased serum GP73 levels were
detected in patients with hepatitis B virus-related HCC (12). Similarly, Marrero et al
demonstrated that serum GP73 levels were significantly increased in
patients with hepatitis C virus-related HCC compared with cirrhotic
controls (15). In addition, serum
GP73 levels have been shown to be more sensitive at the early
stages of HCC (14), and Liu et
al reported that GP73 may represent an effective serum marker
for monitoring the progression of liver diseases (21). In a previous Chinese study, a
significant correlation was identified between the overexpression
of GP73 at the protein and/or mRNA levels and the aggressive
behavior of HCC. However, no correlation was reported with the
overall patient survival rates (22). In addition, Mao et al
identified that GP73 may also be utilized for the surveillance of
HCC recurrence in post-operative patients (17). To investigate the diagnostic
abilities of serum GP73 and AFP for HCC, Zhou et al
performed a diagnostic meta-analysis with the following results:
Sensitivity, 76 (95% CI, 51–91%) vs. 70% (95% CI, 47–86%) and
specificity, 86 (95% CI, 65–95%) vs. 89% (95% CI, 69–96%),
respectively, This indicated that serum GP73 has a comparable
accuracy to AFP for the diagnosis of HCC (8). The elevated levels of GP73 expression
identified in HCC may be due to an increase in the cytokine
response and the level of the viral infection itself. Kladney et
al identified that GP73 expression increased in response to
interferon γ and that it was inhibited by tumor necrosis factor α
(11). Kawamoto et al
demonstrated that α1,6-fucosyltransferase enhanced the expression
of GP73 (23). However, the precise
mechanism of GP73 elevation in HCC remains unclear.
TRIFA was used in the current study to determine the
level of serum GP73 and was validated as an ideal measure for serum
GP73 in liver diseases, particularly in HCC, due to its high
sensitivity. The diagnostic agreement between ELISA and TRFIA was
evaluated using various cut-off values, according to ROC curves.
Although ELISA kit detection is extensively used, the sensitivity
of serum GP73 detection, with a cut-off value of 78.1 ng/l, by
TRIFA was notably higher when compared with that of ELISA in HCC.
The higher sensitivity of TRFIA was likely to be due to the use of
a highly detectable ‘tracer’ The sensitivity of labeled reagent
techniques may be substantially increased by improving the
signal-to-noise ratio. In comparison to conventional methods, TRFIA
technology is simple to perform, reproducible, accurate and
amenable to automation. Therefore, due to its improved analytical
ability, we hypothesized that TRIFA represents an alternative to
traditional technologies for the sensitive determination of serum
GP73 in HCC.
In the present study, the sensitivities of GP73, AFP
and GGT-II were 73.4, 55.6 and 68.4%, respectively. The differences
in the sensitivity of these markers was likely to be due to the
various synthetic and secretion pathways in the cells. Of the 3
markers, GP73 exhibited the highest sensitivity (73.4%) and GGT-II
the highest specificity (97.1%). The diagnostic accuracy of GP73 in
combination with the conventional serum markers, AFP and GGT-II,
was improved and has been demonstrated by ROC analysis. Previously,
no studies have analyzed the correlation between the serum
expression levels and GGT-II and GP73 in HCC patients, and no
correlation was identified in the present novel study. However, the
results indicated that the combined determination of the three
complementary markers represents a higher sensitivity level for the
diagnosis of HCC, but the discriminating ability of these markers
must be verified in an additional cohort of HCC patients by
investigating sensitivity and specificity independently. The
bootstrap estimate of bias in the AUROC was relatively small and
therefore, the resulting AUROC showed only a slight decrease. In
conclusion, the current data obtained, with regard to sensitivity
and specificity, may serve as a measurement of the diagnostic
ability.
Acknowledgements
The present study was supported by grants of the
National Natural Science Foundation of China (no. 81272708), the
Foundation for Talents in Six Fields of Jiangsu Province (no.
2012-WSN-065), the Health Project of Jiangsu Province (no. H201318)
and the Social Development Foundation of Nantong City (no.
S2010012, HS2011004 and BK2013069).
References
|
1
|
Thun MJ, DeLancey JO, Center MM, Jemal A
and Ward EM: The global burden of cancer: priorities for
prevention. Carcinogenesis. 31:100–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bosch FX, Ribes J, Cléries R and Díaz M:
Epidemiology of hepatocellular carcinoma. Clin Liver Dis.
9:191–211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fattovich G, Stroffolini T, Zagni I and
Donato F: Hepatocellular carcinoma in cirrhosis: incidence and risk
factors. Gastroenterology. 127(5 Suppl 1): S35–S50. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zoli M, Magalotti D, Bianchi G, Gueli C,
Marchesini G and Pisi E: Efficacy of a surveillance program for
early detection of hepatocellular carcinoma. Cancer. 78:977–985.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pateron D, Ganne N, Trinchet JC,
Aurousseau MH, Mal F, Meicler C, et al: Prospective study of
screening for hepatocellular carcinoma in Caucasian patients with
cirrhosis. J Hepatol. 20:65–71. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oka H, Tamori A, Kuroki T, Kobayashi K and
Yamamoto S: Prospective study of alpha-fetoprotein in cirrhotic
patients monitored for development of hepatocellular carcinoma.
