Introduction
Despite a worldwide decline in incidence and
mortality in the last 60 years, gastric cancer remains the fourth
most common type of cancer and the second most frequent cause of
cancer mortality. Gastric cancer continues to be a major health
concern due to the slow decrease in incidence in Asia and the high
mortality from diagnosed gastric carcinomas in the West, even
though advanced diagnostic and operative techniques are widely
applied in clinical practice (1,2).
Increased understanding of the proliferative and apoptotic changes
in gastric cancer, particularly the identification of novel
biomarkers for cancer diagnosis and targets for treatment, may
result in the improvement of diagnosis, treatment and
prevention.
Ki-67 antigen (also known as MKI67) is in the nuclei
of cells in the G1, S, G2 and mitosis phases
of the cell cycle and is associated with ribosomal RNA
transcription. During interphase, Ki-67 antigen is exclusively
detected within the cell nucleus, whereas in mitosis, the majority
of the protein relocates to the surface of the chromosomes.
Quiescent or resting cells in the G0-phase do not
express the Ki-67 antigen (3),
making the Ki-67 antigen an excellent operational marker for
determining the proliferation of a given cell population and the
aggressiveness of malignancies (4).
In experimental and clinical practice, Ki-67 and MIB-1 monoclonal
antibodies are directed against different epitopes of the same
proliferation-related antigen; whereas Ki-67 works only on frozen
sections, MIB-1 may also be used on fixed sections (5).
The caspase-3 (CASP3) protein is a member of the
cysteine-aspartic acid protease (caspase)/interleukin-1β-converting
enzyme (ICE) family. CASP3 is activated directly by caspase-8, -9
and -10 in the apoptotic cell by extrinsic (death ligand) and
intrinsic (mitochondrial) pathways to initiate apoptosis. CASP3 is
synthesized as an inactive 32 kDa proenzyme and processed during
apoptosis into its active form, which is composed of two subunits,
p17–20 and p10–12. Activated CASP3 is responsible for the cleavage
of poly(ADP-ribose) polymerase (PARP), actin and sterol regulatory
element binding protein (SREBP), which are associated with
apoptosis (6–8).
The p53 tumor suppressor gene is considered to be
central in protecting against the development of cancer. The
encoded protein is a master switch that coordinates and
concentrates a plethora of stress signals, transforming them into a
series of responses, including apoptosis or cell cycle arrest, in
response to DNA damage, thereby maintaining genetic stability in
the organism (9). Therefore, p53
has been described as ‘the guardian of the genome’ (9,10). The
p53 pathway is also involved in regulating metastasis-associated
genes, including maspin, kai1, integrin, nm23, matrix
metalloproteinase (MMP)-2, MMP-13 and the tissue inhibitor of
metalloproteinase-3 (TIMP-3) (10–16).
Although p53 inactivation in human cancer is a complex process that
depends on the tissue type, p53 dysfunction may disorder the
biological events of cancer cells, giving rise to their aggressive
phenotypes.
Previously, we observed that p53 and Ki-67 were
gradually increased from gastrointestinal mucosa to adenocarcinoma
through adenoma. Accumulated p53 expression showed a positive
association with the depth of invasion, local invasion via vessels
and lymph node metastasis of gastrointestinal adenocarcinoma (GIA).
Ki-67 expression was positively correlated with local invasion via
vessels and negatively correlated with the dedifferentiation and
liver metastasis of GIA (17). The
present study investigated the clinicopathological and prognostic
significance of Ki-67, CASP3 and p53 to clarify their roles in the
regulation of the balance between proliferation and apoptosis.
Materials and methods
Patients
This retrospective study was performed on
curatively-resected gastric carcinoma specimens collected in Toyama
University Hospital (Toyama, Japan) between 1993 and 2006. The
patients with gastric carcinomas consisted of 130 males and 301
females (age range, 38–88 years; mean age, 66.4 years). Archival
materials were obtained from the Department of Pathology. In 165
cases, tumor development was accompanied by lymph node metastasis.
None of the patients underwent chemotherapy, radiotherapy and
adjuvant treatment prior to surgery. All patients were followed up
by consulting their case documents and by telephone.
Pathology
All tissues were fixed in 10% neutralized formalin,
embedded in paraffin and cut into 4-μm sections stained with
hematoxylin and eosin (HE) to confirm the histological diagnosis
and microscopic characteristics. The staging for each gastric
carcinoma was evaluated according to the Union Internationale le
Contre Cancer (UICC) system, indicating the extent of tumor spread
(17). The histological
architecture was defined in terms of Lauren’s classification
(18,19). Furthermore, the tumor size, depth of
invasion, lymphatic and venous invasion and lymph node metastasis
of the tumors were determined.
Tissue microarray (TMA)
Representative areas of solid tumor were selected
for sampling from HE-stained sections of the selected tumor cases,
and 2-mm diameter tissue cores per donor block were punched out and
transferred to a recipient block, with a maximum of 48 cores, using
a Tissue Microarrayer (KIN-1; Azumaya, Tokyo, Japan). Sections
(4-μm thick) were consecutively cut from the microarrays and
transferred to poly-lysine-coated glass slides. HE staining was
performed for the confirmation of the tumor tissue.
