Introduction
Hepatocellular carcinoma (HCC) is one of the most
frequently occurring cancers in the world, resulting in
approximately one million deaths every year (1). The majority of liver cancers are
diagnosed at later stages due to the absence of symptoms in
patients and an incorrect liver disease diagnosis. Surgical options
for patients with HCC include a resection of the primary tumor and
liver transplantation (2). As HCC
is typically diagnosed at an advanced stage, a resection of the
primary tumor is typically not an option and >80% of HCC
patients have recurrent disease within two years following the
surgery. Recurrence and metastasis are the two main causes of
patient mortality. Recent advances in our understanding of the
biology and signaling pathways of HCC have led to apoptosis
induction being considered as a new treatment strategy for HCC
(3).
Studies have focused on Rabdosia rubescens,
which is used as a herbal medicine, due to its antitumor effects
and lack of serious side-effects (4,5).
Oridonin, a diterpenoid that is isolated from R. rubescens,
has shown antitumor effects in several malignant tumors, including
breast and cervical carcinoma and lymphoma (6,7).
Oridonin has been demonstrated to induce apoptosis in HepG2 HCC
cells, which have a moderate metastatic potential (8,9).
However, the effect of oridonin on human HCC cell lines with a high
metastatic potential has not been determined. Therefore, the
present study investigated the effect of oridonin on the apoptosis
of the highly metastatic MHCC97-H HCC cell line and the underlying
molecular mechanism involved.
Materials and methods
Reagents
High glucose Dulbecco’s modified Eagle’s medium
(DMEM) and fetal calf serum (FCS) were purchased from HyClone
(Beijing, China). The Annexin V Alexa Fluor 488/propidium iodide
(PI) Apoptosis, MTS/PMS Cell Proliferation Assay, Active Caspase-3
Staining and Cytoplasmic and Mitochondrial Protein Extraction kits
were purchased from Invitrogen (Carlsbad, CA, USA), Promega
(Madison, WI, USA), Biovision (Milpitas, CA, USA) and Sangon
Biotech Co. Ltd (Shanghai, China), respectively. The Z-LEHD-FMK
caspase-9 inhibitor, rhodamine-123 and 3,3′-diaminobenzidine
tetrahydrochloride (DAB) were purchased from R&D Systems
(Minneapolis, MN, USA), Sigma Chemical Co. (St. Louis, MO, USA) and
Dako (Glostrup, Denmark), respectively. Cytochrome c, Bcl-2 and Bax
monoclonal antibodies and horseradish peroxidase (HRP)-conjugated
secondary antibodies (goat-anti-rabbit and goat-anti-mouse) were
purchased from Epitomics (Burlingame, CA, USA). Caspase-9 and
glyceraldehyde 3-phosphate dehydrogenase monoclonal antibodies were
purchased from Cell Signaling (Danvers, MA, USA). Oridonin (Lot,
111721–200501; 97% purity) was obtained from the Beijing Institute
of Biological Products (Beijing, China). Oridonin was prepared in
dimethyl sulfoxide (DMSO).
Cell culture
The human MHCC97-H HCC cell line was obtained from
the Hepatic Carcinoma Institute, Fudan University (Shanghai,
China). The MHCC97-H cells were cultured in DMEM supplemented with
10% FCS at 37°C in a humidified atmosphere, with 5%
CO2(10). All the
experiments were performed with cells in the logarithmic growth
phase. The DMSO concentration in the cell cultures (<0.5%) did
not affect the cell viability.
MTS/PMS assay for cell proliferation
The MHCC97-H cells were seeded into 96-well plates
at a density of 1×105 cells/ml. The cells were treated
with oridonin at concentrations of 6.25, 12.5, 25, 50 and 100 μM
for 24, 48 and 72 h. The untreated cells served as the controls.
Proliferation was determined using the MTS/PMS Cell Proliferation
Assay kit, according to the manufacturer’s instructions. MTS/PMS
(10 μl) was added to each well and incubated at 37°C for 2 h. The
absorbance was measured at 490 nm on a multi-well plate reader. The
effect of oridonin on cell proliferation was reported as the cell
survival percentage, calculated as absorbance (oridonin-treated
group)/absorbance (control group) × 100. The background absorbance
of the medium in the absence of the cells was subtracted from the
absorbance values for the control and oridonin-treated groups. Each
assay was performed in triplicate and the results are presented as
the mean ± SD.
