Introduction
Numerous types of gram-negative bacteria possess the
type III secretion system (TTSS), which delivers bacterial proteins
from the bacterial outer membrane into the host. The bacterial
proteins that influence the host physiology by translocation into
the host cells via the TTSS are generally called ‘effectors’
(1). A TTSS effector, AexT, with
homology to Pseudomonas aeruginosa bifunctional toxins,
ExoT/S, was originally identified from a fish pathogen,
Aeromonas salmonicida(2),
while AexU, an AexT-like TTSS effector, has more recently been
characterized from the small subunit (SSU) of Aeromonas
hydrophila(3). The AexU gene is
1,539 bp in length, encodes a protein with 512 amino acid residues
and has 38% identity with the AexT gene over the entire length
(3). Although the activity of
adenosine diphosphate (ADP)-ribosyltransferase in AexU has been
well documented (4), AexU has also
been shown to possess guanosine triphosphate (GTP)ase-activating
protein (GAP) activity, which is mainly responsible for host cell
apoptosis and the disruption of actin filaments (5). However, the cytotoxic impact of AexU
on host cells and its molecular mechanism remain largely
unknown.
The integrins, a family of transmembrane
heterodimers, function as receptors for extracellular matrix
molecules and mediate signal transduction to control a wide variety
of cell functions, including survival, proliferation, angiogenesis
and migration (6). Furthermore, the
aberrant expression of integrins has been shown to play significant
roles in the acquisition of an aggressive phenotype in cancer cells
(7–9). In our previous study, the cytotoxic
effect of AexU was shown to be exaggerated in the presence of
increased β4-integrin expression (10), thus suggesting a significant role
for the association between AexU and β4-integrin and indicating
that AexU may be applicable to cancer therapy.
To date, several studies have reported a significant
role for β4-integrin in the regulation of the malignant phenotype
of various types of cancer cells, including prostate cancer
(11–15). Drake et al(13) reported that the upregulation of
β4-integrin that is induced by the stable knockdown of ZEB1, a
major regulator of the epithelial-mesenchymal transition, resulted
in a significant enhancement of the invasive potential of human
prostate cancer cells. Based on these previous findings, the
cytotoxicity of AexU was evaluated in the present study either
alone or in combination with chemotherapeutic agents against human
prostate cancer PC3 cells, and the mechanism underlying
AexU-induced antitumor activity was analyzed, focusing on the
significance of β4-integrin protein expression.
Materials and methods
Tumor cell lines
PC3, LNCaP and DU145 cells, derived from human
prostate cancer, were purchased from the American Type Culture
Collection (ATCC; Rockville, MD, USA). The cell lines were
maintained in RPMI-1640 (Life Technologies, Inc., Gaithersburg, MD,
USA) supplemented with 5% heat-inactivated fetal bovine serum.
Preparation of AexU protein
The AexU protein was produced as described
previously (10). Briefly, the AexU
gene was amplified using primers, including a C-terminal
hemagglutinin (HA) epitope tag. The gene was then sub-cloned into a
BamHI and SalI-digested pGEX-5X-1 vector (GE
Healthcare, Hino, Japan) and the AexU protein was produced as a
recombinant glutathione-S-transferase-AexU-HA fusion protein.
Expression plasmid and transfection into
PC3 cells
A chemically synthesized oligonucleotide encoding a
β4-integrin short hairpin RNA (shRNA) with a loop motif was
inserted downstream of the U6 promoter of the pBAsi hU6 Pur DNA
vector (Takara Bio, Inc., Otsu, Japan). The sequence of the shRNA
targeting β4-integrin was 5′-CTACGACAGCTT CCTTATGTA-3′. Similarly,
a control plasmid was constructed by randomizing the sequence of
the shRNA (5′-GGAATCTCA TTCGATGCATAC-3′).
The expression vectors were transfected into the PC3
cells by liposome-mediated gene transfer methods, as described
previously (16). Briefly, either
the purified expression plasmid that contained the shRNA targeting
β4-integrin or the control plasmid was added to the PC3 cells
following pre-incubation for 30 min with Lipofectamine™ 2000
(Invitrogen, San Diego, CA, USA) and serum-free
Opti-MEM® (Life Technologies, Inc.). Drug selection in
1.5 μg/ml Puromycin (Sigma, St. Louis, MO, USA) was performed for
three days following transfection and the colonies were harvested
and expanded to generate the cell lines.