Immunohistochemistry
Serial sections of TMA were deparaffinized with
xylene, rehydrated with alcohol and subjected to
immunohistochemical staining with intermittent microwave radiation,
as previously described (20).
Rabbit anti-human CASP3, rabbit anti-human Ki-67 (NovoCastra, Leica
Biosystems Newcastle Ltd., Newcastle Upon Tyne, UK) and mouse
anti-human p53 (Dako, Carpinteria, CA, USA) antibodies were used at
1:100 dilution to detect the respective proteins, with anti-rabbit
or anti-mouse Envison-PO (Dako) as the secondary antibody. Binding
was visualized with 3,3′-diaminobenzidine (DAB) and counterstaining
with Mayer’s hematoxylin was performed to aid orientation. Omission
of the primary antibody was used as a negative control.
Immunoreactivity for Ki-67, p53 and
CASP3
In total, 100 cells from five representative fields
of each section were randomly selected and counted in a blinded
manner by two independent observers (L. Xiao and H.C. Zheng).
Inconsistent data were discussed by the observers until final
agreements were reached. The expression positivity was graded and
counted as follows: 0, negative; 1, 1–50%; 2, 50–74%; and 3, ≥75%.
The staining intensity score was graded as follows: 1, weak; 2,
intermediate; and 3, strong. The scores for Ki-67, CASP3 and p53
positivity and staining intensity were multiplied to obtain a final
score, which determined their expression as follows: −, 0; +, 1–2;
++, 3–4; and +++, 6–9.
Statistical analysis
The statistical evaluation was performed using
Spearman’s correlation test to analyze rank data. Kaplan-Meier
survival plots were generated and comparisons between survival
curves were made with the log-rank statistic. Cox’s proportional
hazards model was used for the multivariate analysis. SPSS 17.0
software (SPSS Inc., Chicago, IL, USA) was applied to analyze all
data and P<0.05 was considered to indicate a statistically
significant difference.
Results
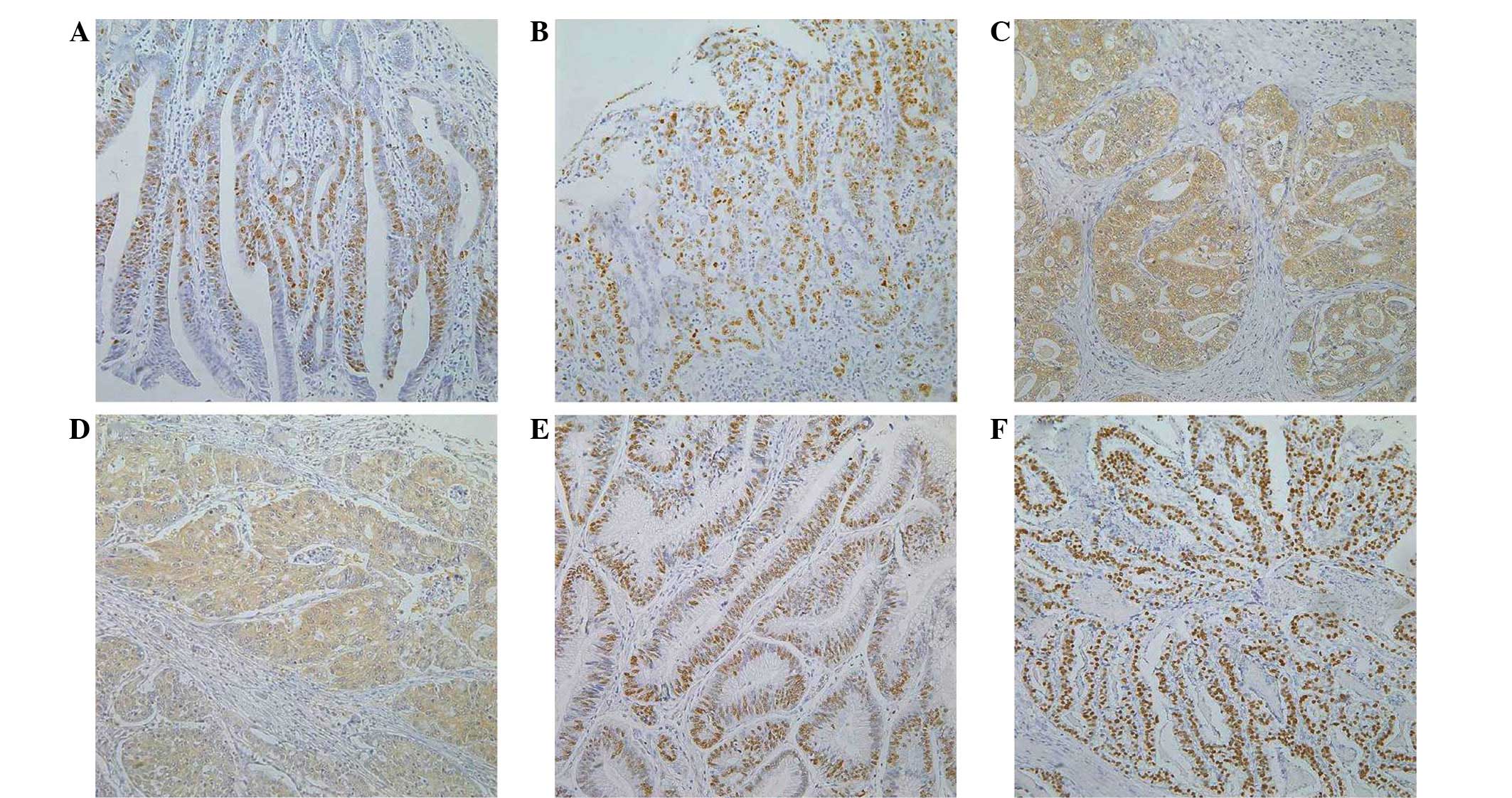
Ki-67 and p53 positivity was clearly localized in
the nuclei of the gastric cancer cells, while CASP3 was detected in
the cytoplasm of the cancer cells (Fig.
