Introduction
Glioblastoma multiforme (GBM) is the most common and
invasive adult malignant brain tumor in humans. Despite standard
treatments, including surgery and radiotherapy, which have been
administered to patients with GBM in the past decade, the prognosis
remains poor, owing to the acquired resistance of the GBM cells to
apoptosis (1–3).
In the past decade, the induction of apoptosis
(programmed cell death type I) has become the major strategy to
combat cancer. However, resistance to apoptosis is considered to be
a characteristic of several types of cancers, particularly primary
GBM. Therefore, the identification of innovative strategies other
than the induction of apoptosis is urgently required. Studies have
demonstrated the potential of autophagy (programmed cell death type
II) as a target for cancer therapy (4). The most useful chemotherapeutic agent
for GBM, temozolomide, has also been reported to exert a
proautophagic effect, indicating the importance of autophagy
modulation in GBM treatment (5).
With the exception of traditionally synthetic
compounds, numerous phytochemicals have been identified to exert
anticancer effects. Honokiol, a small molecule biphenolic compound
purified from the medicinal herb Magnolia officinalis
(Magnoliae Cortex), has been known to exert antithrombotic,
antibacterial and anxiolytic effects (6–9).
Studies have revealed that honokiol is able to inhibit tumor growth
in animals and induce apoptosis in various types of cancer cells,
including leukemia, hepatoma, prostate, lung and colon cells
(10–13). More recently, honokiol has been
shown to be capable of crossing the blood-brain barrier (BBB) and
blood-cerebrospinal fluid barrier (BCSFB) and inhibiting brain
tumor growth in the human U251 xenograft glioma model (14). However, little is known about the
molecular mechanisms underlying the effects of honokiol against
glioma cells.
The present study explored the effects of honokiol
in DBTRG-05MG GBM cells to investigate whether autophagy is
involved in the anticancer effects of the compound.
Materials and methods
Cell line and cell culture
The human GBM DBTRG-05MG cell line was maintained in
RPMI-1640 medium (Gibco, Carlsbad, CA, USA) containing 10% fetal
bovine serum, 0.01 M HEPES and 1 mM sodium pyruvate in a 37°C
incubator with 5% CO2.
Chemicals and reagents
Honokiol was dissolved in dimethyl sulfoxide (DMSO)
at a concentration of 50 mM and stored at −20°C. Sulforhodamine B
(SRB) was dissolved in phosphate buffer at a concentration of 5
mg/ml and stored at 4°C. SRB, DMSO and cadmium acetate were
purchased from Sigma (St. Louis, MO, USA).
Cell viability assay
The DBTRG-05MG cells were seeded at a density of
3×103 cells/well in 96-well plates for 24 h. The cells
were then treated with various concentrations of honokiol (6.25,
12.5, 25 and 50 μM) for 72 h. The cell numbers were determined
using the SRB assay. Briefly, the cells were fixed in 10%
trichloroacetic acid and stained with 0.4% SRB, a protein binding
dye. Following incubation and washing with 1% acetic acid, the
bound SRB was dissolved in 10 mM unbuffered Tris base and the
optical density was measured at 562 nm using a microtiter plate
reader.
Analysis of sub-G1 apoptotic
population and cell cycle distribution
One day after being seeded in a 6-well plate
(1×105 cells/ml, 2 ml/well), the cells were incubated
with various concentrations of honokiol (12.5, 25 and 50 μM) for 72
h. The control groups were treated with phosphate-buffered saline
(PBS) only. Upon harvesting, the cells were fixed in 70% ice-cold
ethanol and stored at −20°C. The cells were then washed twice with
ice-cold PBS and incubated with RNase and the DNA intercalating
dye, propidium iodide (50 μg/ml). The percentages of the
sub-G1 apoptotic population and cell cycle distribution
were then analyzed using a flow cytometer (BD Biosciences, San
Jose, CA, USA).
