Introduction
Signal transducers and activators of transcription
(STAT) proteins are transcription factors that regulate critical
cell functions. STAT3, in particular, is constitutively active in a
wide variety of human cancer cells and plays significant roles
cancer cell growth (1–6). STAT3 has the ability to promote
malignancy, and deficiency in STAT3 leads to the resistance to
transformation. Thus, STAT3 is considered to be an oncogene
(7–10). Blocking STAT3 signaling through
STAT3 antisense oligonucleotides or RNA interference may induce
growth arrest and apoptosis in various tumor types. Blocking STAT3
is neither harmful nor toxic to normal cells (11–13),
providing further evidence for the potential of STAT3 as a target
for cancer treatment.
Numerous efforts have been made to identify
effective agents against cancer, particularly from herbal
medicines. In China, traditional Chinese medicine has been widely
used in cancer therapy, and has been shown to effectively control
cancer progression and prolong survival times in cancer patients.
Based on the principles of traditional Chinese medicine and the
progress in the pharmacological characterization of anticancer
herbs, we developed a saponin from Veratrum (Prosapogenin A)
for the treatment of cancer.
Veratrum is a type of traditional Chinese
medicine. Previous research has shown that Veratrum
significantly inhibits the growth of human leukemia HL-60, human
gastric carcinoma BGC-823, human liver carcinoma BEL-7402 and human
nasopharyngeal carcinoma KB (14–16)
cells.
In the present study, it was observed that PSA from
Veratrum inhibited proliferation and induced apoptosis in
human cervical carcinoma (HeLa), hepatocellular carcinoma (HepG2)
and breast adenocarcinoma (MCF-7) cells. PSA induced cell cycle
arrest in the G2/M phase in the HepG2 cells, together
with the downregulation of STAT3 expression and the modulation of
STAT3 downstream genes. PSA also modulated the expression of
glycometabolism-related genes.
Materials and methods
Materials and reagents
RPMI-1640, fetal bovine serum (FBS),
penicillin-streptomycin, trypsin-EDTA and TRIzol reagent were
purchased from Invitrogen Life Technologies (Carlsbad, CA, USA).
MTT, Hoechst 33342 and propidium iodide (PI) were obtained from
Sigma (St. Louis, MO, USA). The RT-PCR kit was provided by Promega
(Madison, WI, USA). STAT3, pSTAT3, β-actin primary antibodies and
horseradish peroxidase (HRP)-conjugated secondary antibodies were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA). A
Human STAT3-Regulated cDNA Plate Array kit (AP-0151) was purchased
from Signosis, Inc. (Santa Clara, CA, USA). PSA was extracted from
Veratrum using 70% ethanol and then separated, purified and
characterized.
Cell culture and cell viability
Cell lines were obtained from the American Type
Culture Collection (Manassas, VA, USA). Human cervical carcinoma
(HeLa), hepatocellular carcinoma (HepG2), breast adenocarcinoma
(MCF-7) and human non-cancer 7701 and 293 cells were cultured in
RPMI-1640 supplemented with 10% FBS and penicillin/streptomycin
(100 U/ml) at 37°C in an atmosphere of 5% CO2. The cells
were exposed to PSA of varying concentrations (0–15 μM) for 24, 48
and 72 h. The ability to reduce survival was assessed as
IC50 values. Ginsenoside Rh2 was used as a positive
control.
Cell apoptosis and cell cycle
analysis
Cell apoptosis was studied by double staining with
Hoechst 33342 and PI and using Array Scan VTIHCS600 High-Contents
(Thermo Scientific, Waltham, MA, USA). The cells were incubated in
96-well plates in either the presence (5 or 10 μM) or absence of
PSA for 48 h. The cells were then incubated with 5 mg/l Hoechst
33342 for 10 min and 5 mg/l PI for another 1 h in the dark,
followed by washing twice with ice-cold PBS and subjection to Array
Scan VTIHCS600 High-Contents to record the red fluorescence
produced by the PI. The cells treated with Rh2 were used as a
positive control.