1). Ki-67 expression was positively correlated with
tumor-node-metastasis (TNM) staging and p53 expression of gastric
cancer (P<0.05), but not with the patients’ age or gender, tumor
size, depth of invasion, lymphatic or venous invasion, lymph node
metastasis or Lauren’s classification (P>0.05; Table I). CASP3 expression was positively
correlated with Ki-67 expression in gastric carcinoma (P<0.05),
but not with the patients’ age or gender, tumor size, depth of
invasion, lymphatic or venous invasion, lymph node metastasis or
TNM staging (P>0.05). There was higher CASP3 expression in the
intestinal-type compared with the diffuse-type of carcinoma
(P<0.05). The elder or male patients with gastric cancer showed
higher p53 expression compared with the younger or female patients,
respectively (P<0.05; Table
II). p53 expression was positively correlated with TNM staging
and the CASP3 expression of gastric cancer (P<0.05), but not
with the tumor size, depth of invasion, lymphatic or venous
invasion, or lymph node metastasis (P>0.05). The intestinal-type
carcinoma samples showed higher p53 expression compared with the
diffuse-type samples (P<0.05; Table III).
 | Table ICorrelation between Ki-67 expression
and clinicopathological features of gastric carcinomas. |
Table I
Correlation between Ki-67 expression
and clinicopathological features of gastric carcinomas.
| Clinicopathological
features | n | Ki-67 expression,
n | PR, % | P-value |
|---|
|
|---|
| − | + | ++ | +++ |
|---|
| Age, years | | | | | | | 0.062 |
| <65 | 186 | 60 | 28 | 36 | 62 | 67.7 | |
| ≥65 | 245 | 55 | 29 | 57 | 104 | 77.6 | |
| Gender | | | | | | | 0.539 |
| Female | 130 | 38 | 14 | 26 | 52 | 70.8 | |
| Male | 301 | 77 | 43 | 67 | 114 | 74.4 | |
| Tumor size, cm | | | | | | | 0.146 |
| <4 | 222 | 66 | 25 | 48 | 83 | 70.3 | |
| ≥4 | 209 | 49 | 32 | 45 | 83 | 76.6 | |
| Depth of
invasion | | | | | | | 0.380 |
|
Tis-1 | 220 | 57 | 28 | 48 | 87 | 74.1 | |
| T2–4 | 211 | 58 | 29 | 45 | 79 | 72.5 | |
| Lymphatic
invasion | | | | | | | 0.534 |
| − | 271 | 74 | 33 | 60 | 104 | 72.7 | |
| + | 159 | 41 | 24 | 33 | 61 | 74.2 | |
| Venous
invasion | | | | | | | 0.864 |
| − | 373 | 99 | 52 | 86 | 136 | 73.5 | |
| + | 58 | 16 | 5 | 7 | 30 | 72.4 | |
| Lymph node
metastasis | | | | | | | 0.566 |
| − | 262 | 72 | 32 | 62 | 96 | 72.5 | |
| + | 165 | 43 | 25 | 31 | 66 | 73.9 | |
| TNM staging | | | | | | | 0.047 |
| 0–I | 247 | 59 | 32 | 58 | 98 | 76.1 | |
| II–IV | 184 | 56 | 25 | 35 | 68 | 69.6 | |
| Lauren’s
classification | | | | | | | 0.372 |
|
Intestinal-type | 214 | 50 | 32 | 46 | 86 | 76.6 | |
| Diffuse-type | 203 | 62 | 23 | 46 | 72 | 69.5 | |
| p53 expression | | | | | | | <0.001 |
| − | 179 | 80 | 31 | 36 | 32 | 55.3 | |
| + | 37 | 6 | 9 | 8 | 14 | 83.8 | |
| ++ | 66 | 7 | 10 | 15 | 34 | 89.4 | |
| +++ | 123 | 11 | 4 | 31 | 77 | 91.1 | |
 | Table IICorrelation between nuclear caspase-3
expression and clinicopathological features of gastric
carcinomas. |
Table II
Correlation between nuclear caspase-3
expression and clinicopathological features of gastric
carcinomas.
