Introduction
Inflammatory bowel disease (IBD) is characterized by
a chronic inflammation of the gastrointestinal tract. Ulcerative
colitis (UC) is the most common form of IBD and patients with UC
are predisposed to developing colorectal cancer. A longer duration
of disease and a greater extent of colitis, i.e. pan- or left-sided
colitis, are believed to increase the risk of developing
UC-associated cancer (UCAC) (1).
Studies have shown that the risk of developing colorectal cancer in
UC patients is 2, 8 and 18% following 10, 20 and 30 years of active
disease, respectively (2). Despite
epidemiological and experimental evidence demonstrating the
increased risk of developing UCAC, the mechanisms of neoplastic
transformation and progression remain unclear. The development of
UCAC is believed to arise from widespread alterations that are
caused by a combination of genetic and epigenetic factors, in
addition to host and microbial affects. UCAC is occasionally
referred to as an ‘inflammation dysplasia carcinoma sequence’,
which differs from sporadic colon cancer (3,4).
Furthermore, previous studies have suggested that the ‘field
effect’, in which genetic and molecular alterations that are caused
by chronic inflammation are identified in neoplastic lesions and
non-neoplastic epithelia, is common in epithelial carcinogenesis
(5,6).
Chemokines that are produced by colonic epithelial
cells play significant roles in the maintenance and repair of the
epithelial barrier and in cancer progression (7,8).
CCL20, also known as macrophage inflammatory protein (MIP) 3α or
liver and activation regulated chemokine, is predominantly
expressed in the inflamed intestinal epithelium and plays a
significant role in lymphocyte and dendritic cell activation and
recruitment to the colonic epithelium (9,10).
Previous studies have demonstrated that CCL20 expression levels in
the colonic epithelia of patients with IBD were higher than in the
normal colonic epithelia (11,12).
Furthermore, neutralization of CCL20 expression using its
monoclonal antibody has been shown to reduce 2,4,6-trinitrobenzen
sulfonic acid (TNBS)-mediated colonic injury and T-cell recruitment
(13). CCR6 is a functional
receptor for CCL20 that is expressed in lymphocytes, immature
dendritic cells, activated neutrophils and lymphoid tissues,
including the lymph node, spleen and appendix (9,14). In
addition, Varona et al(15)
conducted an in vivo study demonstrating that CCR6 plays a
crucial role in the development of IBD. These findings suggest that
the CCL20/CCR6 axis may contribute to chronic inflammation of the
colonic mucosa.
The present study investigated whether an evaluation
of CCL20 and CCR6 expression in the rectal mucosa would be useful
for predicting the development of UC-associated neoplasia.
Materials and methods
Patients and samples
A total of 93 formalin-fixed, paraffin-embedded
(FFPE) tissue samples were obtained from patients with UC who
underwent proctocolectomies between 2003 and 2011 in Mie University
Hospital (Tsu, Mie, Japan). The patients with right-sided or
segmental colitis and proctitis, acute fulminating, first
attack-type disease or those that were <15 or >60 years old
at the onset of the disease were excluded from the study. Approval
for this study was obtained from the ethics review board of Mie
University Hospital. All the patients provided written informed
consent to allow the collection and use of their tissues for the
present study.
Immunohistochemistry (IHC)
The FFPE specimens were sliced into 2-μm thick
sections. Following deparaffinization and dehydration, the sections
were incubated in 10 mM sodium citrate buffer (pH 6.0) and
autoclaved at 121°C for 10 min for antigen retrieval. Following an
additional incubation in 3% hydrogen peroxide for 10 min, the
sections were blocked and incubated with a primary antibody
overnight at 4°C. Human CCL20/MIP-3α antibodies (monoclonal mouse
IgG1 clone no. 67310; dilution, 1:250; R&D Systems,
Minneapolis, MN, USA) and human CCR6 antibodies (monoclonal mouse
IgG2B clone no. 53103; dilution, 1:50; R&D Systems)
were used as the primary antibodies for the implementation of the
labeled streptavidin-biotin method using the Envision™+ Dual Link
System-horseradish peroxidase (HRP) and 3,3′-diaminobenzidine
(DakoCytomation, Glostrup, Denmark) staining. All the sections were
counterstained with hematoxylin, then dehydrated and mounted. A
minimum of three sections/specimen were stained to confirm
reproducibility. Negative controls were run simultaneously using
pre-immune immunoglobulin.