Annexin V/PI assay for apoptosis
The MHCC97-H cells (1×105 cells/ml) were
seeded onto 6-well plates and treated with oridonin at
concentrations of 12.5, 25, 50 and 100 μM for 24 h. In addition,
the cells were treated with 100 μM oridonin in the presence of 20
μM Z-LEHD-FMK. The apoptotic cells were detected using the Annexin
V Alexa Fluor 488/PI Apoptosis kit, according to the manufacturer’s
instructions. The cells were washed twice with ice-cold
phosphate-buffered saline (PBS), then resuspended in PBS (100 μl)
and incubated with Annexin V labeling solution (5 μl) for 30 min at
4°C in the dark. The cells were incubated in 1X buffer solution
(200 μl) and labeled with PI. The percentage of the apoptotic cells
was determined by flow cytometry (FACScan; Becton Dickinson
Corporation, Franklin Lakes, NJ, USA).
Mitochondrial membrane potential
The MHCC97-H cells were treated with oridonin (12.5,
25, 50 and 100 μM) for 24 h, washed twice with PBS, labeled with
rhodamine-123 (1 μg/ml) for 10 min at 37°C and washed twice again
with PBS. The mitochondrial membrane potential was determined by
flow cytometry.
Caspase-3 activity
The MHCC97-H cells were treated with oridonin (12.5,
25, 50 and 100 μM) for 24 h, washed twice with PBS and then
resuspended in PBS (300 μl). Caspase-3 activity was determined
using the Active Caspase-3 Staining kit, according to the
manufacturer’s instructions.
Western blot
Following oridonin treatment, the MHCC97-H cells
were washed twice with PBS, lysed with RIPA buffer on ice and
centrifuged at 10,000 × g for 30 min at 4°C. The supernatant was
collected and stored at −80°C. The cytoplasmic and mitochondrial
proteins were extracted using the Cytoplasmic and Mitochondrial
Protein Extraction kit, according to the manufacturer’s
instructions. The protein concentration was determined using the
BCA Protein Assay kit (Sangon Biotech Co. Ltd). Proteins (20 μg) in
an equal volume of 2X sample loading buffer were denatured by
boiling for 5 min. Electrophoresis was performed at 70 V for 20 min
and then at 100 V for 1 h. The proteins were transferred onto
polyvinylidene difluoride (PVDF) membranes (0.22 μm) using the
semi-dry transfer method (Trans-blot SD; Bio-Rad Laboratories,
Hercules, CA, USA). The PVDF membrane was incubated with primary
antibody (1:1,000) overnight, washed three times with PBS
supplemented with Tween 20 (PBST) for 10 min, incubated with
secondary antibody (1:3,000) for 2 h at room temperature and washed
twice with PBST for 10 min. Western blot bands were developed using
DAB as the HRP substrate and analyzed using Quantity One 4.6
(Bio-Rad).
Cell morphology
The MHCC97-H cells were treated with 50 μM oridonin
at time-points ranging from 24 to 72 h, washed in PBS, dried and
stained using a Wright-Giemsa stain. The cell morphology was
observed under a light microscope (Leica, Solms, Germany). The
untreated cells were used as the controls.
Statistical analysis
The data are presented as the mean ± SD of three
independent experiments. Fisher’s least significant difference
(LSD) tests were performed using SPSS version 13.0 software (SPSS,
Inc., Chicago, IL, USA). The t-test was applied to compare the
means from the two groups. An LSD t-test was utilized to compare
the means from multiple groups. The correlation between caspase-3
activity and inducing concentrations of oridonin was analyzed by
linear correlation analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of oridonin on the proliferation
of MHCC97-H cells
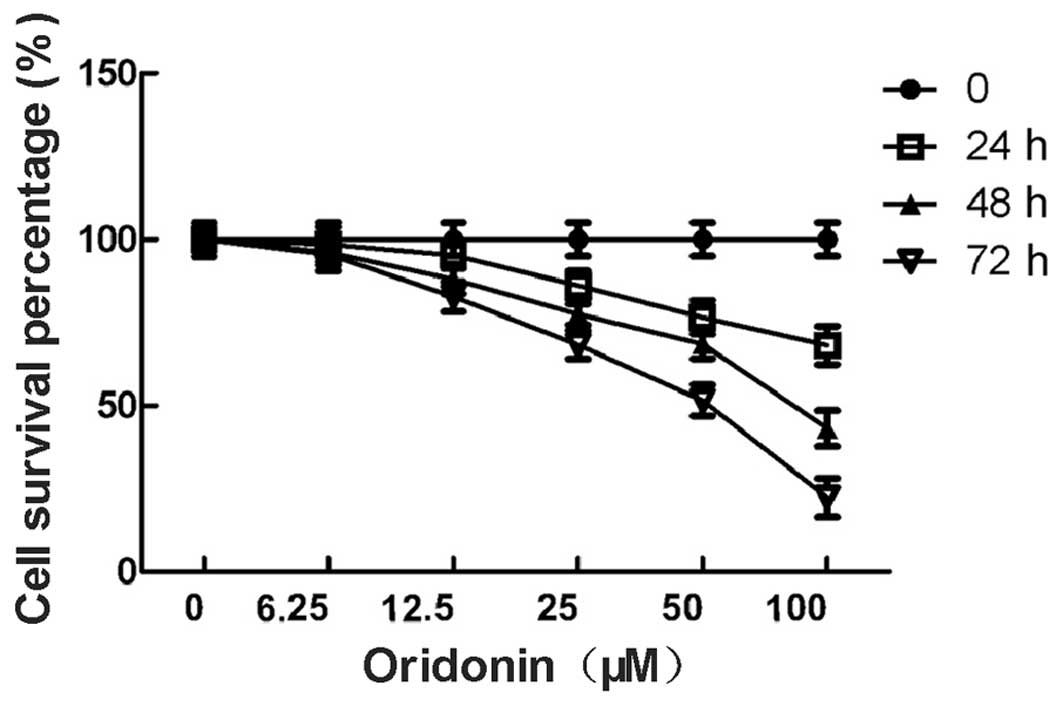
The percentage of viable MHCC-97-H cells in the
oridonin-treated group is shown in Fig.