Western blot analysis
The cells were seeded at density of 1×106
cells/dish in a 10-cm dish for 24 h. To prepare the total cell
extract, the cells were harvested at 72 h following the treatment
described previously, then washed, lysed with lysis buffer [50 mM
Tris-HCl (pH 7.4) 150 mM NaCl, 1% Triton X-100, 0.5% deoxycholate,
1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM
Na3VO4, 1 mM NaF and 2% cocktail] and cleared
by centrifugation at 12,000 × g for 30 min at 4°C. Briefly, the
cell pellets were lysed with protein extraction solution and
incubated at −20°C for 20 min. Next, the cell lysates were
centrifuged at 15,000 × g for 5 min and the total protein was
collected. The protein concentration was measured using a protein
assay kit (Strong Biotech, Taipei, Taiwan). Total protein (20 μg)
was separated on a 10% SDS-PAGE gel and transferred to a PVDF
membrane. Non-specific binding was blocked using 5% skimmed milk.
Primary antibodies to detect RB (sc-102), poly(ADP-ribose)
polymerase (PARP; sc-7150) and Bcl-x (S/L; sc-8392) were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Primary
antibodies to phospho-RB (Ser807/811), Beclin-1 (#3738), LC3
(#4108) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; #2118)
were purchased from Cell Signaling Technology (Beverly, MA, USA).
The primary antibodies were detected using horseradish peroxidase
(HRP)-conjugated anti-mouse, anti-rabbit or anti-goat secondary
antibodies as appropriate, for 1 h at 25°C. The bound
HRP-conjugated secondary antibodies were visualized using an
Enhanced Chemiluminescence (ECL) Plus System (Millipore, Billerica,
MA, USA).
Results
Honokiol inhibits the growth of GBM cells
and induces apoptosis
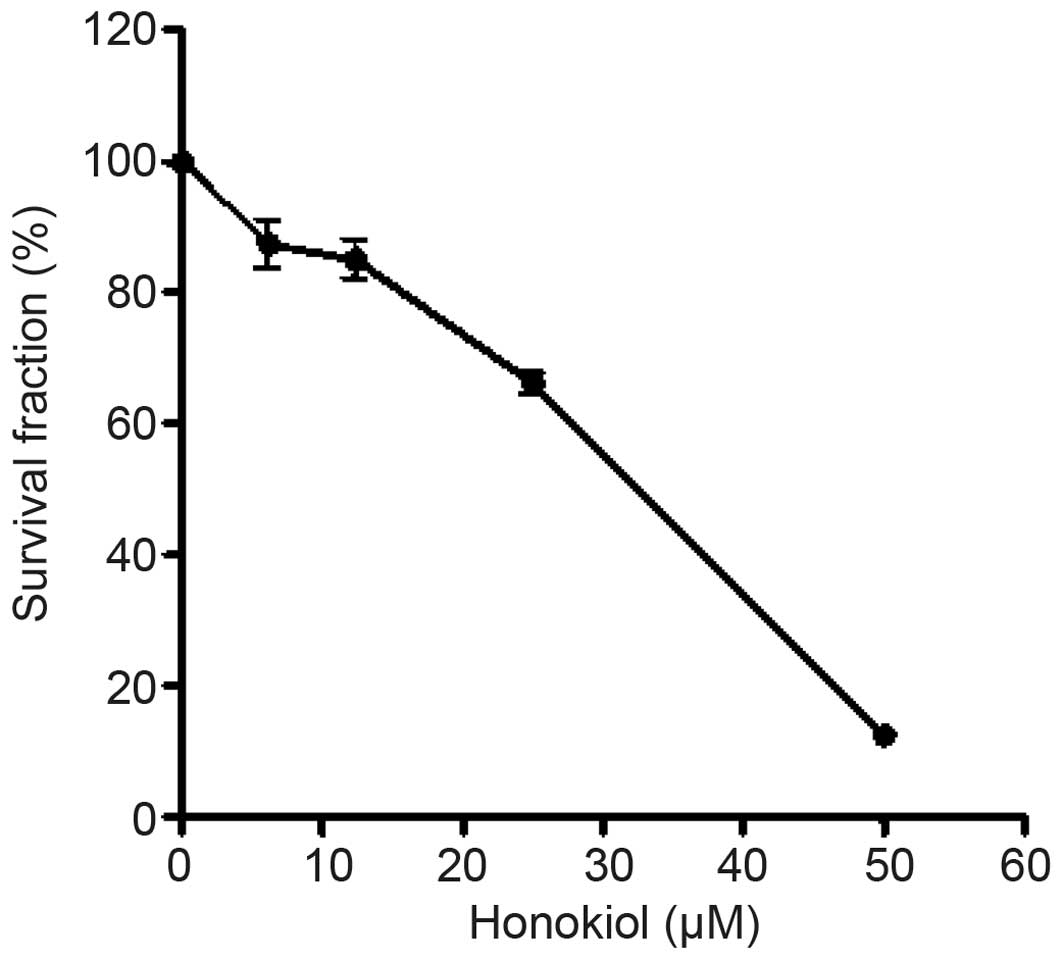
The SRB assay revealed that honokiol inhibited the
growth of the DBTRG-05MG cells in a dose-dependent manner.