Immunohistochemical evaluation
The sections were observed under a light microscope.
The IHC scores were calculated by multiplying the percentage of the
positive epithelial cells (0–100%) by the staining intensity, as
described in a previous study (16). The staining intensity was scored as
follows: 0, negative; 1, weak; 2, moderate; and 3, strong. The IHC
score ranged from 0–300. Each sample was scored in a blinded manner
by two investigators who did not have any clinical or pathological
information with regard to the origin of the samples.
Statistical analysis
All statistical analyses were performed using Stat
View 5.0 for Windows (SAS Institute Inc., Cary, NC, USA). The
contingency tables were analyzed using Fisher’s exact probability
test or the χ2 test with Yates’ correction. The
correlation between the continuous and categorical variables was
evaluated using the Mann-Whitney U test for two groups and the
Kruskal-Wallis test for more than three groups. A non-parametric
receiver operating characteristic (ROC) analysis was performed to
calculate the best cut-off value for each IHC score that was
predictive of the development of UC-associated neoplasia, using
MedCalc 7.2 for Windows (MedCalc, Mariakerke, Belgium). The
logistic regression analysis was used to evaluate whether CCL20 and
CCR6 expression in the rectal mucosa predicted the development of
UC-associated neoplasia. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient demographics and disease
characteristics
The characteristics of the patients are shown in
Table I. The median age at UC
diagnosis was 29 years (range, 17–59 years). A total of 74 patients
presented with pancolitis and the remainder presented with
left-sided colitis. With regard to the degree of inflammation, 46,