1. The percentage of viable cells was 98.6, 95.3, 86.2, 76.6
and 68.2% for the cells that were treated with 6.25, 12.5, 25, 50
and 100 μM oridonin for 24 h, respectively. The percentage of
viable cells was 96.2, 88.1, 77.5, 68.5 and 43.2% for the cells
that were treated with 6.25, 12.5, 25, 50 and 100 μM oridonin for
48 h, respectively. The percentage of viable cells was 95.5, 82.8,
68.3, 51.6 and 22.4% for the cells that were treated with 6.25,
12.5, 25, 50 and 100 μM oridonin for 72 h, respectively. The
difference between the test group and the control group was
significant (P<0.05). The data indicated that the growth
inhibitory effect of oridonin on the MHCC97-H cells was dependent
on the concentration and the duration of the treatment. The 6.25 μM
concentration of oridonin was not included in the further
experiments due to the weak anti-proliferative effect.
Effect of oridonin on the apoptosis of
MHCC97-H cells
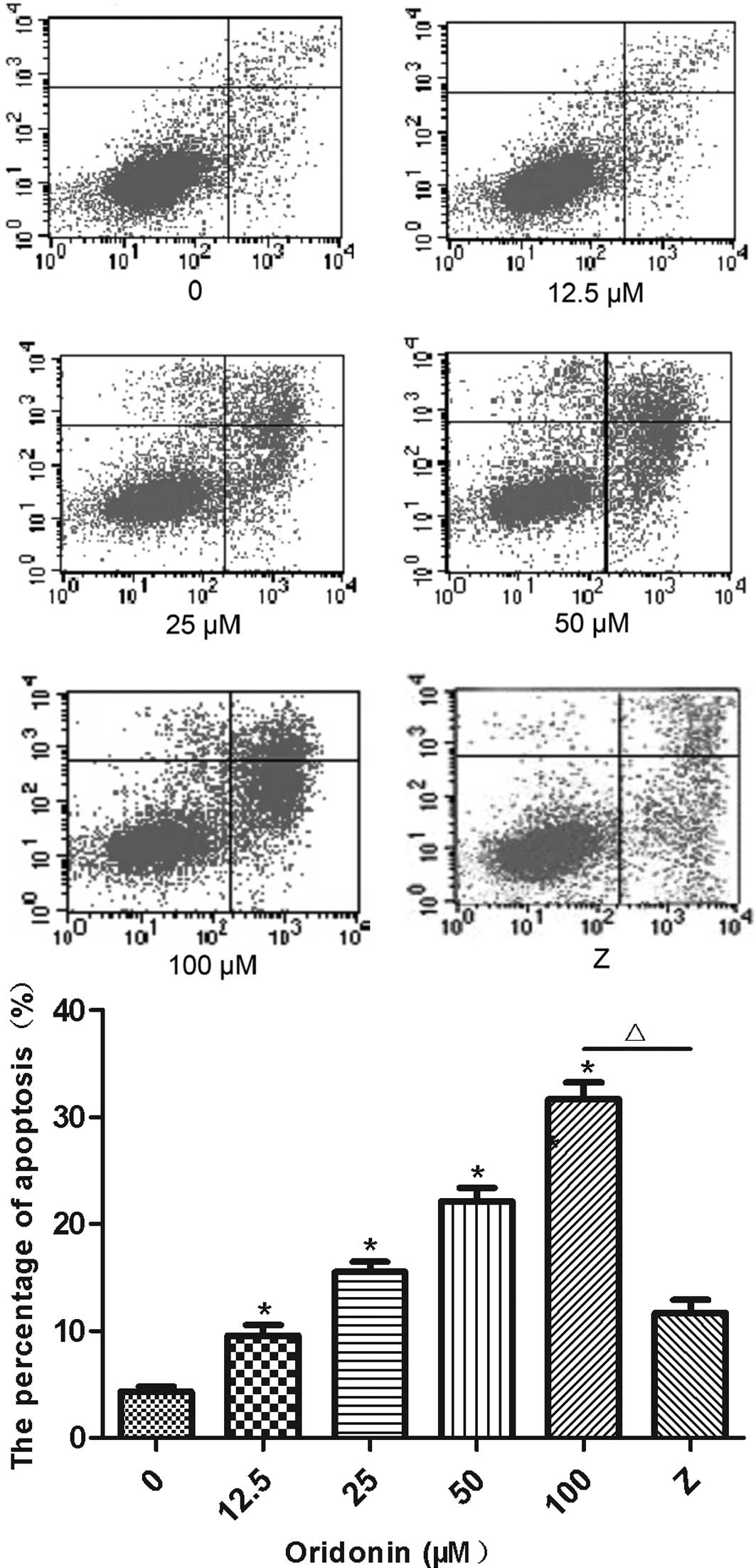
The percentage of Annexin V-positive and PI-negative