| Clinicopathological
features | n | CASP3 expression,
n | PR, % | P-value |
|---|
|
|---|
| − | + | ++ | +++ |
|---|
| Age, years | | | | | | | 0.100 |
| <65 | 173 | 75 | 38 | 32 | 28 | 56.6 | |
| ≥65 | 232 | 81 | 56 | 42 | 53 | 65.1 | |
| Gender | | | | | | | 0.412 |
| Female | 125 | 54 | 24 | 22 | 25 | 56.8 | |
| Male | 280 | 102 | 70 | 52 | 56 | 63.6 | |
| Tumor size, cm | | | | | | | 0.255 |
| <4 | 207 | 88 | 51 | 31 | 37 | 57.5 | |
| ≥4 | 198 | 68 | 43 | 43 | 44 | 65.7 | |
| Depth of
invasion | | | | | | | 0.141 |
|
Tis-1 | 205 | 88 | 42 | 31 | 44 | 57.1 | |
|
T2–4 | 200 | 68 | 52 | 43 | 37 | 66.0 | |
| Lymphatic
invasion | | | | | | | 0.447 |
| − | 254 | 101 | 51 | 52 | 50 | 60.2 | |
| + | 150 | 55 | 42 | 22 | 31 | 63.3 | |
| Venous
invasion | | | | | | | 0.537 |
| − | 347 | 132 | 81 | 67 | 67 | 62.0 | |
| + | 58 | 24 | 13 | 7 | 14 | 58.6 | |
| Lymph node
metastasis | | | | | | | 0.564 |
| − | 246 | 100 | 55 | 40 | 51 | 59.3 | |
| + | 157 | 56 | 39 | 34 | 28 | 64.3 | |
| TNM staging | | | | | | | 0.919 |
| 0–I | 232 | 91 | 48 | 37 | 56 | 60.8 | |
| II–IV | 173 | 65 | 46 | 37 | 25 | 62.4 | |
| Lauren’s
classification | | | | | | | <0.001 |
|
Intestinal-type | 201 | 56 | 51 | 36 | 58 | 72.1 | |
| Diffuse-type | 199 | 98 | 43 | 35 | 23 | 50.8 | |
| Ki-67
expression | | | | | | | <0.001 |
| − | 103 | 58 | 28 | 14 | 3 | 43.7 | |
| + | 52 | 28 | 8 | 9 | 7 | 46.2 | |
| ++ | 87 | 29 | 24 | 18 | 16 | 66.7 | |
| +++ | 147 | 34 | 29 | 30 | 54 | 76.9 | |
 | Table IIIRelationship between p53 expression
and clinicopathological features of gastric carcinomas. |
Table III
Relationship between p53 expression
and clinicopathological features of gastric carcinomas.
| Clinicopathological
features | n | p53 expression,
n | PR, % | P-value |
|---|
|
|---|
| − | + | ++ | +++ |
|---|
| Age, years | | | | | | | 0.032 |
| <65 | 175 | 90 | 16 | 29 | 40 | 48.6 | |
| ≥65 | 247 | 99 | 22 | 38 | 88 | 59.9 | |
| Gender | | | | | | | 0.027 |
| Female | 127 | 65 | 12 | 20 | 30 | 48.8 | |
| Male | 295 | 124 | 26 | 47 | 98 | 58.0 | |
| Tumor size, cm | | | | | | | 0.697 |