39 and 8 patients demonstrated mild, moderate and severe
inflammation, respectively. A total of 16 patients (17.2%) had
UC-associated neoplasia, seven of whom developed UCAC with
dysplastic lesions and nine who had only dysplastic lesions. The
median disease duration was six years in the patients with
non-neoplasia, eight years in the patients with dysplasia and 19
years in the patients with UCAC.
 | Table IPatient characteristics (n=93). |
Table I
Patient characteristics (n=93).
| A,
Characteristics | Value |
|---|
| Gender, n |
| Male | 49 |
| Female | 44 |
| Age at UC diagnosis,
years (range) | 29 (17–59) |
| Extent of disease,
n |
| Pancolitis | 74 |
| Left-sided
colitis | 19 |
| Duration of disease,
years (range) | 7 (1–28) |
| Degree of
inflammation, n |
| Mild | 46 |
| Moderate | 39 |
| Severe | 8 |
| Neoplasia
classification, n |
| Without
neoplasia | 77 |
| Dysplasia | |
| LGD | 8 |
| HGD | 1 |
| UC-associated
cancer | 7 |
|
| B, TNM stage |
|
| Stage | Histological
differentiation |
|
| T1N0M0 with HGD | Poor |
| T3N0M0 with HGD | Well |
| T3N0M0 with
LGD | Poor |
| T3N0M0 with
HGD | Moderate |
| T3N0M0 with
LGD | Well |
| T4N0M0 with
LGD | Moderate |
| T4N0M0 with
HGD | Well |
Immunohistochemical findings and
evaluation of CCL20 and CCR6 expression
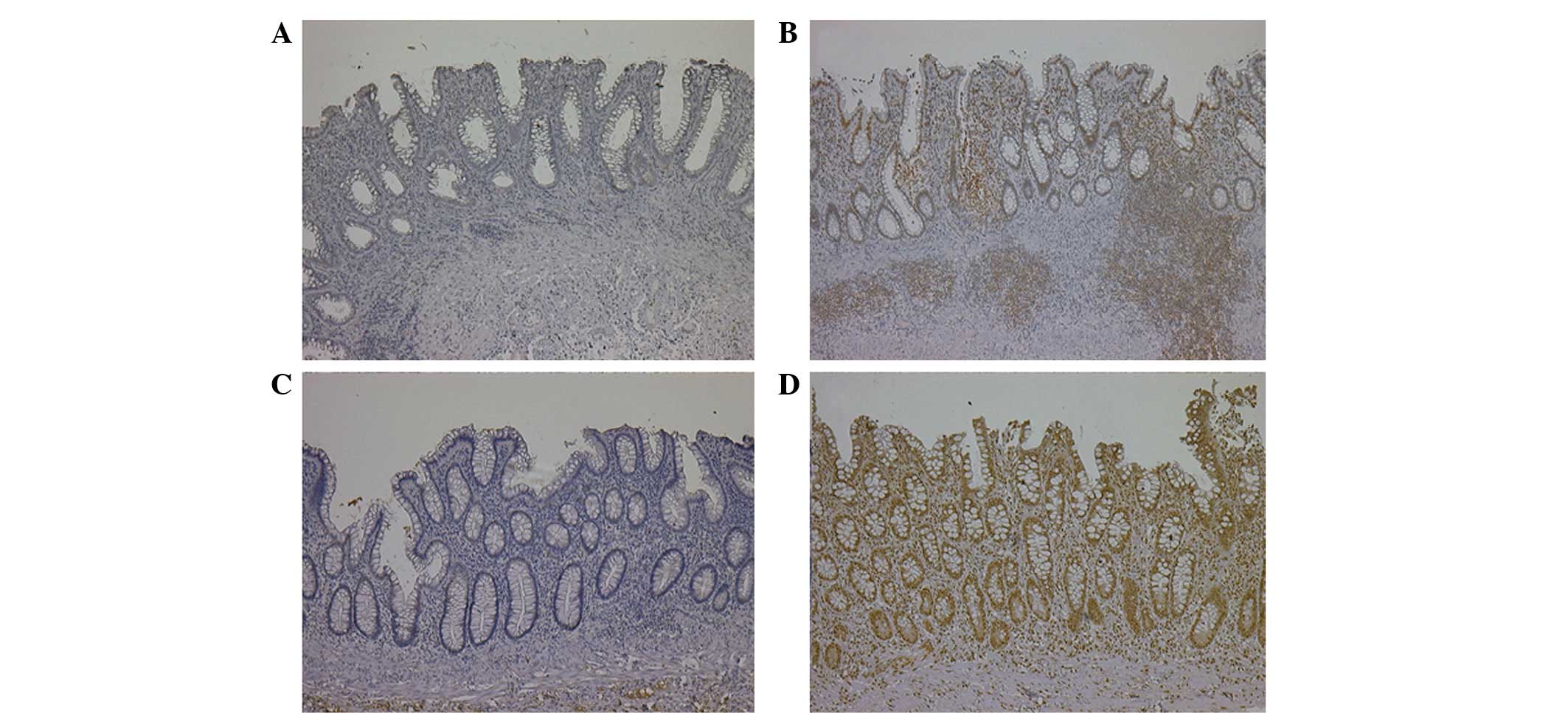
CCL20 expression was observed in the nuclei of the
epithelial cells, the inflammatory cells and the lymphoid follicles
(Fig. 1A and B). CCR6 expression
was observed in the nuclei or cytoplasm of the epithelial cells,
the infiltrating inflammatory cells and the endothelial cells
(Fig. 1C and D). The median IHC
scores of CCL20 and CCR6 were recorded as 20 (range, 0–285) and 40
(range, 0–270), respectively.