MHCC97-H cells that were treated with oridonin is shown in Fig. 2. The percentage of the apoptotic
cells was significantly higher (P<0.05) for the oridonin-treated
cells than for the untreated control cells. Furthermore, oridonin
increased the percentage of apoptotic cells in a
concentration-dependent manner. The percentage of apoptotic cells
(Annexin V-positive and PI-negative) was 9.5, 15.6, 22.2 and 31.7%
in the MHCC97-H cells that were treated with 12.5, 25, 50 and 100
μM oridonin for 24 h. Addition of the caspase-9 inhibitor,
Z-LEHD-FMK, significantly lowered (P<0.05) the percentage of
apoptotic cells that were induced by the 100-μM concentration of
oridonin (11.7 vs. 31.7%).
Effect of oridonin on the mitochondrial
membrane potential of MHCC97-H cells
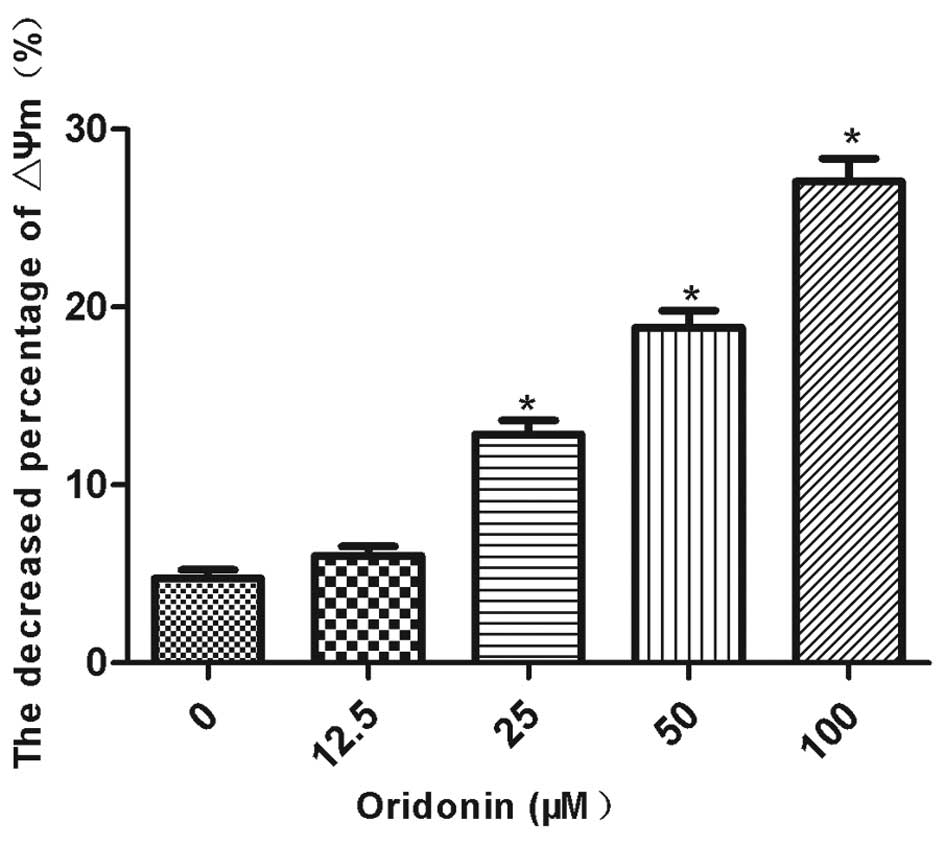
The mitochondrial membrane potential was decreased
by oridonin in a concentration-dependent manner. The mitochondrial
membrane potential was significantly decreased (P<0.05) by 12.9,
18.9 and 27.1% in the MHCC97-H cells that were treated with 25, 50
and 100 μM oridonin, respectively (Fig.