Following 72 h of treatment, the IC50 of honokiol on the
DBTRG-05MG cells was ~30 μM (Fig.
1). To investigate the apoptotic effects of honokiol on the GBM
cells, the percentage of the apoptotic sub-G1 fraction
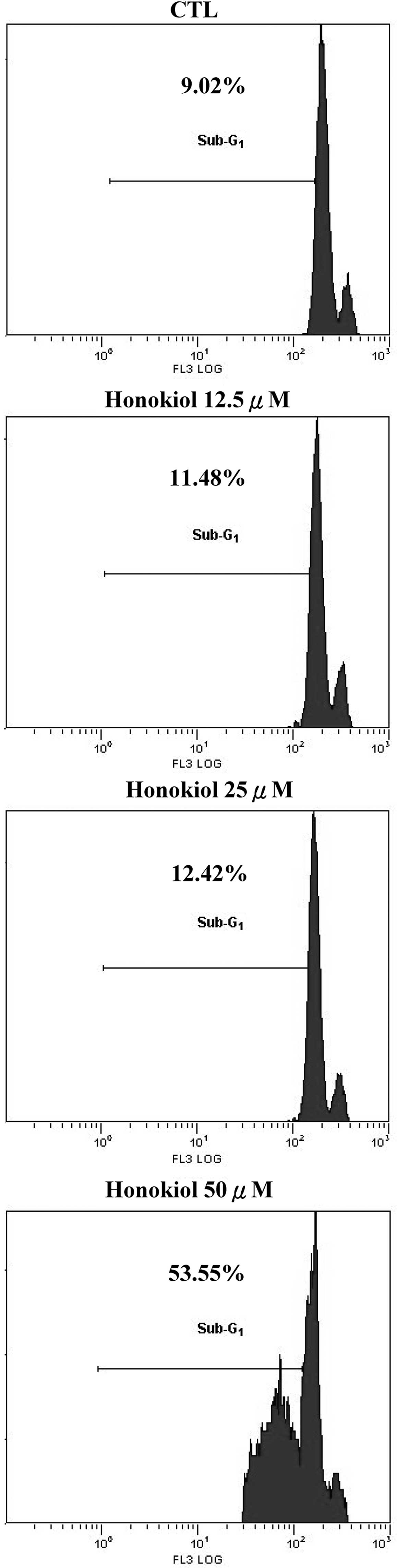
was analyzed using a flow cytometer. Following the treatment with
honokiol for 72 h, the percentage of the apoptotic
sub-G1 fraction was 9.02, 11.48, 12.42 or 53.55% in the
control and the cells treated with 12.5, 25 and 50 μM of honokiol,
respectively (Fig. 2). A marked
increase in the apoptotic fraction was not observed in the cells
that were treated with ≤25 μM honokiol. When the dose increased to
50 μM, a massive sub-G1 fraction was observed,
indicating that apoptosis only occurred at higher doses of honokiol
(Fig. 2).