To analyze the cell cycle profile, cells treated
with PSA (0, 5 or 10 μM) for 48 h were fixed with 70% ethanol at
4°C for 12 h and then stained in PI solution (50 μg/ml PI, 100
μg/ml RNase A and 0.2% v/v Triton X-100) for 20 min at 37°C. The
stained cells were then subjected to DNA content analysis by flow
cytometric analysis using FACScalibur flow cytometer (BD
Biosciences, San Jose, CA, USA). and the data obtained were
processed using Summit software.
RNA extraction and RT-PCR analysis
Total RNA was isolated from the cancer cells using
TRIzol reagent. Oligo(dT)-primed RNA (1 μg) was reverse-transcribed
and the obtained cDNA was used to determine the mRNA quantity of
STAT3, using the RT-PCR kit according to the manufacturer’s
instructions. β-actin was used as an internal control. The primers
used for amplification of the STAT3 and β-actin transcripts are as
follows: STAT3 forward, 5′-CCAAGGAGGAGGCATTCG-3′ and reverse,
5′-ACATCGGCAGGTCAATGG-3′; size, 147 bp; and β-actin forward,
5′-CTTCTACAATGAGCTGCGTG-3′ and reverse, 5′-TCATGAGGTAGTCAGTCAGG-3′;
size, 305 bp. The expression of the glycometabolism-related genes
was also detected by RT-PCR: GLUT1 forward,
5′-CAACGCTGTCTTCTATTACTC-3′ and reverse, 5′-GCCACGATGCTCAGATAG-3′;
size, 252 bp; HK forward, 5′-CCAGAAGGCTCAGAAGTC-3′ and reverse,
5′-ATGCTTGTCCAGGAAGTC-3′; size, 216 bp; PFKL forward,
5′-TCCGCATCTATGGTATTCAC-3′ and reverse, 5′-GTCTTCATC
TTCTCCGTCAT-3′; size, 400 bp.
Western blotting
The tumor cells were lysed by RIPA and centrifuged
at 12,000 × g for 10 min, followed by determination of the protein
concentration in the supernatants. Equal amounts of protein per
lysate were separated by SDS-PAGE and transferred to polyvinylidene
difluoride membranes, followed by blocking in Tris-buffered saline
(TBS) containing skimmed dry milk (3% w/v). The membranes were
incubated with the primary rabbit anti-human STAT3, pSTAT3 and
β-actin monoclonal antibodies (Cell Signaling Technology, Inc.)
diluted to 1:1,000 in PBS at 4°C overnight, then incubated with the
HRP-conjugated secondary antibody (goat anti-rabbit IgG, Cell
Signaling Technology, Inc.) This was followed by enhanced
chemiluminescence detection.
STAT3-regulated downstream gene cDNA
assay
STAT3 mediates the expression of its target genes,
which are involved in a diverse array of biological processes,
including oncogenesis, cell growth, differentiation and apoptosis.
A STAT3-regulated cDNA plate array (Signosis, Inc.) was used to
detect the expression of the STAT3 target genes. The cDNA plate
array is a plate-based hybridization profiling analysis for
monitoring the expression of dozens of genes through the reverse
transcription of mRNA into cDNA. Like array analyses, total RNA is
first reverse-transcribed into cDNA in the presence of biotin-dUTP
in the assay. Targeted genes are then specifically captured onto
individual wells on a plate through a pre-coated gene-specific
oligonucleotide. The captured cDNAs are further detected with
streptavidin-HRP. Luminescence is reported as relative light units
on a microplate luminometer. The expression level of the genes is
directly proportional to the luminescence intensity.