Correlation of CCL20 and CCR6 expression
in the rectal mucosa with clinical outcome
The patients with low- or high-grade dysplasia and
UCAC were classified as the UC-associated neoplasia group and the
remainder were classified as the non-neoplasia group. There were no
significant differences in the gender, extent of disease or degree
of inflammation between the non-neoplasia and UC-associated
neoplasia groups. The patients with UC-associated neoplasia had a
significantly longer disease duration than those without neoplasia
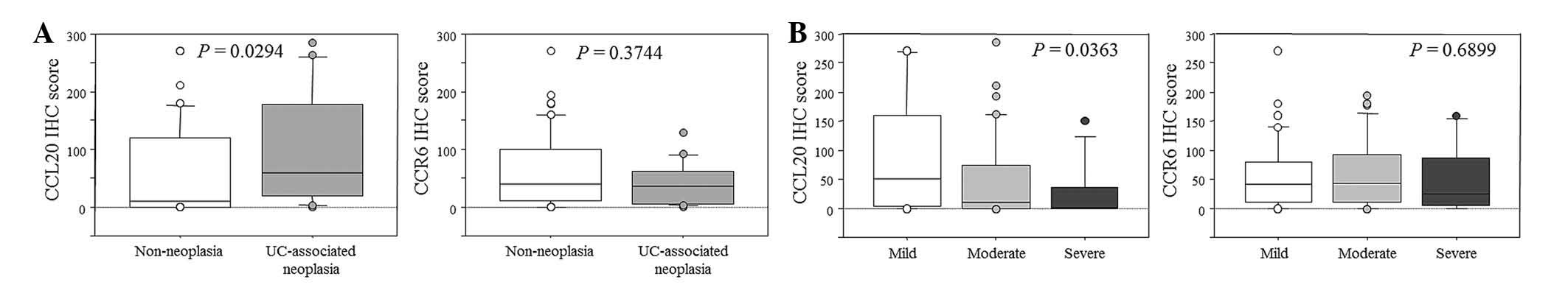
(11 vs. six years, respectively; P=0.0172; Table II). The IHC score for CCL20 in the
UC-associated neoplasia group was higher than in the non-neoplasia
group (P=0.0294). In contrast, there was no significant correlation
in CCR6 expression between the non-neoplasia and UC-associated
neoplasia groups (P=0.3744; Fig.
2A). The IHC score for CCL20, but not CCR6, was significantly
increased in the patients with a mild form of the disease
(P=0.0363; Fig. 2B). The ROC
analysis determined that the optimal cut-off values for the IHC
score of CCL20 and CCR6 were 20 and 92, respectively. In the
logistic regression analysis, the duration of the disease (>8
years) (2,17,18)
and the IHC scores of CCL20 above the cut-off value were
significantly associated with the development of UC-associated
neoplasia (P=0.0287; Table
III).
 | Table IICharacteristics of patients with and
without UC-associated neoplasia. |
Table II
Characteristics of patients with and
without UC-associated neoplasia.
| Non-neoplasia,
n=77 | UC-associated
neoplasia, n=16 | P-value |
|---|
| Gender, n
(male/female) | 39/38 | 10/6 | 0.4235 |
| Age at UC
diagnosis, years (range) | 29 (17–59) | 27 (17–55) | 0.6357 |
| Extent of disease,
n (%) |
| Pancolitis | 63 (82) | 11 (69) | 0.3056 |
| Left-sided
colitis | 14 (18) | 5 (31) | |
| Duration of
disease, years (range) | 6 (1–28) | 11 (1–28) | 0.0172 |
 | Table IIIMultivariate analysis of the utility
of disease duration and high CCL20 expression in the rectal mucosa
for predicting the risk of developing UC-associated neoplasia. |
Table III
Multivariate analysis of the utility
of disease duration and high CCL20 expression in the rectal mucosa
for predicting the risk of developing UC-associated neoplasia.