Honokiol-induced apoptosis of GBM cells
is associated with the downregulation of the Rb protein and
cleavage of PARP and Bcl-x (S/L)
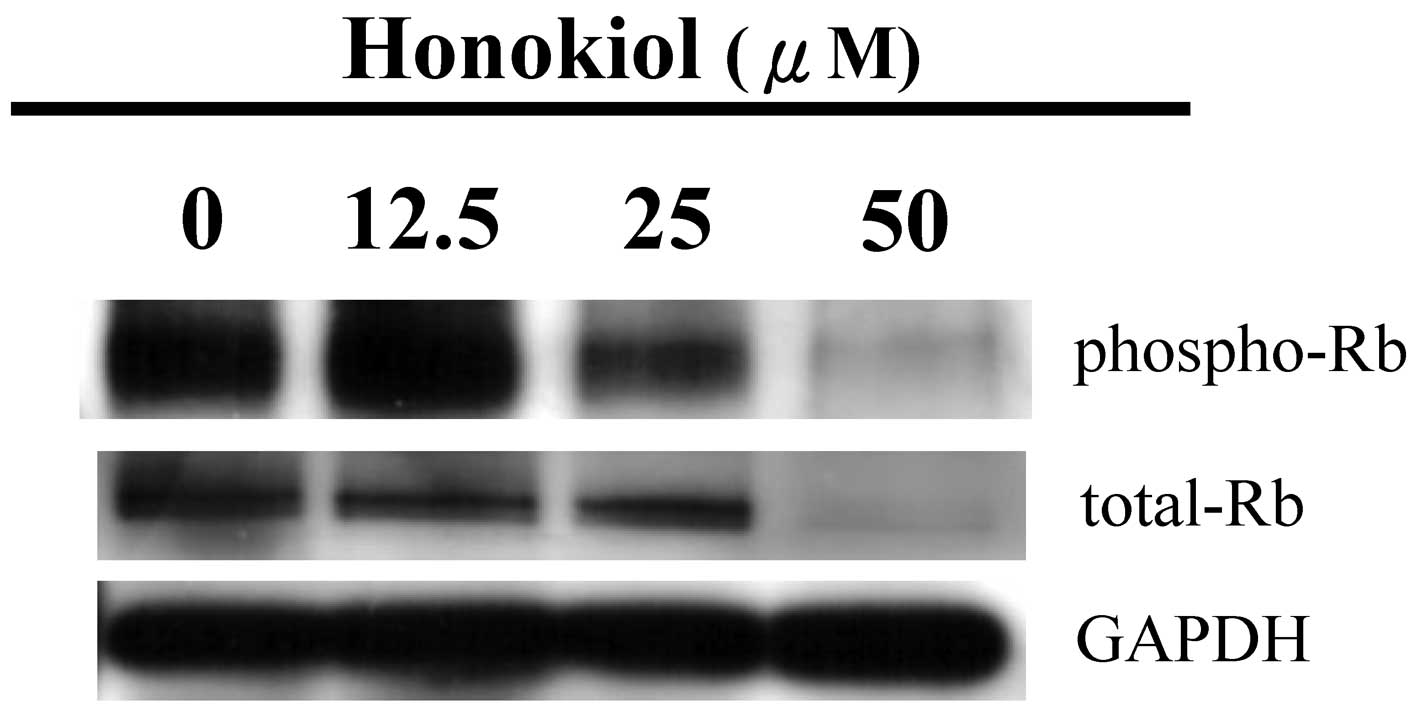
To improve our understanding of the events that are
involved in honokiol-induced apoptosis, the effects of honokiol on
the Rb, PARP and Bcl-x (S/L) proteins, which are known to regulate
cell apoptotic cascades, were examined. At a dose of 25 μM,
honokiol markedly decreased the phospho-Rb level, but did not