Statistical analysis
All data were expressed as the mean ± standard
deviation. Statistical differences between groups were analyzed by
one-way ANOVA then a post-hoc t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
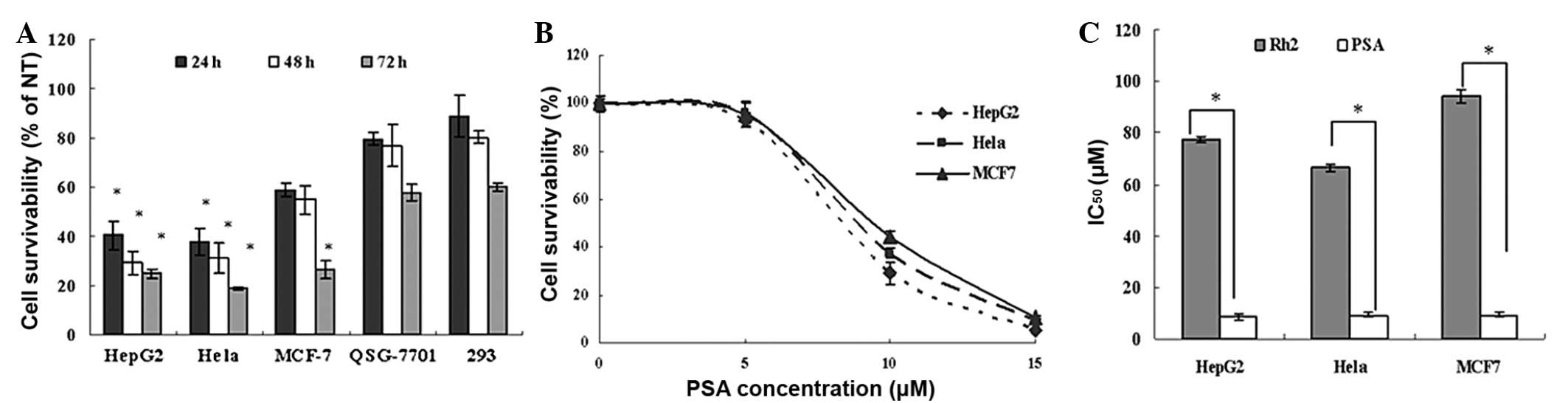
Effect of PSA on cell viability
To determine the effect of PSA and the positive
control Rh2 on a wider panel of human cancer cell lines, the cell
survival of three cancer cell lines of different origins [cervical
carcinoma (HeLa), hepatocellular carcinoma (Hep G2) and breast
adenocarcinoma (MCF-7) cells] was determined in comparison with
that of normal human hepatocyte 7701 and 293 cells. The effect of
10 μM PSA treatment for 24, 48 and 72 h on a range of cancer and
non-cancer cells was determined by MTT assay. The results showed
that 10 μM PSA significantly inhibited the growth of the cancer
cells in a time-dependent manner. Notably, PSA did not
significantly affect the survival of the human non-cancer 7701 and
293 (Fig. 1A) cells. The
concentration-response correlation between PSA (0–15 μM) and
reduced cell survival was also examined in the HeLa, HepG2 and
MCF-7 cells. PSA significantly inhibited the growth of the cancer
cells in a concentration-dependent manner (Fig. 1B). The IC50 values for
this compound were 8.41, 9.36 and 9.27 μM (all n=3) in the HepG2,
MCF7 and HeLa cells, respectively, and were significantly lower in
comparison with those for the positive control, Rh2 (Fig. 1C).