3). By contrast, the decrease in the mitochondrial membrane
potential (6.0%) was not significantly different between the 12.5
μM oridonin-treated cells and the control cells. The ratio of the
mitochondrial membrane potential prior to and following the
oridonin treatment is shown in Fig.
3.
Effect of oridonin on caspase-3 activity
of MHCC97-H cells
Oridonin increased caspase-3 activity in the
MHCC97-H cells (Fig. 4). Caspase-3
activity was significantly increased (P<0.05) by 13.2, 21.6,
29.7 and 43.6% in the cells that were treated with 12.5, 25, 50 and
100 μM oridonin for 24 h. Caspase-3 activity was positively
correlated with the oridonin concentration
(r2=0.9538).
Effect of oridonin on the expression of
apoptotic proteins in MHCC97-H cells
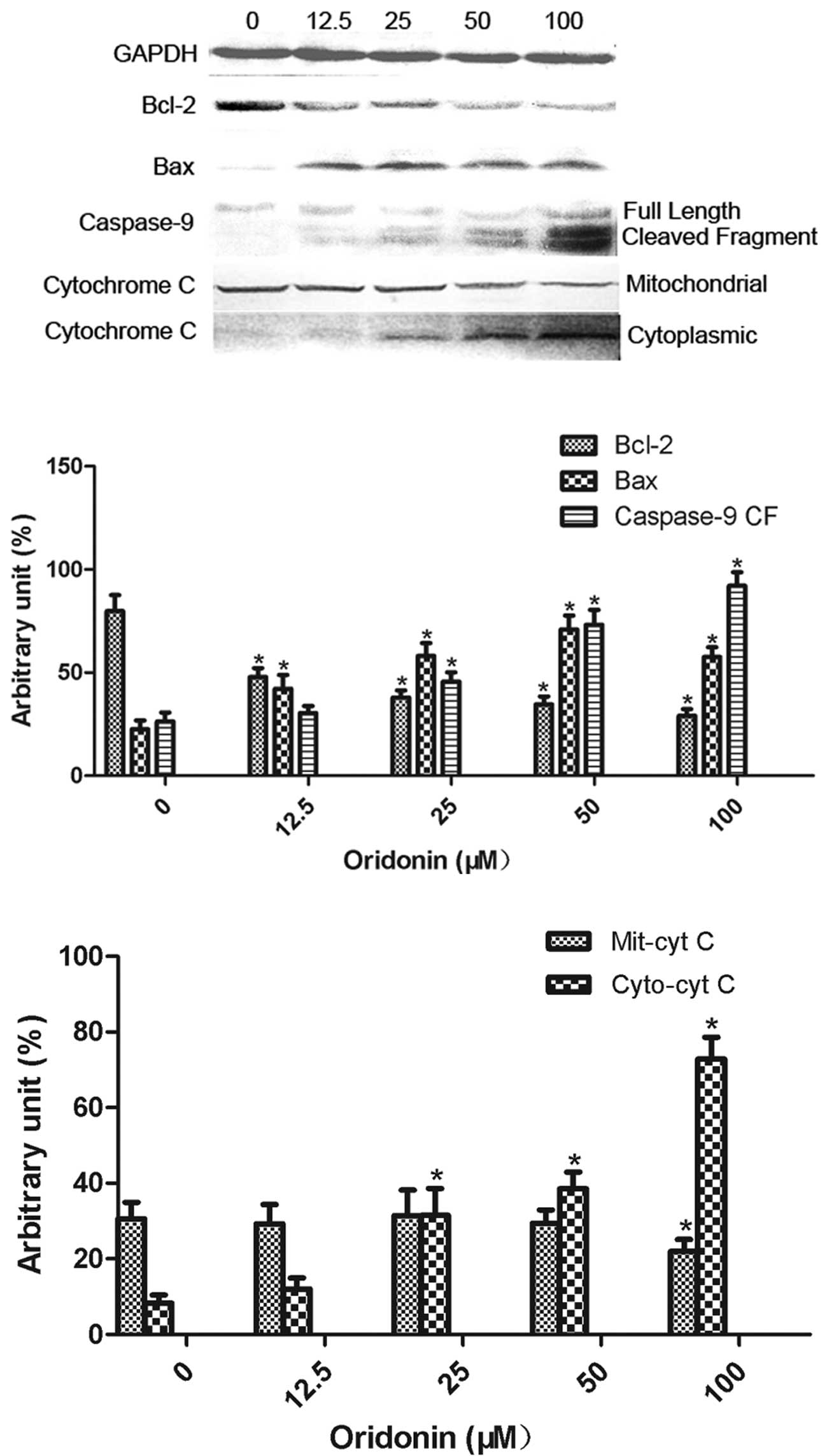
The effect of oridonin at concentrations of 12.5,
25, 50 and 100 μM on the expression of the apoptosis-related
proteins, including Bcl-2, Bax, cytochrome c and caspase-9, is
shown in Fig. 5. Bcl-2 expression
was significantly decreased (P<0.05) and Bax expression was
significantly increased (P<0.05) by all the concentrations of
oridonin. The expression of cleaved caspase-9 and cytoplasmic
cytochrome c was significantly increased (P<0.05) by oridonin at
concentrations of 25, 50 and 100 μM. Oridonin at a concentration of
100 μM significantly decreased (P<0.05) the expression of
mitochondrial cytochrome c.