significantly affect the total Rb level (Fig. 3). However, honokiol significantly
decreased the phospho- and total Rb proteins when the dose reached
50 μM. This phenomenon was paralleled with the high level of
apoptosis induced by the higher dose (50 μM) of honokiol shown in
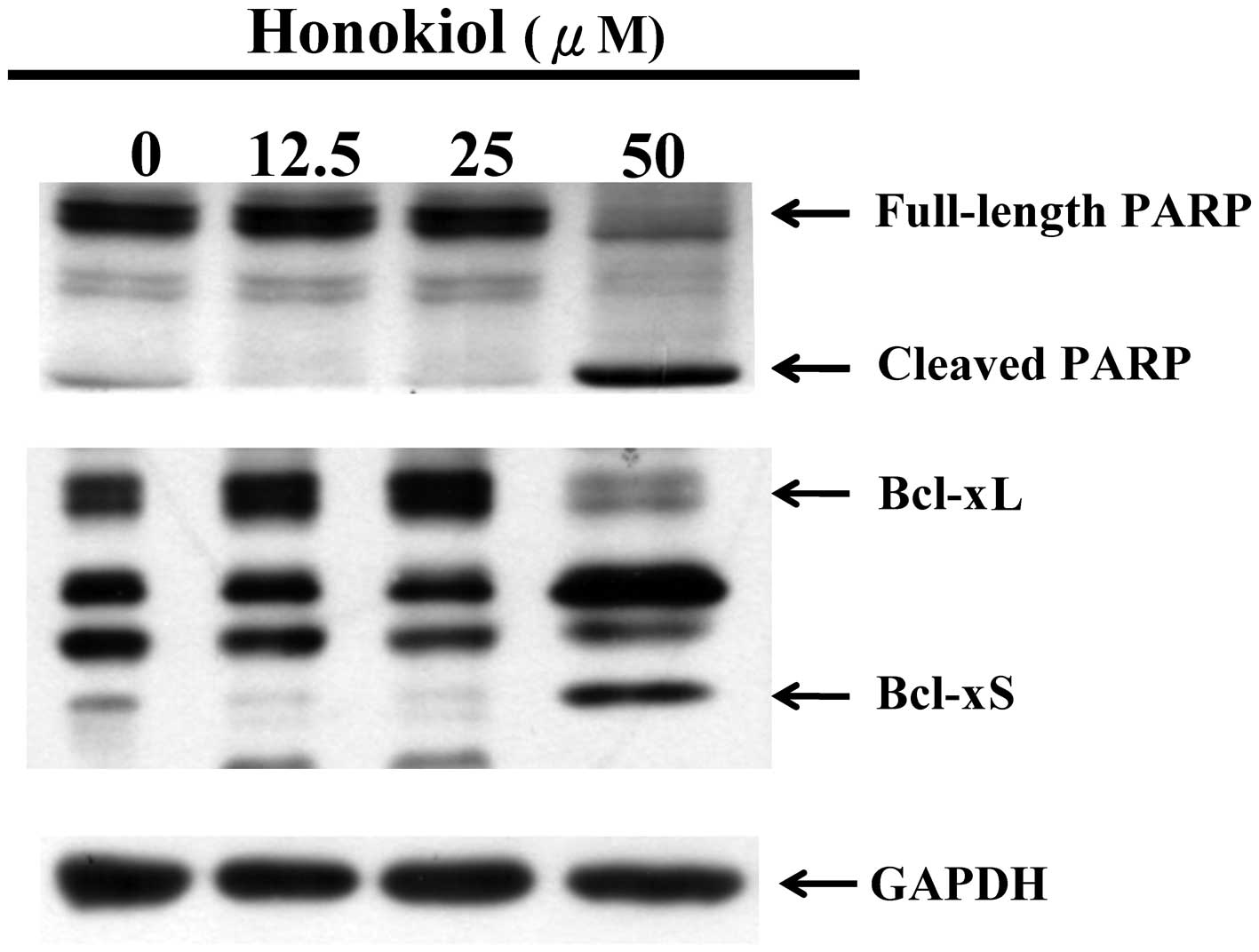
Fig. 2. Consistent with this, the
cleavage of PARP, an apoptotic marker, and anti-apoptotic protein
Bcl-x (S/L) were only observed in the cells that were treated with
50 μM honokiol (Fig. 4).