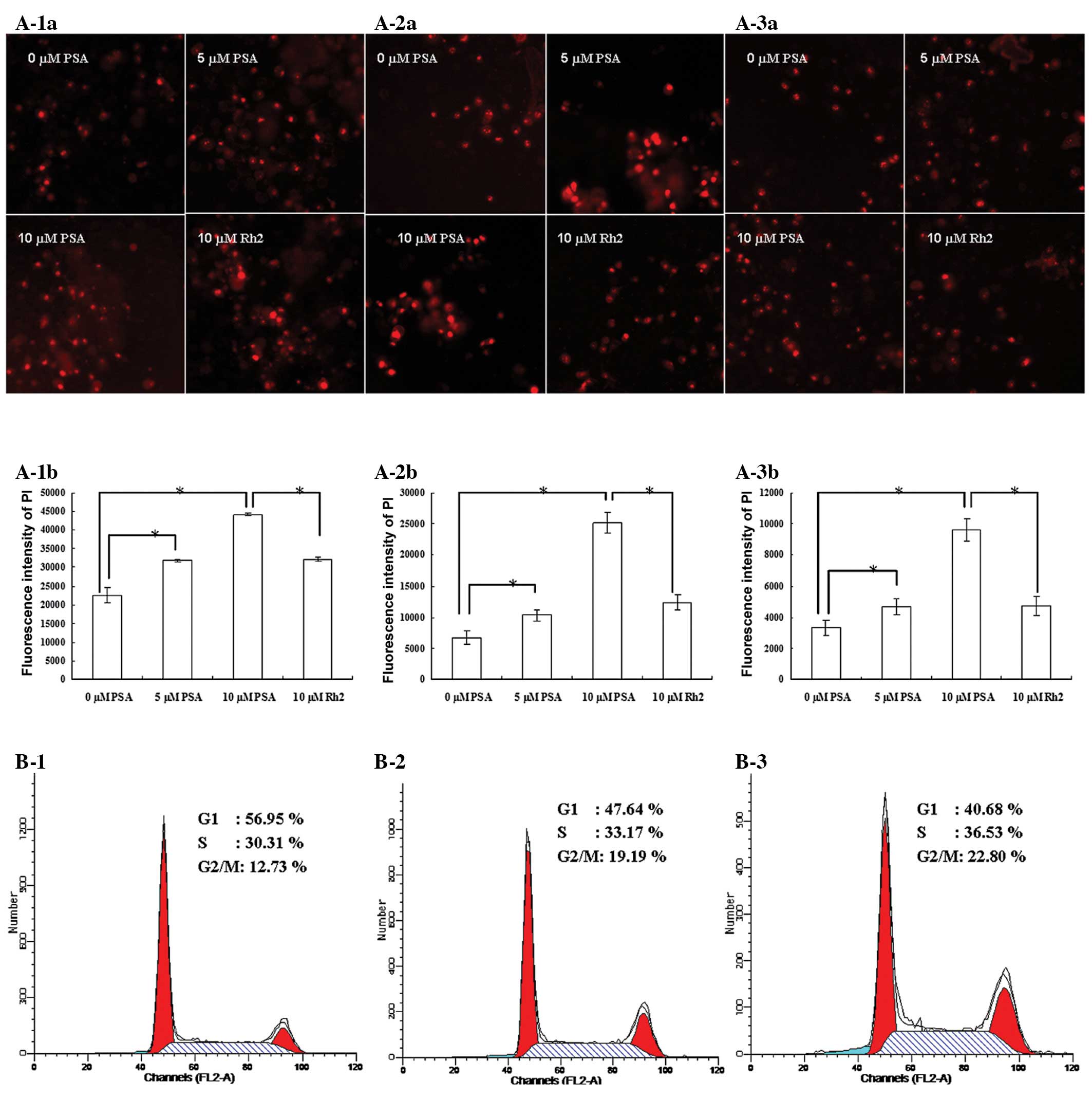
Apoptosis and cell cycle analysis
Apoptosis is programmed cell death that is
characterized by specific structural changes. PI and Hoechst 33342
double staining was used to detect the changes of apoptotic cells
treated with PSA and Rh2. The apoptosis-inducing effects of PSA
were assessed by Array Scan VTIHCS600 High-Contents. All three
cancer cell lines treated with 5 and 10 μM PSA for 48 h showed a
significant increase in apoptotic bodies compared with that in the
untreated group. By comparison, the apoptotic cell count in the
groups treated with 10 μM Rh2 was significantly lower compared with
that in the groups treated with 10 μM PSA (Fig. 2A).
The effect of PSA on the cells was also examined
using a cell cycle analysis. The effect of PSA on cell cycle
activity was analyzed by performing PI staining followed by
detection with flow cytometry. PSA induced cell cycle arrest in the
HepG2 cells in the G2/M phase (Fig. 2B). There was a significant
accumulation of cells in the G2/M phase for those cells
treated with 5 and 10 μM of PSA (19.19 and 22.80%, respectively)
compared with the untreated cells (12.73%). This indicates that
there is a blockage in the G2/M phase, which may cause
cell growth suppression or apoptosis by inhibiting the mitosis of
cells.