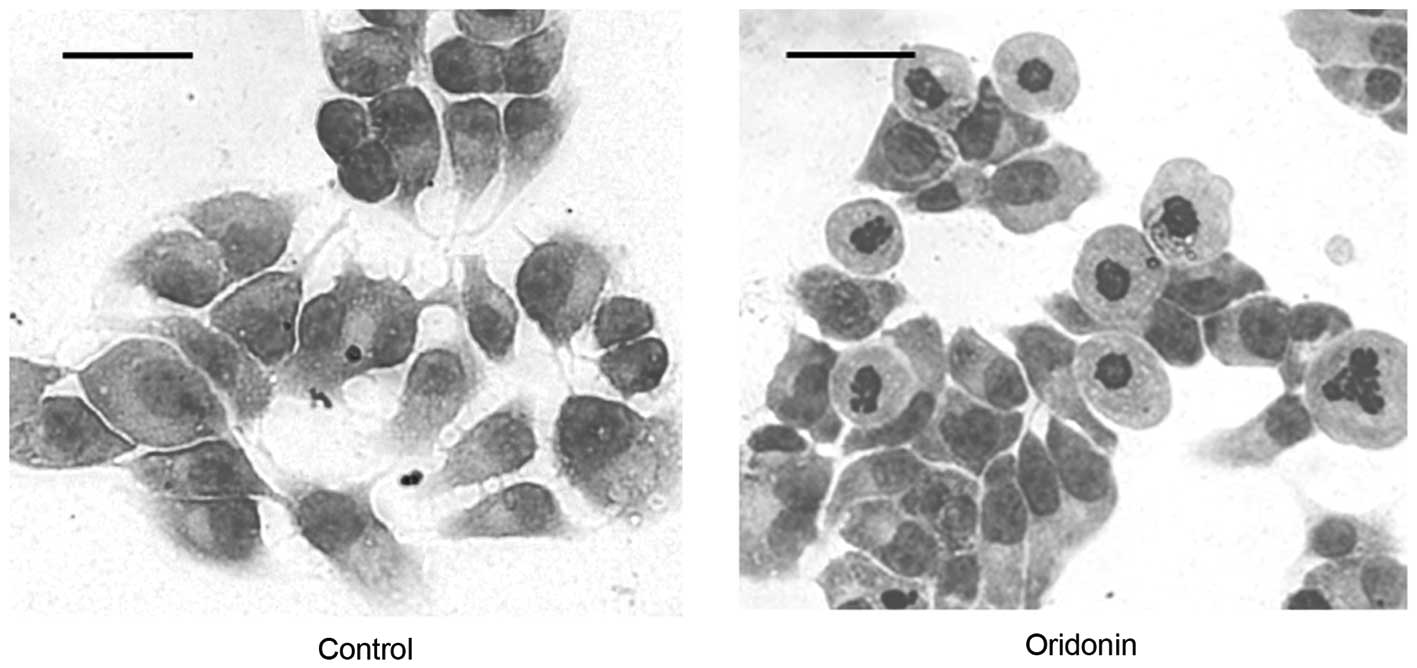
Effect of oridonin on the morphology of
MHCC97-H cells
The untreated control MHCC97-H cells had an
epithelioid morphology with a large, round or oval nucleus and
abundant cytoplasm (Fig. 6; left
panel). The morphological alterations that are associated with
cells undergoing apoptosis, including cell shrinkage, nuclear
fragmentation and chromatin condensation, were observed in the
cells that were treated with 50 μM oridonin for 24 h (Fig. 6; right panel). Necrosis was evident
in the cells that were treated with 50 μM oridonin for ≥48 h.