In vitro cell growth assay
To evaluate the cytotoxicity of AexU on the in
vitro proliferation of the tumor cell lines, 5×104
cells from each cell line were seeded into 12-well plates and the
number of cells was assessed daily in triplicate using Cell
Counting kit-8 (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan). In addition, the effects of the treatment with cisplatin or
docetaxel (Sigma) in combination with AexU on the proliferation of
the PC3 cells were also examined.
Western blot analysis
The western blot analyses were performed as
described previously (17). In
brief, samples that contained equal amounts of protein (25 μg) from
the lysates of the cultured tumor cell lines were analyzed using
antibodies against total and phosphorylated Akt, β4-integrin,
cleaved caspase-3 (Cell Signaling Technology, Inc., Danvers, MA,
USA), Bax, β-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) and horseradish peroxidase-conjugated secondary antibodies
(Santa Cruz Biotechnology, Inc.).
Statistical analysis
The differences between the two groups were compared
using unpaired t-tests. All the statistical calculations were
performed using the StatView 5.0 software program (Abacus Concepts,
Inc., Berkeley, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Cytotoxic effects of AexU on prostate
cancer cells depends on the expression of β4-integrin
The cytotoxic effects of AexU on the three human
prostate cancer cell lines were examined. AexU exhibited
dose-dependent cytotoxic activity in the PC3 cells, whereas there
were no significant growth inhibitory effects of AexU on the LNCaP
or DU145 cells (Fig. 1A).
β4-integrin protein expression levels were examined in these cells
using western blot analysis, and abundant β4-integrin expression