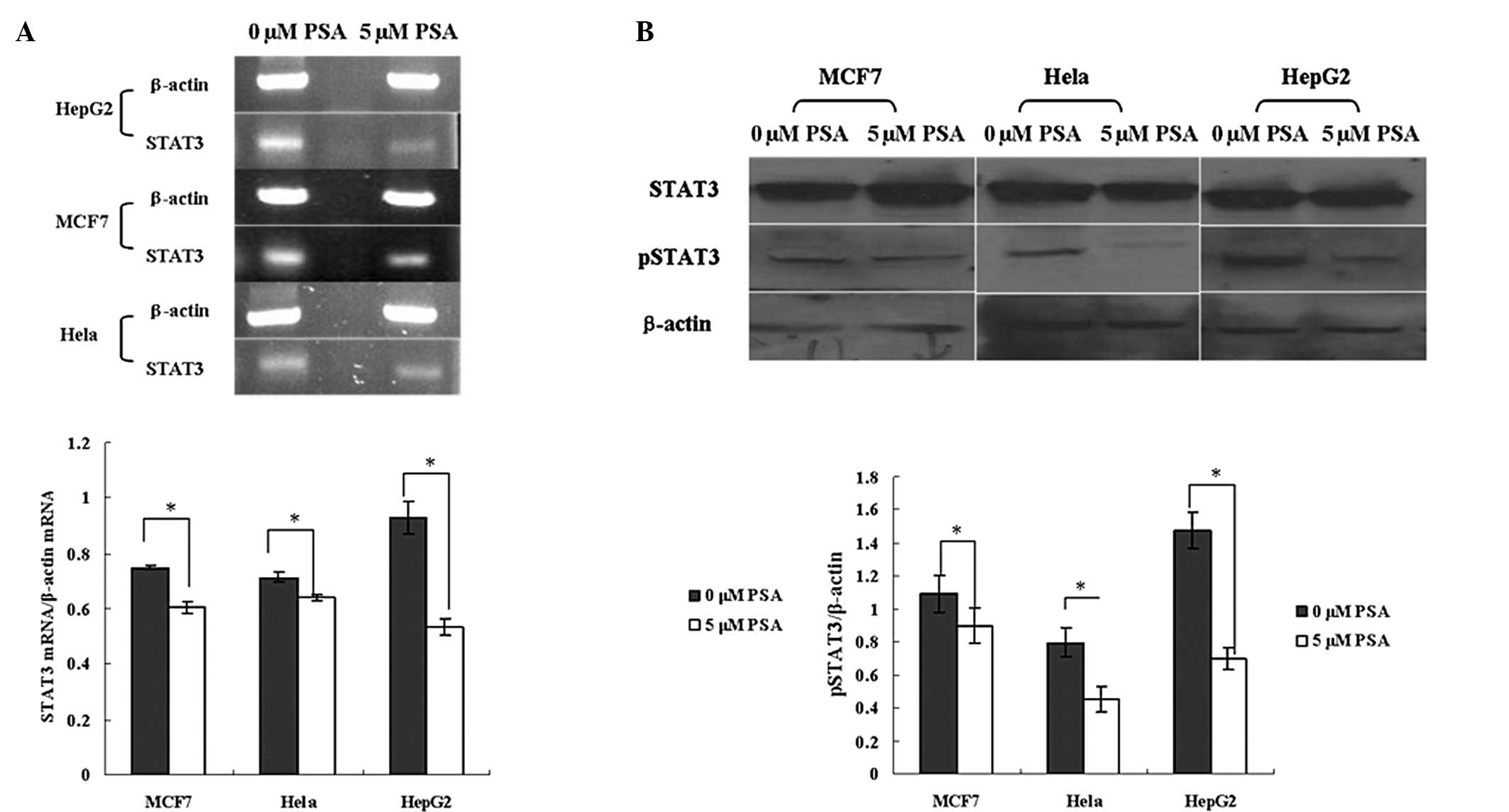
PSA inhibits the expression of STAT3 mRNA
and pSTAT3
The expression of STAT3 mRNA and pSTAT3 in the
HepG2, HeLa and MCF7 cells was significantly decreased by PSA
treatment. The RT-PCR results showed that 5 μM PSA decreased the
STAT3 mRNA level of the HepG2, MCF7 and HeLa cells by ~42, 19 and
10%, respectively (Fig. 3A). PSA
did not significantly affect the expression of the STAT3 protein,
but 5 μM PSA significantly decreased the level of pSTAT3 (Fig. 3B). The results of these experiments
therefore indicate that the anticancer effect of PSA is mediated by
the inhibition of the STAT3 pathway.