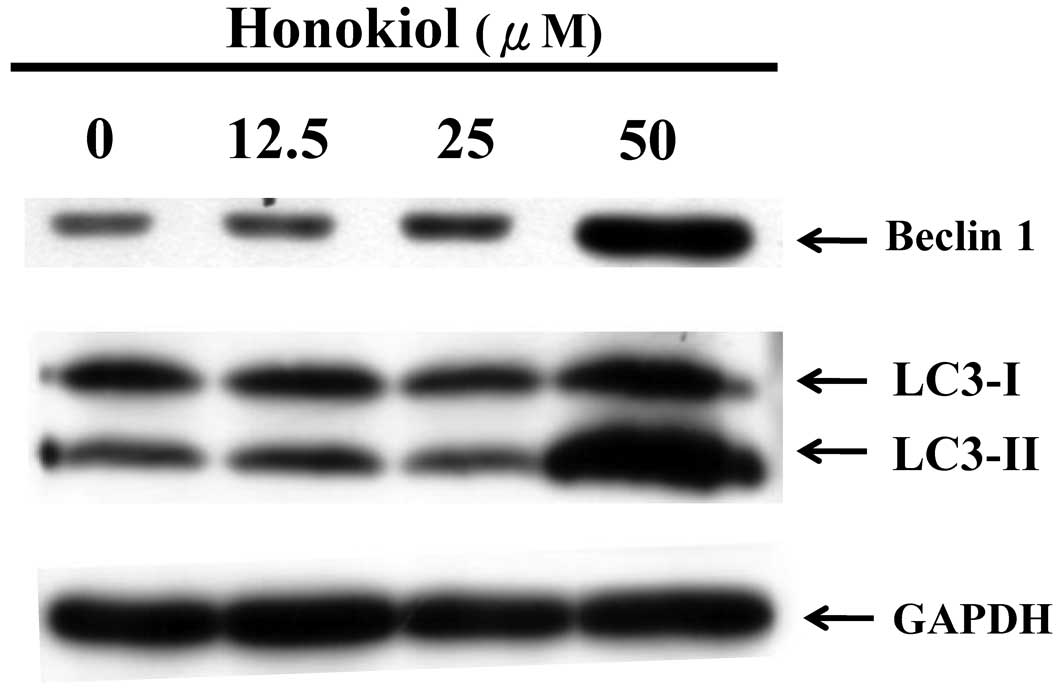
Honokiol increases the level of autophagy
markers in GBM cells
To investigate if autophagy was involved in the
honokiol-induced effects against GBM cells, the protein levels of
two hallmarks of autophagy, namely Beclin-1 and MAP1LC3A
(microtubule-associated protein 1A/1B light chain 3), were examined
in the honokiol-treated cells. Honokiol markedly increased the
levels of Beclin-1 and LC3-II at 50 μM (Fig. 5). Compared with the results that are
shown in Figs. 2–4, as in the induction of apoptosis,
autophagy was also markedly triggered by honokiol at a dose of 50
μM. The proautophagic effects of honokiol may play a crucial role
in the anticancer effects against GBM cells.
Discussion
To date, there have been no effective therapeutics
for the treatment of GBM. Innovative strategies and agents must be
investigated in order to conquer this life threatening disease.
Honokiol has been shown to exert antitumor effects
in various types of cancer in animals (15,16).
Furthermore, the toxicity of honokiol in normal peripheral blood
mononuclear cells (PBMNCs) or primary cultured human cells is
relatively low (17). As honokiol
has been demonstrated to be able to cross the BBB and inhibit the
growth of glioma in animal models, it may be regarded as a
potential innovative therapy for treating GBM. However, little is
known about the molecular mechanisms underlying the effects against
glioma cells (14).
The present study explored the effects of honokiol
in DBTRG-05MG GBM cells and demonstrated that type I (apoptosis)
and II (autophagy) programmed cell death were involved in the
effects against the cells. Consistent with other studies, the
present data revealed that honokiol was able to induce apoptosis in
the GBM cells, as reflected by the appearance of a massive
apoptotic sub-G1 fraction. Hyperphosphorylation is able
to inactivate the inhibitory effect of Rb on the E2F-1
transcription factor, leading to the progression of the cell cycle.