| Variables | Odds ratio | 95% confidence
interval | P-value |
|---|
| Duration of disease
(<8 years vs. ≥8 years) | 4.786 | 0.071–0.865 | 0.0287 |
| CCL20 IHC score
(low vs. high) | 4.786 | 0.071–0.865 | 0.0287 |
Comparison of CCL20 and CCR6 expression
in UCAC and sporadic cancer
Next, the expression of the two markers, CCL20 and
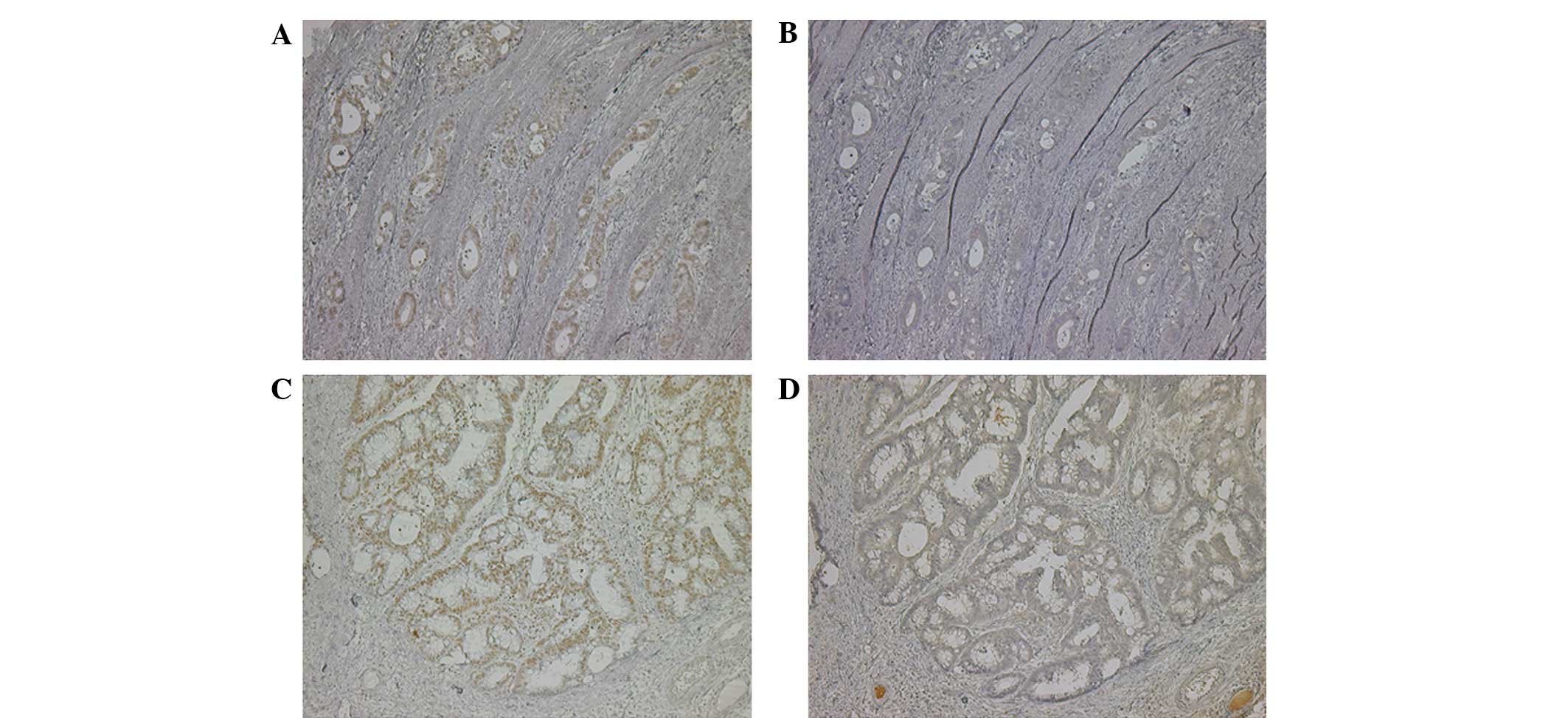
CCR6, were compared in UCAC and sporadic colon cancer. Fig. 3 shows the immunohistochemical
results for CCL20 and CCR6 in UC-associated and sporadic colon
cancer. CCL20 expression was observed in the nuclei of the
UC-associated and sporadic colon cancer cells. In contrast, CCR6
expression was observed in the cytoplasm of the cancer cells. The
expression of CCR6, but not CCL20, was increased in UCAC compared
with sporadic colon cancer (P=0.0316; Table IV).