Effect of PSA on the expression of the
STAT3 target genes
The effect of PSA on the expression of the STAT3
target genes was analyzed with a Human STAT3-Regulated cDNA Plate
Array kit. The results showed that 5 μM PSA downregulated the ratio
of Bcl-2/Bax and the expression of survivin and glycoprotein 130
(gp130), and upregulated the expression of c-myc, C-reactive
protein (CRP), cyclin E, glycogen synthase kinase 3β (GSK-3β),
IL-10, oncostatin M (OSM), p21 and p27, subsequently promoting cell
apoptosis. Therefore, PSA is able to promote cell apoptosis by
inhibiting the STAT3 pathway.
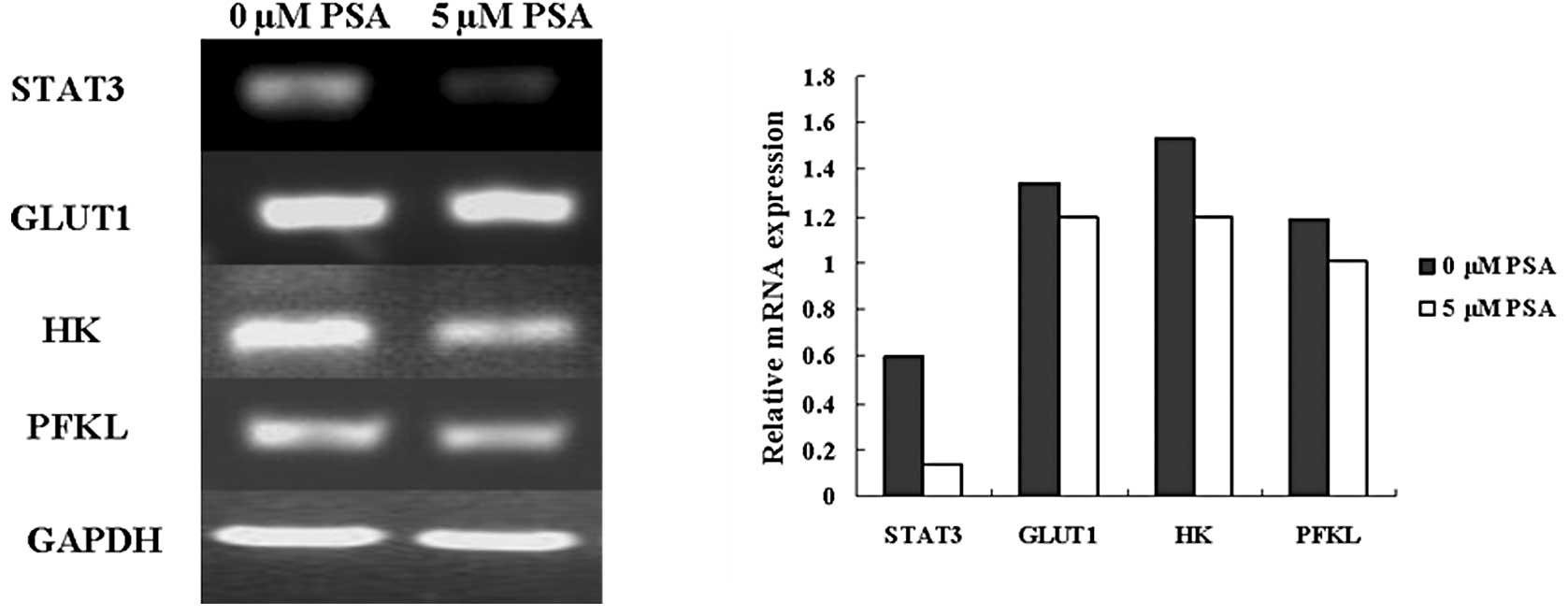
Effect of PSA on glycometabolism-related
genes
Cancer cells utilize the glycolytic pathway to
maintain cell growth even when adequate oxygen is present; this is
known as the Warburg effect (17).