was identified in the PC3 cells, but not in the LNCaP or DU145
cells (Fig. 1B).
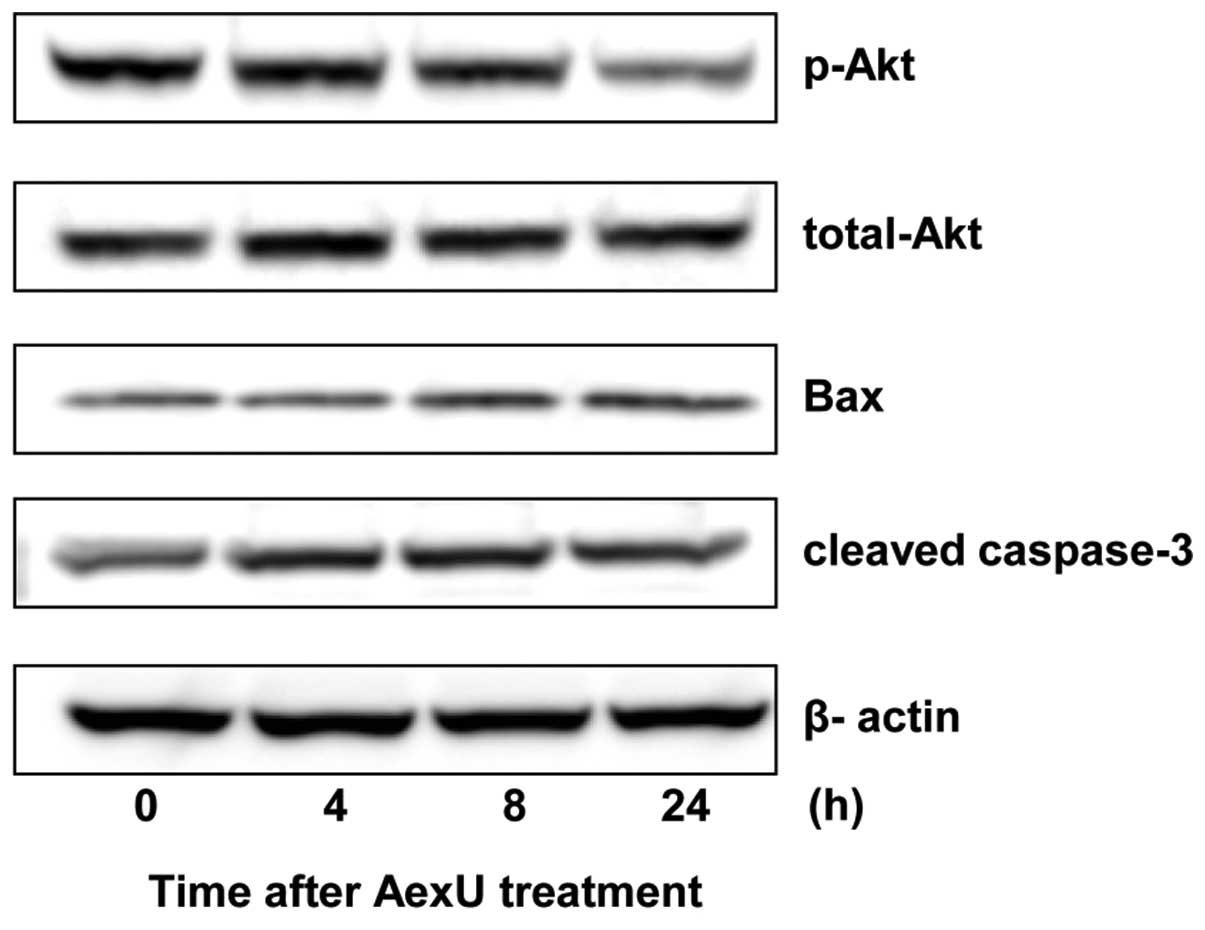
Inactivation of Akt in PC3 cells
following treatment with AexU
Despite the lack of change in the total Akt
expression, the phosphorylated Akt in the PC3 cells was
downregulated following treatment with AexU (Fig. 2). Furthermore, AexU treatment
induced an increase in the expression levels of Bax and caspase-3
in the PC3 cells.
Chemosensitization of PC3 cells by AexU
treatment
To determine whether AexU enhances the
chemosensitivity of PC3 cells, the cells were treated with various
concentrations of cisplatin or docetaxel in combination with AexU.
AexU significantly enhanced the sensitivity of the PC3 cells to
cisplatin and docetaxel (Fig. 3).
The IC50 values of the two agents in the PC3 cells
following treatment with AexU decreased by ~90% compared with those
of the PC3 cells that were treated with either of the
chemotherapeutic agents alone.
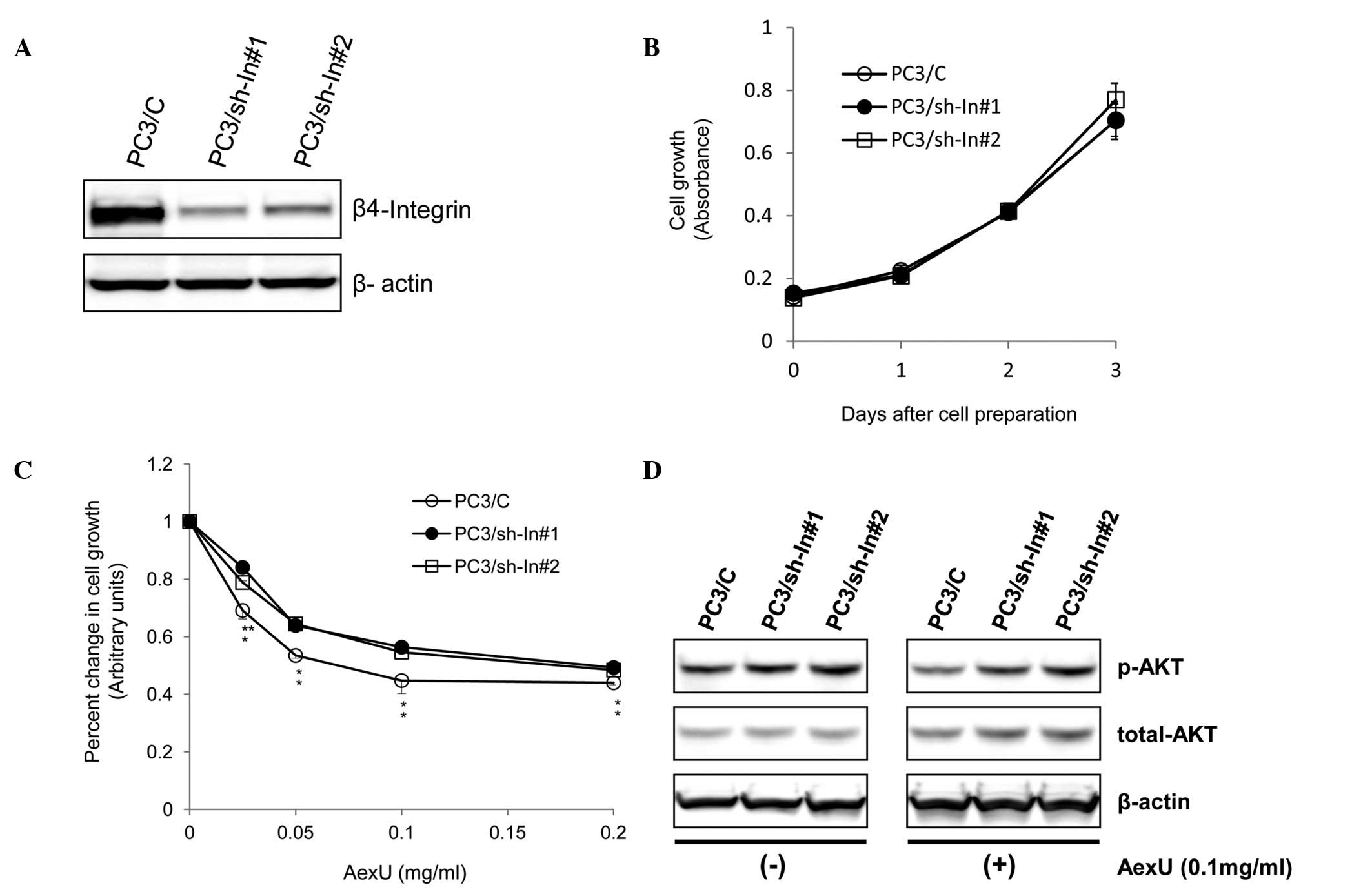
Establishment of PC3 sublines transduced
with a vector-based shRNA targeting β4-integrin
To further characterize the association between the
cytotoxicity of AexU and the expression of β4-integrin in prostate
cancer, the PC3 sublines, PC3/sh-In#1 and PC3/sh-In#2, were
established by transfecting the cells with a vector-based shRNA
targeting β4-integrin. The expression levels of β4-integrin in the
PC3/sh-In#1 and PC3/sh-In#2 cells were markedly inhibited by
>50% compared with the negative control vector-transfected
sublines (PC3/C) (Fig. 4A).
The growth patterns of the PC3 sublines with and
without AexU treatment were investigated. There were no significant
differences in the growth between the untreated PC3 sublines
(Fig. 4B). However, treatment of