| <4 | 214 | 95 | 20 | 35 | 64 | 55.6 | |
| ≥4 | 208 | 94 | 18 | 32 | 64 | 54.8 | |
| Depth of
invasion | | | | | | | 0.169 |
|
Tis-1 | 215 | 89 | 20 | 39 | 67 | 58.6 | |
|
T2–4 | 207 | 100 | 18 | 28 | 61 | 51.7 | |
| Lymphatic
invasion | | | | | | | 0.861 |
| − | 264 | 119 | 22 | 46 | 77 | 54.9 | |
| + | 157 | 70 | 16 | 20 | 51 | 55.4 | |
| Venous
invasion | | | | | | | 0.721 |
| − | 362 | 160 | 34 | 61 | 107 | 55.8 | |
| + | 60 | 29 | 4 | 6 | 21 | 51.7 | |
| Lymph node
metastasis | | | | | | | 0.532 |
| − | 253 | 113 | 21 | 45 | 74 | 55.3 | |
| + | 164 | 76 | 16 | 21 | 51 | 53.7 | |
| TNM staging | | | | | | | 0.047 |
| 0–I | 241 | 98 | 22 | 43 | 78 | 59.3 | |
| II–IV | 181 | 91 | 16 | 24 | 50 | 49.7 | |
| Lauren’s
classification | | | | | | | <0.001 |
|
Intestinal-type | 212 | 66 | 22 | 42 | 82 | 68.9 | |
| Diffuse-type | 200 | 119 | 13 | 24 | 44 | 40.5 | |
| CASP3
expression | | | | | | | 0.004 |
| − | 142 | 95 | 11 | 12 | 24 | 33.1 | |
| + | 90 | 42 | 4 | 18 | 26 | 53.3 | |
| ++ | 72 | 25 | 8 | 13 | 26 | 65.3 | |
| +++ | 81 | 12 | 9 | 18 | 42 | 85.2 | |
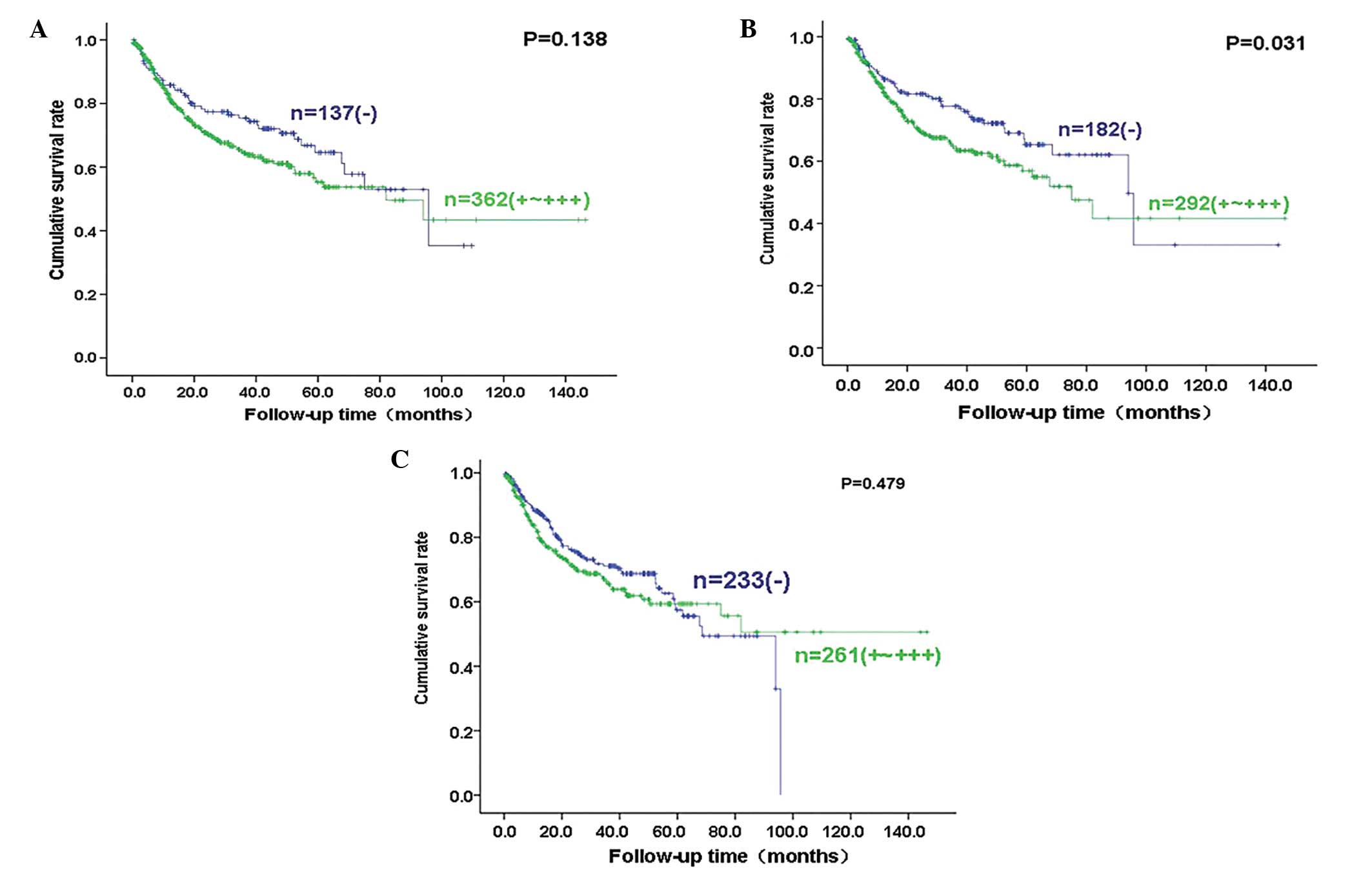
Follow-up information was available for 499 of the
gastric carcinoma patients for periods ranging between 0.2 months
and 12.2 years (mean, 70.1 months). Fig. 2 shows survival curves stratified
according to Ki-67, CASP3 and p53 expression. Univariate analyses
using the Kaplan-Meier method indicated that there was a higher
cumulative survival rate among carcinoma cases with negative CASP3
expression compared with weak, moderate and strong CASP3 expression
(P<0.05), whereas there was no correlation between Ki-67 or p53
expression and the survival rate of the patients with gastric
cancer (P>0.05). Cox’s proportional hazard model indicated that
the patient age, gender, depth of invasion, lymphatic invasion,
lymph node metastasis, TNM staging, Lauren’s classification and
CASP3 expression, but not the tumor size, venous invasion and Ki-67
or p53 expression, were independent prognostic factors for gastric
carcinomas (P<0.05; Table
IV).