 | Table IVComparison of CCL20 and CCR6
expression in UCAC (n=7) and sporadic cancer (n=15). |
Table IV
Comparison of CCL20 and CCR6
expression in UCAC (n=7) and sporadic cancer (n=15).
| Marker | Sporadic colon
cancer, n (%) | UCAC, n (%) | P-value |
|---|
| CCL20 |
| Positive | 12 (80) | 5 (71) | 0.6593 |
| Negative | 3 (20) | 2 (29) | |
| CCR6 |
| Positive | 2 (13) | 4 (57) | 0.0316 |
| Negative | 13 (87) | 3 (43) | |
Discussion
Current surveillance guidelines recommend that a
colonoscopy with random biopsies should be collected at 10-cm
increments along the colonic mucosa or that target biopsy testing
should be conducted every 1–2 years for chronic UC (17,19,20).
However, this type of surveillance has demonstrated several
limitations, including sampling errors and difficulties in the
macroscopic diagnosis of neoplastic lesions that are flat or
diffusely infiltrative (21,22).
Novel optical techniques, including chromoendoscopy, narrow band
imaging, confocal laser endoscopy and partial-wave spectroscopic
microscopy, have been reported to aid in the detection and
diagnosis of neoplastic lesions in patients with UC (23–26).
Further reliable and effective diagnostic approaches are urgently
required to improve the surveillance of UCAC.
In the present study, a high expression level of
CCL20 and a low expression level of CCR6 in the rectal mucosa were
correlated with the development of UC-associated neoplasia. These
results suggested that an evaluation of CCL20/CCR6 expression in
the rectal mucosa may be useful in the identification of patients
who are at high risk for developing UC-associated neoplasia.
Several studies have demonstrated that CCL20
expression is increased under inflammatory conditions and that CCR6
expression, while not affected by inflammation, is upregulated
during epithelial differentiation (10,27,28).
In the present study, CCR6 expression was not observed to be
correlated with the severity of UC, which was similar to the
results of previous studies, and CCL20 expression was shown to be
affected by the severity of UC. Notably, the CCL20 expression in
the epithelial cells of patients with mild or moderate disease
forms was elevated compared with patients with severe disease. This
suggested that the patients with a good control of the mucosal
inflammation or repeated active and remission by conventional
treatments for UC may have a high level of CCL20 expression.
Cook et al(14) reported that CCL20 and CCR6 were
separately expressed by adjacent cell populations within the
Peyer’s patch, suggesting that CCL20 may have paracrine functions.
In contrast, the co-expression of CCL20 and CCR6 in the same cells
suggested that a CCL20/CCR6 interaction mediated the autocrine or
paracrine effects on intestinal epithelial cells (27). However, there was no significant
correlation between CCL20 and CCR6 expression in the rectal mucosa
in the present study. This may be explained by the presence of
multiple receptors for CCL20 (data not shown). The percentage of
rectal epithelial cells co-expressing CCL20 and CCR6 was 20.4% of
all the patients, including 23.4% (18/77) of patients without
neoplasia and 6.3% (1/16) of patients with UC-associated neoplasia.
Thus, the present data suggested that CCL20/CCR6 signaling may have
various physiological functions and that these proteins may exhibit
differential interactions in the context of UC compared with
non-UC.
In sporadic colorectal cancer, CCL20/CCR6 signaling
has been shown to play a role in the proliferation and migration of
colorectal cancer cells and in the promotion of liver metastasis
via the autocrine and paracrine functions (27,29).
Several studies have suggested that the prognoses of UCAC and
sporadic colon cancer are similar (30–32).
However, this remains a controversial topic. Furthermore, Mikami
et al(33) reported that
poorly-differentiated UCAC with invasion of the submucosa or with a
greater dependence on CD44 cleavage may influence a poor prognosis.