the PC3 sublines with AexU revealed that the PC3/sh-In#1 and
PC3/sh-In#2 cells were significantly less sensitive to AexU than
the PC3/C cells (Fig. 4C).
Furthermore, there were no significant differences in the
expression levels of either total or phosphorylated Akt among the
PC3 sublines, but AexU treatment resulted in a marked inhibition of
phosphorylated Akt in the PC3/C cells compared with the PC3/sh-In#1
and PC3/sh-In#2 cells (Fig.
4D).
Discussion
Prostate cancer is the most common malignancy and
the second leading cause of cancer-related mortality in males in
Western industrialized countries (18). Although androgen withdrawal remains
the only effective therapy for advanced prostate cancer, the
majority of patients with advanced disease undergo a progression to
castration-resistant prostate cancer (CRPC) within a few years
following the initiation of this therapy (19). In recent years, several novel agents
have demonstrated a prognostic advantage in males with CRPC and
have been introduced into clinical practice (20). However, these improvements have been
modest, suggesting the requirement for the development of new
agents with alternative mechanisms of action to those of the
currently available agents. Therefore, the present study
investigated the cytotoxicity of a TTSS effector, AexU, which has
been shown to have a therapeutic effect against cancer cells
(10) and human CRPC PC3 cells. The
present study focused on the involvement of β4-integrin expression
on the AexU-mediated antitumor activity.
The study demonstrated the potent dose-dependent
cytotoxic activity of AexU against the PC3 cells. Several previous
studies have addressed the mechanism of action underlying the
cytotoxic effects that are induced by AexU (3,4).