 | Table IVMultivariate analysis of
clinicopathological variables for the survival of the patients with
gatric carcinomas. |
Table IV
Multivariate analysis of
clinicopathological variables for the survival of the patients with
gatric carcinomas.
| Clinicopathological
parameters | Relative risk (95%
CI) | P-value |
|---|
| Age (≥65
years) | 1.962
(1.342–2.870) | 0.001 |
| Gender
(female) | 1.679
(1.079–2.612) | 0.022 |
| Tumor size (>4
cm) | 1.511
(0.890–2.566) | 0.126 |
| Depth of invasion
(T2–4) | 5.255
(2.376–11.622) | <0.001 |
| Lymphatic invasion
(+) | 2.193
(1.421–3.383) | <0.001 |
| Venous invasion
(+) | 1.158
(0.756–1.774) | 0.500 |
| Lymph node
metastasis (+) | 3.629
(1.848–7.126) | <0.001 |
| TNM staging
(III–IV) | 0.309
(0.138–0.694) | 0.004 |
| Lauren’s
classification | 2.251
(1.457–3.477) | <0.001 |
| Ki-67 expression (+
to +++) | 0.982
(0.822–1.172) | 0.837 |
| CASP3 expression (+
to +++) | 1.277
(1.064–1.533) | 0.009 |
| p53 expression (+
to +++) | 1.112
(0.947–1.306) | 0.194 |
Discussion
Cell proliferative activity is an important factor
for assessing the biological behavior of carcinoma, and the
identification of proliferating activities in tumors may be useful
for predicting clinicopathological and prognostic significance.
Ki-67 is a nuclear non-histone protein, which is required for
maintaining the cell cycle (22).
Our previous study showed gradually increasing expression of Ki-67
from the gastrointestinal mucosa to adenocarcinoma through adenoma
(17). In the present study, it was
observed that Ki-67 was expressed in 30.4% of gastric cancers and
that Ki-67 expression was positively correlated with TNM staging
and p53 expression, but not with aggressive parameters such as
local invasion, lymph node metastasis and differentiation, which is
consistent with previous studies (23–27).
Xu et al(28) noted that the
expression of Ki-67 antigen was significantly associated with
distant metastases to the liver, ovary and adrenal gland, but not
to the histological type, growth pattern, depth of invasion,
histological differentiation or the metastases to local lymph
nodes. Several studies have reported that Ki-67 expression was a
more valuable independent prognostic predictor for the survival of
patients with gastric cancer (24,27,29),
which is contrary to the present data.
Normal cells contain only a small amount of caspases
in the form of inactive zymogens, and activated caspases have been
transformed to proteases via the catalytic activity of enzymes
capable of cleaving a number of substrate proteins, resulting in
apoptosis. CASP3 is activated by a series of cascade reactions,
until eventually DNase (CAD, CPAN or DEF40) is activated, which
belongs to the Mg2+-dependent endonucleases, and acts as
a killer in apoptosis (6,31). Reportedly, CASP3 expression is
higher in normal tissues compared with gastric carcinoma tissue
(6,32). Hoshi et al(32) also observed that the positive rate
of CASP3 expression was lower in gastric cancers compared with
their adjacent mucosa and gastric adenoma. In the present study,
there was higher CASP3 expression in intestinal-type compared with
diffuse-type carcinoma, indicating that its aberrant expression
underlies the molecular mechanisms of the differentiation of
gastric cancer. Additionally, CASP3 was also demonstrated to
correlate with the poor prognosis of patients with gastric cancer
as an independent factor, in agreement with the study by Isobe
et al(33). The lack of
correlation between CASP3 and aggressive behaviors of gastric
cancer was consistent with our previous findings (6). Notably, the positive association
between CASP3 and Ki-67 expression suggested the hypothesis that
highly proliferating carcinomas may have a high apoptotic
potential, contrary to results observed in a previous study
(32).
The p53 gene is a tumor suppressor gene located on
chromosome 17p13.1 and the single most common target for genetic
alterations in human cancer, which is activated in response to
genotoxic and non-genotoxic insults to cells (34). Mutated p53 lacks the DNA repair
regulation of the cell cycle and results in metabolically stable
abnormal protein that accumulates in the nucleus, which may be
detected by immunohistochemistry (35). Our previous studies showed that
aberrant p53 overexpression was more common in gastric carcinoma
than adenoma (17) or intestinal
metaplasia (36). In the present
study, it was shown that p53 expression was positively correlated
with the TNM staging and CASP3 expression of gastric cancer, and
that expression was higher in intestinal-type compared with
diffuse-type carcinomas, indicating that p53 expression may be
involved in the progression and differentiation of gastric cancer.
Gonçalves et al(35) also
noted that p53 expression was more frequent among gastric
intestinal-type, differentiated and macroscopically elevated
cancers. Significantly shorter survival times were observed in
p53-negative patients compared with p53-positive patients. Tzanakis
et al(37) demonstrated that
a more marked expression of p53 was associated with a tumor size of
>5 cm, and that advanced stage p53 expression was significantly
decreased in poorly-differentiated adenocarcinoma compared with
well- or moderately-differentiated adenocarcinoma, which is
consistent with the present results. Unlike the present survival
data, p53 overexpression was an independent adverse prognostic
factor for survival (37).