In the present study, CCR6 expression was observed to be more
frequent in UCAC compared with sporadic colon cancer. The results
suggested that CCR6 expression may be associated with the malignant
potential of UCAC. However, it should be noted that the data did
not demonstrate whether CCL20/CCR6 signaling played a role in the
carcinogenesis of UC. In future studies, this possibility may be
investigated using transcriptional and in vivo studies.
In conclusion, the results of the present study
suggested that an evaluation of CCL20/CCR6 expression in the rectal
mucosa may be useful for the identification of high-risk patients
with UC-associated neoplasia. However, the data in this study
should be interpreted with caution. Firstly, a selection bias was
present due to the fact that the patient population that was
enrolled in the study was not representative of a typical
surveillance patient population. Furthermore, this study included a
small number of UC patients. Hence, further research, including a
prospective and multicenter study using a collection of biopsy
samples from patients with UC, is required to assess the utility of
these markers for routine clinical use.
Acknowledgements
The authors would like to thank Motoko Ueeda and
Chihiro Hibi for providing their technical assistance during the
realization of this study.
References
|
1
|
Danese S and Fiocchi C: Ulcerative
colitis. N Engl J Med. 365:1713–1725. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eaden JA, Abrams KR and Mayberry JF: The
risk of colorectal cancer in ulcerative colitis: a meta-analysis.
Gut. 48:526–535. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cho JH and Brant SR: Recent insights into
the genetics of inflammatory bowel disease. Gastroenterology.
140:1704–1712. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thompson AI and Lees CW: Genetics of
ulcerative colitis. Inflamm Bowel Dis. 17:831–848. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Slaughter DP, Southwick HW and Smejkal W:
Field cancerization in oral stratified squamous epithelium;
clinical implications of multicentric origin. Cancer. 6:963–968.
1953. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chai H and Brown RE: Field effect in
cancer-an update. Ann Clin Lab Sci. 39:331–337. 2009.PubMed/NCBI
|
|
7
|
Zimmerman NP, Vongsa RA, Wendt MK and
Dwinell MB: Chemokines and chemokine receptors in mucosal
homeostasis at the intestinal epithelial barrier in inflammatory
bowel disease. Inflamm Bowel Dis. 14:1000–1011. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koelink PJ, Overbeek SA, Braber S, et al:
Targeting chemokine receptors in chronic inflammatory diseases: an
extensive review. Pharmacol Ther. 133:1–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Williams IR: CCR6 and CCL20: partners in
intestinal immunity and lymphorganogenesis. Ann NY Acad Sci.
1072:52–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Izadpanah A, Dwinell MB, Eckmann L, et al:
Regulated MIP-3alpha/CCL20 production by human intestinal
epithelium: mechanism for modulating mucosal immunity. Am J Physiol
Gastrointest Liver Physiol. 280:G710–G719. 2001.PubMed/NCBI
|
|
11
|
Kwon JH, Keates S, Bassani L, et al:
Colonic epithelial cells are a major site of macrophage
inflammatory protein 3alpha (MIP-3alpha) production in normal colon
and inflammatory bowel disease. Gut. 51:818–826. 2002. View Article : Google Scholar
|
|
12
|
Kaser A, Ludwiczek O, Holzmann S, et al:
Increased expression of CCL20 in human inflammatory bowel disease.
J Clin Immunol. 24:74–85. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Katchar K, Kelly CP, Keates S, et al:
MIP-3alpha neutralizing monoclonal antibody protects against
TNBS-induced colonic injury and inflammation in mice. Am J Physiol
Gastrointest Liver Physiol. 292:G1263–G1271. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cook DN, Prosser DM, Forster R, et al:
CCR6 mediates dendritic cell localization, lymphocyte homeostasis,
and immune responses in mucosal tissue. Immunity. 12:495–503. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Varona R, Cadenas V, Flores J, et al: CCR6
has a non-redundant role in the development of inflammatory bowel
disease. Eur J Immunol. 33:2937–2946. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wong SC, Lo SF, Lee KC, et al: Expression
of frizzled-related protein and Wnt-signalling molecules in
invasive human breast tumours. J Pathol. 196:145–153. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Itzkowitz SH and Present DH; Crohn’s and
Colitis Foundation of America Colon Cancer in IBD Study Group.