Sierra et al(4) revealed a
characteristic rounded morphology of HeLa cells that were
transfected with the AexU gene, which was attributed mainly to the
NH2-terminal portion of this toxin. This phenomenon may be
explained by the pronounced ADP-ribosyltransferase activity in the
NH2-terminal domain of AexU, which was demonstrated to induce the
rounded cell morphology by disrupting the function of actin
filaments (21), thus interfering
with significant host cell functions, including motility,
phagocytosis, secretion, proliferation and mitosis (3,4). The
actin cytoskeleton in human cancer cells has been reported to be
disrupted by AexU, which appears to co-localize with β4-integrin
and is more effective in cells overexpressing β4-integrin (10). In fact, in the present study, the
sensitivity of the LNCAP and DU145 cells, which express
undetectable levels of β4-integrin, was shown to be much lower than
that of the PC3 cells, which have an abundant expression of
β4-integrin. Consistent with the previous study, this finding
suggests that β4-integrin expression is involved in the
AexU-mediated cytotoxicity of AexU against cancer cells.
The mechanism by which AexU induces cytotoxic
effects in the PC3 cells is worthy of attention. Among the various
major signaling pathways that we examined (data not shown), Akt was
identified to be markedly inactivated in the PC3 cells following
treatment with AexU. To date, there have been several studies that
have investigated the changes in host cell signaling elicited by
bacterial proteins that are delivered via the TTSS (22,23).
However, the present study is the first to characterize the changes
in signal transduction that are caused by AexU. The present study
subsequently assessed whether AexU treatment altered the
apoptosis-related molecules in the PC3 cells and revealed that
there was upregulation of Bax and cleaved caspase-3. Sierra et
al(4) also reported the
activation of caspase-3 and caspase-9 in HeLa cells producing the
AexU toxin. Collectively, these findings suggest that the
cytotoxicity of AexU results from apoptotic cell death via the
mitochondrial stress pathway.
To the best of our knowledge, there have not been
any previous studies that have evaluated the efficacy of TTSS
effectors as anticancer agents, including AexU. Therefore, the
antitumor activity of AexU in combination with other agents has not
been examined. The present study assessed whether the additional
treatment of cells with chemotherapeutic agents, including
cisplatin and docetaxel, was able to increase AexU-induced
cytotoxicity in the PC3 cells. A markedly enhanced sensitivity of
the PC3 cells to the chemotherapeutic agents was observed following
treatment with AexU. Considering these findings, it may be worth
exploring agents that enhance the cytotoxicity of AexU against
cancer cells more effectively than these chemotherapeutic
agents.
The present study established PC3 cells in which
β4-integrin expression was markedly inhibited by introducing an
expression plasmid. The plasmid contained shRNA targeted against
the β4-integrin gene in order to elucidate the role of β4-integrin
in AexU-induced cytotoxicity in the PC3 cells. Despite the fact
that no significant differences in proliferation were observed
between the PC3 sublines without AexU, the suppression of the
β4-integrin protein resulted in a decrease in the sensitivity of
the PC3 cells to AexU. Furthermore, the activation of Akt following
the treatment of the control PC3 cells, but not the β4-integrin
knockdown PC3 cells, appeared to be markedly inhibited. These
findings suggest that AexU-induced cytotoxicity in the PC3 cells is
mediated, at least in part, by the inactivation of Akt signaling
regulated by the expression of the β4-integrin protein.
In conclusion, a TTSS effector, AexU, was shown to
exert potent cytotoxic activity against PC3 cells through the
inactivation of the Akt signaling pathway. This effect may be
significantly enhanced by an additional treatment with
chemotherapeutic agents. Furthermore, the β4-integrin protein
appears to be involved in the mechanism underlying AexU-induced
apoptotic cell death. Collectively, the results of the present
study suggest that AexU-based therapy may be a promising novel
strategy for CRPC. However, additional experiments, particularly
in vivo studies, are required to further investigate the
potential of AexU as a therapeutic agent for this condition.