Discussion
The present study investigated the apoptotic
potential of oridonin in MHCC97-H cells, a human hepatoma cell line
with a high metastatic capacity (11,12).
Oridonin decreased the number of viable cells and increased the
percentage of apoptotic cells. Therefore, oridonin inhibited the
proliferation of the MHCC97-H cells by inducing apoptosis. The
IC50 of oridonin, which was calculated using the Bliss
method (13), was 142.2, 80.8 and
44.6 μM for the 24-, 48- and 72-h treatments, respectively. These
data indicate that the growth inhibitory effect of oridonin is
concentration- and time-dependent. Zhang et al(14) reported that the MHCC97-H cell line
has a higher invasive and metastatic potential than HepG2 and
SMMC7721 HCC cell lines. Previously, we reported that the 24 h
IC50 of oridonin in HepG2 cells was 27.6 μM (8). These findings are in agreement with
those of Huang et al(9).
Taken together, these findings indicate that the effective
inhibitory concentration of oridonin is higher in MHCC97-H cells
than in HepG2 cells and is likely to be the result of the various
metastatic potentials of these cell lines.
The present study indicates that a mitochondrial
pathway is involved in oridonin-induced apoptosis in MHCC97-H
cells. Cell apoptosis or programmed cell death is significant in
the maintenance of the intrinsic stability of multicellular
organisms (15). Previous studies
have indicated that interactions between multiple genes and their
composite regulation were involved in the induction of apoptosis
(15–19). It is now known that membrane
receptor and mitochondrial pathways are the main regulators of
apoptosis. Mitochondrial pathways play significant roles in the
regulation of apoptosis by inducing the mitochondrial permeability
transition pore (20).
Pro-apoptotic factors induce mitochondrial permeability transition
pore formation, which leads to the loss of membrane potential and
cytochrome c release into the cytoplasm. Cytochrome c binds
caspase-9 and Apaf-1 to form a complex that activates other caspase
family members, including caspase-3 and caspase-6, to induce
apoptosis (21). The present study
identified that oridonin decreased the mitochondrial membrane
potential and mitochondrial cytochrome c expression and increased
cytoplasmic cytochrome c expression in the MHCC97-H cells.
Furthermore, the activity of caspase-9 and caspase-3 was increased
by oridonin, while the caspase-9 inhibitor, Z-LEHD-FMK, decreased
oridonin-induced apoptosis. Taken together, these findings indicate
that a mitochondrial pathway is involved in oridonin-induced
apoptosis in MHCC97-H cells.
Members of the Bcl-2 family, including Bcl-2 and
Bax, play a significant role in the mitochondrial pathway of
apoptosis (22,23). Bax is a pro-apoptotic factor that is
located in the mitochondrial matrix. Bcl-2 is an anti-apoptotic
factor that is located in the outer layer of the mitochondrial
membrane. Bax and Bcl-2 regulate apoptosis by controlling the
activity of proteases and nucleases. Bax promotes apoptosis in
response to certain mitochondrial stimuli by inducing the opening
of the mitochondrial permeability transition pore to release
cytochrome c. Bcl-2 antagonizes the action of Bax by preventing the
opening of the mitochondrial permeability transition pore (23). The present study demonstrated that
oridonin decreased Bcl-2 expression and increased Bax expression in
a concentration-dependent manner, resulting in a decreased
Bcl-2/Bax ratio. The study indicated that oridonin induced
apoptosis in the MHCC97-H cells. Therefore, these proteins may be
involved in oridonin-induced apoptosis.
In summary, oridonin inhibited the proliferation of
the MHCC97-H cells by promoting apoptosis. Oridonin induced
apoptosis via a mitochondrial pathway that involved a reduction in
the mitochondrial membrane potential to promote the release of
cytochrome c and the activation of caspase-3 and -9. Further
investigation of the molecular mechanisms by which oridonin induces
apoptosis is required.