Consensus conference: Colorectal cancer screening and surveillance
in inflammatory bowel disease. Inflamm Bowel Dis. 11:314–321. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eaden JA and Mayberry JF; British Society
for Gastroenterology; Association of Coloproctology for Great
Britain and Ireland. Guidelines for screening and surveillance of
asymptomatic colorectal cancer in patients with inflammatory bowel
disease. Gut. 51(Suppl 5): V10–V12. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ullman T, Odze R and Farraye FA: Diagnosis
and management of dysplasia in patients with ulcerative colitis and
Crohn’s disease of the colon. Inflamm Bowel Dis. 15:630–638.
2009.
|
|
20
|
Velayos FS, Liu L, Lewis JD, et al:
Prevalence of colorectal cancer surveillance for ulcerative colitis
in an integrated health care delivery system. Gastroenterology.
139:1511–1518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen R, Rabinovitch PS, Crispin DA, et al:
DNA fingerprinting abnormalities can distinguish ulcerative colitis
patients with dysplasia and cancer from those who are
dysplasia/cancer-free. Am J Pathol. 162:665–672. 2003. View Article : Google Scholar
|
|
22
|
Neumann H, Vieth M, Langner C, et al:
Cancer risk in IBD: how to diagnose and how to manage DALM and ALM.
World J Gastroenterol. 17:3184–3191. 2011.PubMed/NCBI
|
|
23
|
Matsumoto T, Kudo T, Jo Y, et al:
Magnifying colonoscopy with narrow band imaging system for the
diagnosis of dysplasia in ulcerative colitis: a pilot study.
Gastrointest Endosc. 66:957–965. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Neumann H, Kiesslich R, Wallace MB and
Neurath MF: Confocal laser endomicroscopy: technical advances and
clinical applications. Gastroenterology. 139:388–392. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kiesslich R: Chromoendoscopy: what is its
true value for ulcerative colitis surveillance? Dig Dis.
28:445–451. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bista RK, Brentnall TA, Bronner MP, et al:
Using optical markers of nondysplastic rectal epithelial cells to
identify patients with ulcerative colitis-associated neoplasia.
Inflamm Bowel Dis. 17:2427–2435. 2011. View Article : Google Scholar
|
|
27
|
Ghadjar P, Rubie C, Aebersold DM and
Keilholz U: The chemokine CCL20 and its receptor CCR6 in human
malignancy with focus on colorectal cancer. Int J Cancer.
125:741–745. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brand S, Olszak T, Beigel F, et al: Cell
differentiation dependent expressed CCR6 mediates ERK-1/2,
SAPK/JNK, and Akt signaling resulting in proliferation and
migration of colorectal cancer cells. J Cell Biochem. 97:709–723.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rubie C, Oliveira V, Kempf K, et al:
Involvement of chemokine receptor CCR6 in colorectal cancer
metastasis. Tumour Biol. 27:166–174. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Connell WR, Talbot IC, Harpaz N, et al:
Clinicopathological characteristics of colorectal carcinoma
complicating ulcerative colitis. Gut. 35:1419–1423. 1994.
View Article : Google Scholar
|
|
31
|
Hinton JM: Risk of malignant change in
ulcerative colitis. Gut. 7:427–432. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lavery IC, Chiulli RA, Jagelman DG, et al:
Survival with carcinoma arising in mucosal ulcerative colitis. Ann
Surg. 195:508–512. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mikami T, Yoshida T, Numata Y, et al:
Invasive behavior of ulcerative colitis-associated carcinoma is
related to reduced expression of CD44 extracellular domain:
comparison with sporadic colon carcinoma. Diagn Pathol. 6:302011.
View Article : Google Scholar
|