The marked inhibition of phospho-Rb by honokiol at 25 μM may
contribute to the significant decrease of GBM cell number at this
dose without the significant induction of apoptosis. Chau and Wang
(18) reported that the loss of
phospho- and total Rb protein may sensitize cells to the induction
of apoptosis. Similarly, in the present study, honokiol decreased
the level of phospho- and total Rb proteins during the induction of
apoptosis at a dose of 50 μM. In addition, the cleavage of PARP and
anti-apoptotic protein Bcl-x (S/L) at this dose further confirmed
this phenomenon. According to the literature, the cleavage of Bcl-x
(S/L) is dependent on the activation of caspase-3-like protease
(19,20). Thus, the cleaved Bcl-x (S/L) that
was observed in the results of the present study indicated that
caspase-3-dependent apoptosis may have occurred in the
honokiol-treated GBM cells.
In addition to apoptosis, autophagy has been shown
to be a potential target for cancer therapy. Proautophagic drugs
are a promising class of compounds for counteracting tumor
progression by favoring cancer cell death (21). A variety of chemicals, including
temozolomide, the most effective agent for GBM treatment, have been
reported to induce autophagy (22).
In the present study, a marked honokiol-induced increase of the two
hallmarks of autophagy, Beclin-1 and LC3-II, indicated the
autophagy-inducing effects on the GBM cells. Depending on the
magnitude of the induction of autophagy, honokiol may exert the
effects of autophagic survival or cell death (23). Autophagy is a double-edged sword in
tumorigenesis (24,25). As the marked autophagy of GBM cells
was also triggered during the induction of apoptosis by honokiol at
a dose of 50 μM in the present study, the modulation of autophagy
by combining the compound with other agents may profoundly affect
honokiol-induced cell death or apoptosis. Therefore, further
investigation into a potential strategy combining the effects of
autophagic modulating agents with honokiol in GBM cells is
warranted.
Acknowledgements
This study was supported by a grant from the
Department of Health, Taiwan (no. DOH99-TD-C-111-008).
References
|
1
|
Maher EA, Furnari FB, Bachoo RM, Rowitch
DH, Louis DN, Cavenee WK and DePinho RA: Malignant glioma: genetics
and biology of a grave matter. Genes Dev. 15:1311–1333. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morandi E, Severini C, Quercioli D, D’Ario
G, Perdichizzi S, Capri M, Farruggia G, Mascolo MG, Horn W, Vaccari
M, Serra R, Colacci A and Silingardi P: Gene expression time-series
analysis of camptothecin effects in U87-MG and DBTRG-05
glioblastoma cell lines. Mol Cancer. 7:662008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chang LF, Lin PC, Ho LI, Liu PY, Wu WC,
Chiang IP, Chang HW, Lin SZ, Harn YC, Harn HJ and Chiou TW:
Overexpression of the orphan receptor Nur77 and its translocation
induced by PCH4 may inhibit malignant glioma cell growth and induce
cell apoptosis. J Surg Oncol. 103:442–450. 2011. View Article : Google Scholar
|
|
4
|
Long JS and Ryan KM: New frontiers in
promoting tumour cell death: targeting apoptosis, necroptosis and
autophagy. Oncogene. 31:5045–5060. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Torres S, Lorente M, Rodríguez-Fornés F,
Hernández-Tiedra S, Salazar M, García-Taboada E, Barcia J, Guzmán M
and Velasco G: A combined preclinical therapy of cannabinoids and
temozolomide against glioma. Mol Cancer Ther. 10:90–103. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hahm ER, Arlotti JA, Marynowski SW and
Singh SV: Honokiol, a constituent of oriental medicinal herb
magnolia officinalis, inhibits growth of PC-3 xenografts in
vivo in association with apoptosis induction. Clin Cancer Res.
14:1248–1257. 2008.PubMed/NCBI
|
|
7
|
Bai X, Cerimele F, Ushio-Fukai M, Waqas M,
Campbell PM, Govindarajan B, Der CJ, Battle T, Frank DA, Ye K,
Murad E, Dubiel W, Soff G and Arbiser JL: Honokiol, a small
molecular weight natural product, inhibits angiogenesis in vitro
and tumor growth in vivo. J Biol Chem. 278:35501–35507. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Munroe ME, Arbiser JL and Bishop GA:
Honokiol, a natural plant product, inhibits inflammatory signals
and alleviates inflammatory arthritis. J Immunol. 179:753–763.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park J, Lee J, Jung E, Park Y, Kim K, Park
B, Jung K, Park E, Kim J and Park D: In vitro antibacterial and
anti-inflammatory effects of honokiol and magnolol against
Propionibacterium sp. Eur J Pharmacol. 496:189–195. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen F, Wang T, Wu YF, Gu Y, Xu XL, Zheng
S and Hu X: Honokiol: a potent chemotherapy candidate for human
colorectal carcinoma. World J Gastroenterol. 10:3459–3463.