In summary, the expression of Ki-67, CASP3 and p53
may be involved in the progression or differentiation of gastric
carcinoma. These expression levels may be utilized as indicators of
the pathobiological behaviors or prognosis of gastric
carcinomas.
Acknowledgements
This study was supported by the Shenyang Science and
Technology Grant (F11-264-1-10 and F12-277-1-01), Liaoning Science
and Technology Grants from the Natural Scientific Foundation of
China (81101885, 81101886 and 81172371) and a Grant-in-aid for
Scientific Research from the Ministry of Education, Culture, Sports
and Technology of Japan (23659958).
References
|
1
|
Rivera F, Vega-Villegas ME and López-Brea
MF: Chemotherapy of advanced gastric cancer. Cancer Treat Rev.
33:315–324. 2007. View Article : Google Scholar
|
|
2
|
Kelley JR and Duggan JM: Gastric cancer
epidemiology and risk factors. J Clin Epidemiol. 56:1–9. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scholzen T and Gerdes J: The Ki-67
protein: from the known and the unknown. J Cell Physiol.
182:311–322. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Endl E and Gerdes J: The Ki-67 protein:
fascinating forms and an unknown function. Exp Cell Res.
257:231–237. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chino O, Osamura Y, Kise Y, Nishi T,
Shimada H, Tanaka M, Kijima H and Makuuchi H: Acceleration of the
proliferative activity of esophageal carcinoma with invasion beyond
the muscularis mucosae; immunohistochemical analysis using MIB-1
for the Ki-67 antigen. Tokai J Exp Clin Med. 32:115–120. 2007.
|
|
6
|
Zheng HC, Sun JM, Wei ZL, Yang XF, Zhang
YC and Xin Y: Expression of Fas ligand and caspase-3 contributes to
formation of immune escape in gastric cancer. World J
Gastroenterol. 9:1415–1420. 2003.PubMed/NCBI
|
|
7
|
Kumar S: The apoptotic cysteine protease
CPP32. Int J Biochem Cell Biol. 29:393–396. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sternberg MJ, Bates PA, Kelley LA and
MacCallum RM: Progress in protein structure prediction: assessment
of CASP3. Curr Opin Struct Biol. 9:368–373. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soussi T: The p53 pathway and human
cancer. Br J Surg. 92:1331–1332. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marreiros A, Dudgeon K, Dao V, Grimm MO,
Czolij R, Crossley M and Jackson P: KAI1 promoter activity is
dependent on p53, junB and AP2: evidence for a possible mechanism
underlying loss of KAI1 expression in cancer cells. Oncogene.
24:637–649. 2005. View Article : Google Scholar
|
|
11
|
Ito R, Nakayama H, Yoshida K, Oda N and
Yasui W: Loss of maspin expression is associated with development
and progression of gastric carcinoma with p53 abnormality. Oncol
Rep. 12:985–990. 2004.PubMed/NCBI
|
|
12
|
Ala-aho R, Grénman R, Seth P and Kähäri
VM: Adenoviral delivery of p53 gene suppresses expression of
collagenase-3 (MMP-13) in squamous carcinoma cells. Oncogene.
21:1187–1195. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yan C, Wang H and Boyd DD: ATF3 represses
72-kDa type IV collagenase (MMP-2) expression by antagonizing
p53-dependent transactivation of the collagenase promoter. J Biol
Chem. 277:10804–10812. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Loging WT and Reisman D: Inhibition of the
putative tumor suppressor gene TIMP-3 by tumor-derived p53 mutants
and wild type p53. Oncogene. 18:7608–7615. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen SL, Wu YS, Shieh HY, Yen CC, Shen JJ
and Lin KH: P53 is a regulator of the metastasis suppressor gene
Nm23-H1. Mol Carcinog. 36:204–214. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de la Fuente MT, Casanova B, Cantero E,
Hernández del Cerro M, Garcia-Marco J, Silva A and Garcia-Pardo A:
Involvement of p53 in alpha4beta1 integrin-mediated resistance of
B-CLL cells to fludarabine. Biochem Biophys Res Commun.
311:708–712. 2003.PubMed/NCBI
|
|
17
|
Zheng H, Tsuneyama K, Cheng C, Takahashi
H, Cui Z, Murai Y, Nomoto K and Takano Y: An immunohistochemical
study of P53 and Ki-67 in gastrointestinal adenoma and
adenocarcinoma using tissue microarray. Anticancer Res.