Glycolysis inhibition is a potential therapeutic modality. The
present study examined whether PSA affected the expression of the
glycometabolism-related genes, glucose transporter 1 (GLUT1),
hexokinase (HK) and phosphofructokinase (PFKL). The western
blotting results showed that 5 μM PSA reduced the expression of the
STAT3, GLUT1, HK and PFKL mRNA (Fig.
5). Therefore, the PSA-induced inhibition of the glycometabolic
pathway may be another anticancer mechanism, and may be due to the
downregulation of STAT3.
Discussion
Chemotherapy remains one of the main therapeutic
approaches for cancer patients. However, the cytotoxicity against
normal cells limits the use of chemotherapeutic drugs. It is
therefore necessary to develop anticancer agents with minimal
side-effects. Traditional Chinese medicine has relatively fewer
side-effects and has been used to treat various diseases in
clinical medicine, including cancer, for numerous years (18–22).
As a well-known traditional Chinese medicine, Veratrum has
shown significant anticancer effects in a variety of carcinomas
(14–16).
The transcription factor STAT3 is, as an oncogene,
crucial for the regulation of cancer cell apoptosis and
proliferation. The phosphorylation of STAT3 at tyrosine 705 results
in its homodimerization, nuclear translocation and DNA binding,
which consequently regulates the expression of various critical
genes involved in cell proliferation and apoptosis (23–25).
The constitutive activation of STAT3 results in the development of
various types of cancer. Therefore, the regulation of the STAT3
pathway and the expression of its target genes has been a promising
area for the development of anticancer therapies.
The present study demonstrated a novel anticancer
effect of PSA from Veratrum on cervical carcinoma (HeLa),
hepatocellular carcinoma (HepG2) and breast adenocarcinoma (MCF-7)
cells, and provided data to indicate its possible mechanism.
Several new points may consequently be reported. First, PSA was
demonstrated to cause a concentration- and time-dependent reduction
in cancer cell survival. PSA caused a smaller reduction in the
survival of normal, i.e. non-cancer, cells. Second, PSA was
identified to be able to induce a large increase in the number of
apoptotic cells, at the same time as a clear cell cycle arrest in
the G2/M phase. Third, PSA was shown to inhibit the
expression of STAT3 mRNA and the phosphorylation of the STAT3
protein. Fourth, PSA was demonstrated to largely suppress the STAT3
downstream genes, Bcl-2/Bax, survivin and gp130, and cause clear
elevation in the expression of c-myc, CRP, cyclin E, GSK-3β, IL-10,
OSM, p21 and p27, subsequently promoting cell apoptosis. Finally,
PSA regulated the expression of the glycometabolism-related genes
to inhibit cell growth and promote cell apoptosis.
In conclusion, PSA exhibits clear anticancer cell
activity in vitro. PSA is able to block the STAT3 pathway
and modulate the expression of glycometabolism-related genes,
resulting in a reduction in cell proliferation and an increase in
cell apoptosis.
Acknowledgements
The authors would like to acknowledge the financial
assistance provided by a grant from the Key Science and Technology
Fund of Henan Province (No. 122102310558) in China.