2004.PubMed/NCBI
|
|
11
|
Wang T, Chen F, Chen Z, Wu YF, Xu XL,
Zheng S and Hu X: Honokiol induces apoptosis through
p53-independent pathway in human colorectal cell line RKO. World J
Gastroenterol. 10:2205–2208. 2004.PubMed/NCBI
|
|
12
|
Konoshima T, Kozuka M, Tokuda H, Nishino
H, Iwashima A, Haruna M, Ito K and Tanabe M: Studies on inhibitors
of skin tumor promotion, IX. Neolignans from Magnolia
officinalis. J Nat Prod. 54:816–822. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu D, Lu Q and Hu X: Down-regulation of
P-glycoprotein expression in MDR breast cancer cell MCF-7/ADR by
honokiol. Cancer Lett. 243:274–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Duan X, Yang G, Zhang X, Deng L,
Zheng H, Deng C, Wen J, Wang N, Peng C, Zhao X, Wei Y and Chen L:
Honokiol crosses BBB and BCSFB, and inhibits brain tumor growth in
rat 9L intracerebral gliosarcoma model and human U251 xenograft
glioma model. PLoS One. 6:e184902011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chilampalli S, Zhang X, Fahmy H, Kaushik
RS, Zeman D, Hildreth MB and Dwivedi C: Chemopreventive effects of
honokiol on UVB-induced skin cancer development. Anticancer Res.
30:777–783. 2010.PubMed/NCBI
|
|
16
|
Li Z, Liu Y, Zhao X, Pan X, Yin R, Huang
C, Chen L and Wei Y: Honokiol, a natural therapeutic candidate,
induces apoptosis and inhibits angiogenesis of ovarian tumor cells.
Eur J Obstet Gynecol Reprod Biol. 140:95–102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Battle TE, Arbiser J and Frank DA: The
natural product honokiol induces caspase-dependent apoptosis in
B-cell chronic lymphocytic leukemia (B-CLL) cells. Blood.
106:690–697. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chau BN and Wang JY: Coordinated
regulation of life and death by RB. Nat Rev Cancer. 3:130–138.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fujita N, Nagahashi A, Nagashima K,
Rokudai S and Tsuruo T: Acceleration of apoptotic cell death after
the cleavage of Bcl-XL protein by caspase-3-like proteases.
Oncogene. 17:1295–1304. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Itoh M, Chiba H, Noutomi T, Takada E and
Mizuguchi J: Cleavage of Bax-alpha and Bcl-x(L) during
carboplatin-mediated apoptosis in squamous cell carcinoma cell
line. Oral Oncol. 36:277–285. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lefranc F, Facchini V and Kiss R:
Proautophagic drugs: a novel means to combat apoptosis-resistant
cancers, with a special emphasis on glioblastomas. Oncologist.
12:1395–1403. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eimer S, Belaud-Rotureau MA, Airiau K,
Jeanneteau M, Laharanne E, Véron N, Vital A, Loiseau H, Merlio JP
and Belloc F: Autophagy inhibition cooperates with erlotinib to
induce glioblastoma cell death. Cancer Biol Ther. 11:1017–1027.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang R, Zeh HJ, Lotze MT and Tang D: The
Beclin 1 network regulates autophagy and apoptosis. Cell Death
Differ. 18:571–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu WK, Coffelt SB, Cho CH, Wang XJ, Lee
CW, Chan FK, Yu J and Sung JJ: The autophagic paradox in cancer
therapy. Oncogene. 31:939–953. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen N and Karantza V: Autophagy as a
therapeutic target in cancer. Cancer Biol Ther. 11:157–168. 2011.
View Article : Google Scholar : PubMed/NCBI
|