References
|
1
|
Ghosh P: Process of protein transport by
the type III secretion system. Microbiol Mol Biol Rev. 68:771–795.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Braun M, Stuber K, Schlatter Y, Wahli T,
Kuhnert P and Frey J: Characterization of an ADP-ribosyltransferase
toxin (AexT) from Aeromonas salmonicida subsp
salmonicida. J Bacteriol. 184:1851–1858. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sha J, Wang SF, Suarez G, et al: Further
characterization of a type III secretion system (T3SS) and of a new
effector protein from a clinical isolate of Aeromonas
hydrophila - part I. Microb Pathog. 43:127–146. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sierra JC, Suarez G, Sha J, et al:
Biological characterization of a new type III secretion system
effector from a clinical isolate of Aeromonas
hydrophila-part II. Microb Pathog. 43:147–160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khajanchi BK, Sha J, Kozlova EV, et al:
N-acylhomoserine lactones involved in quorum sensing control the
type VI secretion system, biofilm formation, protease production,
and in vivo virulence in a clinical isolate of Aeromonas
hydrophila. Microbiology. 155:3518–3531. 2009. View Article : Google Scholar
|
|
6
|
LaFlamme SE, Nieves B, Colello D and
Reverte CG: Integrins as regulators of the mitotic machinery. Curr
Opin Cell Biol. 20:576–582. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gilcrease MZ: Integrin signaling in
epithelial cells. Cancer Lett. 247:1–25. 2007. View Article : Google Scholar
|
|
8
|
Guo W and Giancotti FG: Integrin
signalling during tumour progression. Nat Rev Mol Cell Biol.
5:816–826. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vicente-Manzanares M, Choi CK and Horwitz
AR: Integrins in cell migration - the actin connection. J Cell Sci.
122:199–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abolghait SK, Iida T, Kodama T, Cantarelli
VV, Akeda Y and Honda T: Recombinant AexU effector protein of
Aeromonas veronii bv. sobria disrupts the actin cytoskeleton
by downregulation of Rac1 and induces direct cytotoxicity to
β4-integrin expressing cell lines. Microb Pathog. 51:454–465.
2011.PubMed/NCBI
|
|
11
|
Choi YP, Kim BG, Gao MQ, Kang S and Cho
NH: Targeting ILK and β4 integrin abrogates the invasive potential
of ovarian cancer. Biochem Biophys Res Commun. 427:642–648.
2012.
|
|
12
|
Gerson KD, Shearstone JR, Maddula VS,
Seligmann BE and Mercurio AM: Integrin β4 regulates SPARC protein
to promote invasion. J Biol Chem. 287:9835–9844. 2012.
|
|
13
|
Drake JM, Barnes JM, Madsen JM, Domann FE,
Stipp CS and Henry MD: ZEB1 coordinately regulates laminin-332 and
β4 integrin expression altering the invasive phenotype of prostate
cancer cells. J Biol Chem. 285:33940–33948. 2010.PubMed/NCBI
|
|
14
|
Bonaccorsi L, Carloni V, Muratori M, et
al: Androgen receptor expression in prostate carcinoma cells
suppresses alpha6beta4 integrin-mediated invasive phenotype.
Endocrinology. 141:3172–3182. 2000.
|
|
15
|
Davis TL, Cress AE, Dalkin BL and Nagle
RB: Unique expression pattern of the alpha6beta4 integrin and
laminin-5 in human prostate carcinoma. Prostate. 46:240–248. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Terakawa T, Miyake H, Furukawa J, Ettinger
SL, Gleave ME and Fujisawa M: Enhanced sensitivity to androgen
withdrawal due to overexpression of interleukin-6 in
androgen-dependent human prostate cancer LNCaP cells. Br J Cancer.
101:1731–1739. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kususda Y, Miyake H, Gleave ME and
Fujisawa M: Clusterin inhibition using OGX-011 synergistically
enhances antitumour activity of sorafenib in a human renal cell
carcinoma model. Br J Cancer. 106:1945–1952. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2006. CA Cancer J Clin. 56:106–130. 2006. View Article : Google Scholar
|
|
19
|
Bluemn EG and Nelson PS: The
androgen/androgen receptor axis in prostate cancer. Curr Opin
Oncol. 24:251–257. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haddad H and Garcia JA: Novel agents for
the management of castration-resistant prostate cancer. Curr Opin
Urol. 22:175–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kurita A, Gotoh H, Eguchi M, et al:
Intracellular expression of the Salmonella plasmid virulence
protein, SpvB, causes apoptotic cell death in eukaryotic cells.
Microb Pathog. 35:43–48. 2003.
|
|
22
|
Bhattacharjee RN, Park KS, Kumagai Y, et
al: VP1686, a Vibrio type III secretion protein, induces toll-like
receptor-independent apoptosis in macrophage through NF-kappaB
inhibition. J Biol Chem. 281:36897–36904. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Molero C, Rodríguez-Escudero I, Alemán A,
Rotger R, Molina M and Cid VJ: Addressing the effects of
Salmonella internalization in host cell signaling on a
reverse-phase protein array. Proteomics. 9:3652–3665. 2009.
|