Hepatology. 19:61–66. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Malaguarnera G, Giordano M, Paladina I,
Berretta M, Cappellani A and Malaguarnera M: Serum markers of
hepatocellular carcinoma. Dig Dis Sci. 55:2744–2755. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou Y, Yin X, Ying J and Zhang B: Golgi
protein 73 versus alpha-fetoprotein as a biomarker for
hepatocellular carcinoma: a diagnostic meta-analysis. BMC Cancer.
12:172012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cui R, He J, Zhang F, Wang B, Ding H, Shen
H, et al: Diagnostic value of protein induced by vitamin K absence
(PIVKAII) and hepatoma-specific band of serum gamma-glutamyl
transferase (GGTII) as hepatocellular carcinoma markers
complementary to alpha-fetoprotein. Br J Cancer. 88:1878–1882.
2003. View Article : Google Scholar
|
|
10
|
Kladney RD, Bulla GA, Guo L, Mason AL,
Tollefson AE, Simon DJ, et al: GP73, a novel Golgi-localized
protein upregulated by viral infection. Gene. 249:53–65. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kladney RD, Cui X, Bulla GA, Brunt EM and
Fimmel CJ: Expression of GP73, a resident Golgi membrane protein,
in viral and nonviral liver disease. Hepatology. 35:1431–1440.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Block TM, Comunale MA, Lowman M, Steel LF,
Romano PR, Fimmel C, et al: Use of targeted glycoproteomics to
identify serum glycoproteins that correlate with liver cancer in
woodchucks and humans. Proc Natl Acad Sci USA. 102:779–784. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Comunale MA, Mattu TS, Lowman MA, Evans
AA, London WT, Semmes OJ, et al: Comparative proteomic analysis of
de-N-glycosylated serum from hepatitis B carriers reveals
polypeptides that correlate with disease status. Proteomics.
4:826–838. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu JS, Wu DW, Liang S and Miao XY: GP73, a
resident Golgi glycoprotein, is sensibility and specificity for
hepatocellular carcinoma of diagnosis in a hepatitis B-endemic
Asian population. Med Oncol. 27:339–345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marrero JA, Romano PR, Nikolaeva O, Steel
L, Mehta A, Fimmel CJ, et al: GP73, a resident Golgi glycoprotein,
is a novel serum marker for hepatocellular carcinoma. J Hepatol.
43:1007–1012. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu J, Jiang F, Ni HB, Xiao MB, Chen BY,
Ni WK, et al: Combined analysis of serum γ-glutamyl transferase
isoenzyme II, α-L-fucosidase and α-fetoprotein detected using a
commercial kit in the diagnosis of hepatocellular carcinoma. Exp
Ther Med. 5:89–94. 2013.
|
|
17
|
Mao Y, Yang H, Xu H, Lu X, Sang X, Du S,
et al: Golgi protein 73 (GOLPH2) is a valuable serum marker for
hepatocellular carcinoma. Gut. 59:1687–1693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saffroy R, Pham P, Reffas M, Takka M,
Lemoine A and Debuire B: New perspectives and strategy research
biomarkers for hepatocellular carcinoma. Clin Chem Lab Med.
45:1169–1179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu KC, Meng XY, Shi YC, Ge ZJ, Ye L, Yu ZJ
and Yang DM: The diagnostic value of a hepatoma-specific band of
serum gamma-glutamyl transferase. Int J Cancer. 36:667–669. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu K, Meng XY, Wu JW, Shen B, Shi YC and
Wei Q: Diagnostic value of serum gamma-glutamyl transferase
isoenzyme for hepatocellular carcinoma: a 10-year study. Am J
Gastroenterol. 87:991–995. 1992.PubMed/NCBI
|
|
21
|
Liu X, Wan X, Li Z, Lin C, Zhan Y and Lu
X: Golgi protein 73(GP73), a useful serum marker in liver diseases.
Clin Chem Lab Med. 49:1311–1316. 2011.PubMed/NCBI
|
|
22
|
Sun Y, Yang H, Mao Y, Xu H, Zhang J, Li G,
et al: Increased Golgi protein 73 expression in hepatocellular
carcinoma tissue correlates with tumor aggression but not survival.
J Gastroenterol Hepatol. 26:1207–1212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawamoto S, Moriwaki K, Nakagawa T, Terao
M, Shinzaki S, Yamane-Ohnuki N, et al: Overexpression of
α1,6-fucosyltransferase in hepatoma enhances expression of Golgi
phosphoprotein 2 in a fucosylation-independent manner. Int J Oncol.
39:203–208. 2011.
|