26:2353–2360. 2006.PubMed/NCBI
|
|
18
|
Sobin LH and Wittekind CH: TNM
Classification of Malignant Tumors. 6th edition. John Wiley and
Sons; Hoboken, NJ: 2002
|
|
19
|
Zheng H, Takahashi H, Murai Y, Cui Z,
Nomoto K, Miwa S, Tsuneyama K and Takano Y: Pathobiological
characteristics of intestinal and diffuse-type gastric carcinoma in
Japan: an immunostaining study on the tissue microarray. J Clin
Pathol. 60:273–277. 2007. View Article : Google Scholar
|
|
20
|
Zheng HC, Li XH, Hara T, Masuda S, Yang
XH, Guan YF and Takano Y: Mixed-type gastric carcinomas exhibit
more aggressive features and indicate the histogenesis of
carcinomas. Virchows Arch. 452:525–534. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kumada T, Tsuneyama K, Hatta H, Ishizawa S
and Takano Y: Improved 1-h rapid immunostaining method using
intermittent microwave irradiation: practicability based on 5 years
application in Toyama Medical and Pharmaceutical University
Hospital. Mod Pathol. 17:1141–1149. 2004.
|
|
22
|
Czyzewska J, Guzińska-Ustymowicz K,
Pryczynicz A, Kemona A and Bandurski R: Immunohistochemical
evaluation of Ki-67, PCNA and MCM2 proteins proliferation index
(PI) in advanced gastric cancer. Folia Histochem Cytobiol.
47:289–296. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lazăr D, Tăban S, Sporea I, Dema A,
Cornianu M, Lazăr E, Goldiş A and Vernic C: Ki-67 expression in
gastric cancer. Results from a prospective study with long-term
follow-up. Rom J Morphol Embryol. 51:655–661. 2010.PubMed/NCBI
|
|
24
|
Lee HE, Kim MA, Lee BL and Kim WH: Low
Ki-67 proliferation index is an indicator of poor prognosis in
gastric cancer. J Surg Oncol. 102:201–206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Joo YE, Chung IJ, Park YK, Koh YS, Lee JH,
Park CH, Lee WS, Kim HS, Choi SK, Rew JS, Park CS and Kim SJ:
Expression of cyclooxygenase-2, p53 and Ki-67 in gastric cancer. J
Korean Med Sci. 21:871–876. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oshima CT, Iriya K and Forones NM: Ki-67
as a prognostic marker in colorectal cancer but not in gastric
cancer. Neoplasma. 52:420–424. 2005.PubMed/NCBI
|
|
27
|
Kikuyama S, Kubota T, Shimizu K and
Miyakita M: Ki-67 antigen expression in relation to
clinicopathological variables and prognosis in gastric cancer.
Oncol Rep. 5:867–870. 1998.PubMed/NCBI
|
|
28
|
Xu L, Zhang SM, Wang YP, Zhao FK, Wu DY
and Yan X: Relationship between DNA ploidy, expression of Ki-67
antigen and gastric cancer metastasis. World J Gastroenterol.
5:10–11. 1999.PubMed/NCBI
|
|
29
|
He WL, Li YH, Yang DJ, Song W, Chen XL,
Liu FK, Wang Z, Li W, Chen W, Chen CY, He YL and Zhan WH: Combined
evaluation of centromere protein H and Ki-67 as prognostic
biomarker for patients with gastric carcinoma. Eur J Surg Oncol.
39:141–149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stepień A, Izdebska M and Grzanka A: The
types of cell death. Postepy Hig Med Dosw (Online). 61:420–428.
2007.(In Polish).
|
|
31
|
Li YH, Wang C, Meng K, Chen LB and Zhou
XJ: Influence of survivin and caspase-3 on cell apoptosis and
prognosis in gastric carcinoma. World J Gastroenterol.
10:1984–1988. 2004.PubMed/NCBI
|
|
32
|
Hoshi T, Sasano H, Kato K, Yabuki N, Ohara
S, Konno R, Asaki S, Toyota T, Tateno H and Nagura H:
Immunohistochemistry of Caspase3/CPP32 in human stomach and its
correlation with cell proliferation and apoptosis. Anticancer Res.
18:4347–4353. 1998.PubMed/NCBI
|
|
33
|
Isobe N, Onodera H, Mori A, Shimada Y,
Yang W, Yasuda S, Fujimoto A, Ooe H, Arii S, Kitaichi M and Imamura
M: Caspase-3 expression in human gastric carcinoma and its clinical
significance. Oncology. 66:201–209. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Steele RJ and Lane DP: P53 in cancer: a
paradigm for modern management of cancer. Surgeon. 3:197–205. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gonçalves AR, Carneiro AJ, Martins I, de
Faria PA, Ferreira MA, de Mello EL, Fogaça HS, Elia CC and de Souza
HS: Prognostic significance of p53 protein expression in early
gastric cancer. Pathol Oncol Res. 17:349–355. 2011.PubMed/NCBI
|
|
36
|
Wang L, Zhang XY, Xu L, Liu WJ, Zhang J
and Zhang JP: Expression and significance of p53 and mdm2 in
atypical intestinal metaplasia and gastric carcinoma. Oncol Lett.
2:707–712. 2011.PubMed/NCBI
|
|
37
|
Tzanakis NE, Peros G, Karakitsos P,
Giannopoulos GA, Efstathiou SP, Rallis G, Tsigris C, Kostakis A and
Nikiteas NI: Prognostic significance of p53 and Ki67 proteins
expression in Greek gastric cancer patients. Acta Chir Belg.
109:606–611. 2009.PubMed/NCBI
|