References
|
1
|
Barazani Y, Hiatt JR, Tong MJ and Busuttil
RW: Chronic viral hepatitis and hepatocellular carcinoma. World J
Surg. 31:1243–1248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fancellu A, Rosman AS, Sanna V, Nigri GR,
Zorcolo L, Pisano M and Melis M: Meta-analysis of trials comparing
minimally-invasive and open liver resections for hepatocellular
carcinoma. J Surg Res. 171:e33–e45. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruix J and Sherman M: Management of
hepatocellular carcinoma: an update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meade-Tollin LC, Wijeratne EM, Cooper D,
et al: Ponicidin and oridonin are responsible for the
antiangiogenic activity of Rabdosia rubescens, a constituent
of the herbal supplement PC SPES. J Nat Prod. 67:2–4. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sartippour MR, Seeram NP, Heber D, et al:
Rabdosia rubescens inhibits breast cancer growth and
angiogenesis. Int J Oncol. 26:121–127. 2005.
|
|
6
|
Hsieh TC, Wijeratne EK, Liang JY,
Gunatilaka AL and Wu JM: Differential control of growth, cell cycle
progression, and expression of NF-kappaB in human breast cancer
cells MCF-7, MCF-10A, and MDA-MB-231 byponicidin and oridonin,
diterpenoids from the chinese herb Rabdosia rubescens.
Biochem Biophys Res Commun. 337:224–231. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu YQ, Mu ZQ, You S, Tashiro S, Onodera S
and Ikejima T: Fas/FasL signaling allows extracelluar-signal
regulated kinase to regulate cytochrome c release in
oridonin-induced apoptotic U937 cells. Biol Pharm Bull.
29:1873–1879. 2006. View Article : Google Scholar
|
|
8
|
Li B, Zhu M, Wang C, et al: Oridonin
upregulates PTEN gene expression and induces apoptosis of HepG2
cells. Herald of Medicine. 9:62008.
|
|
9
|
Huang J, Wu L, Tashiro S, Onodera S and
Ikejima T: Reactive oxygen species mediate oridonin-induced HepG2
apoptosis through p53, MAPK, and mitochondrial signaling pathways.
J Pharmacol Sci. 107:370–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu Z, Zhou X, Lu H, et al: Comparative
glycoproteomics based on lectins affinity capture of N-linked
glycoproteins from human Chang liver cells and MHCC97-H cells.
Proteomics. 7:2358–2370. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun H and Liu GT: Inhibitory effect of
anti-hepatitis drug bicyclol on invasion of human hepatocellular
carcinoma MHCC97-H cells with high metastasis potential and its
relative mechanisms. J Asian Nat Prod Res. 11:576–583. 2009.
View Article : Google Scholar
|
|
12
|
Tian J, Tang Z and Ye S: Establishment of
a human hepatocellular carcinoma (HCC) cell line with high
metastatic potential (MHCC97) and its biological characteristics.
Zhonghua Zhong Liu Za Zhi. 20:405–407. 1998.(In Chinese).
|
|
13
|
Xiao SH, Xue J and Zhang HB: Further
studies on mefloquine and praziquantel alone or interaction of both
drugs against Schistosoma japonicum in vitro. Parasitol Res.
110:1239–1248. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Hu MY, Wu WZ, Wang ZJ, Zhou K,
Zha XL and Liu KD: The membrane-cytoskeleton organizer ezrin is
necessary for hepatocellular carcinoma cell growth and
invasiveness. J Cancer Res Clin Oncol. 132:685–697. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Salminen A, Ojala J and Kaarniranta K:
Apoptosis and aging: increased resistance to apoptosis enhances the
aging process. Cell Mol Life Sci. 68:1021–1031. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brenner C, Subramaniam K, Pertuiset C and
Pervaiz S: Adenine nucleotide translocase family: four isoforms for
apoptosis modulation in cancer. Oncogene. 30:883–895. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dunkle A and He YW: Apoptosis and
autophagy in the regulation of T lymphocyte function. Immunol Res.
49:70–86. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hellwig CT, Passante E and Rehm M: The
molecular machinery regulating apoptosis signal transduction and
its implication in human physiology and pathophysiologies. Curr Mol
Med. 11:31–47. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sevrioukova IF: Apoptosis-inducing factor:
structure, function, and redox regulation. Antioxid Redox Signal.
14:2545–2579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sayeed I, Parvez S, Winkler-Stuck K, et
al: Patch clamp reveals powerful blockade of the mitochondrial
permeability transition pore by the D2-receptor agonist
pramipexole. FASEB J. 20:556–558. 2006.
|
|
21
|
Feng R, Han J, Ziegler J, Yang M and
Castranova V: Apaf-1 deficiency confers resistance to
ultraviolet-induced apoptosis in mouse embryonic fibroblasts by
disrupting reactive oxygen species amplification production and
mitochondrial pathway. Free Radic Biol Med. 52:889–897. 2012.
View Article : Google Scholar
|
|
22
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reed JC: Proapoptotic multidomain
Bcl-2/Bax-family proteins: mechanisms, physiological roles, and
therapeutic opportunities. Cell Death Differ. 13:1378–1386. 2006.
View Article : Google Scholar : PubMed/NCBI
|