References
|
1
|
Lin L, Hutzen B, Zuo M, Ball S, Deangelis
S, et al: Novel STAT3 phosphorylation inhibitors exhibit potent
growth-suppressive activity in pancreatic and breast cancer cells.
Cancer Res. 70:2445–2454. 2010. View Article : Google Scholar
|
|
2
|
Hutzen B, Friedman L, Sobo M, Lin L, Cen
L, et al: Curcumin analogue GO-Y030 inhibits STAT3 activity and
cell growth in breast and pancreatic carcinomas. Int J Oncol.
35:867–872. 2009.PubMed/NCBI
|
|
3
|
Groner B, Lucks P and Borghouts C: The
function of Stat3 in tumor cells and their microenvironment. Semin
Cell Dev Biol. 19:341–350. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brantley EC and Benveniste EN: Signal
transducer and activator of transcription-3: a molecular hub for
signaling pathways in gliomas. Mol Cancer Res. 6:675–684. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu H, Pardoll D and Jove R: STATs in
cancer inflammation and immunity: a leading role for STAT3. Nat Rev
Cancer. 9:798–809. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu A, Liu Y, Xu Z, Yu W, Wang H, et al:
Novel small molecule, XZH-5, inhibits constitutive and
interleukin-6-induced STAT3 phosphorylation in human
rhabdomyosarcoma cells. Cancer Sci. 102:1381–1387. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bromberg JF, Horvath CM, Besser D, Lathem
WW and Darnell JE Jr: Stat3 activation is required for cellular
transformation by v-src. Mol Cell Biol. 18:2553–2558.
1998.PubMed/NCBI
|
|
8
|
Schlessinger K and Levy DE: Malignant
transformation but not normal cell growth depends on signal
transducer and activator of transcription 3. Cancer Res.
65:5828–5834. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Inghirami G, Chiarle R, Simmons WJ, Piva
R, Schlessinger K and Levy DE: New and old functions of STAT3: a
pivotal target for individualized treatment of cancer. Cell Cycle.
4:1131–1133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bromberg JF, Wrzeszczynska MH, Devgan G,
Zhao Y, Pestell RG, et al: Stat3 as an oncogene. Cell. 98:295–303.
1999. View Article : Google Scholar
|
|
11
|
Buettner R, Mora LB and Jove R: Activated
STAT signaling in human tumors provides novel molecular targets for
therapeutic intervention. Clin Cancer Res. 8:945–954.
2002.PubMed/NCBI
|
|
12
|
Turkson J and Jove R: STAT proteins: novel
molecular targets for cancer drug discovery. Oncogene.
19:6613–6626. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiong H, Zhang ZG, Tian XQ, Sun DF, Liang
QC, et al: Inhibition of JAK1, 2/STAT3 signaling induces apoptosis,
cell cycle arrest, and reduces tumor cell invasion in colorectal
cancer cells. Neoplasia. 10:287–297. 2008.PubMed/NCBI
|
|
14
|
Tang J, Li HL, Shen YH, et al: Antitumor
and antiplatelet activity of alkaloids from veratrum
dahuricum. Phytother Res. 24:821–826. 2010.PubMed/NCBI
|
|
15
|
Ivanova A, Serly J, Christov V, et al:
Alkaloids derived from genus Veratrum and Peganum of
Mongolian origin as multidrug resistance inhibitors of cancer
cells. Fitoterapia. 82:570–575. 2011.
|
|
16
|
Tang J, Li HL, Shen YH, et al: Antitumor
activity of extracts and compounds from the rhizomes of Veratrum
dahuricum. Phytother Res. 22:1093–1096. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wittig R and Coy JF: The role of glucose
metabolism and glucose-associated signalling in cancer. Perspect
Medicin Chem. 1:64–82. 2007.PubMed/NCBI
|
|
18
|
Zhao J, Jiang P and Zhang W: Molecular
networks for the study of TCM Pharmacology. Brief Bioinform.
11:417–430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pon D, Wang Z, Le KN and Chow MS:
Harnessing traditional Chinese medicine to improve cancer therapy:
issues for future development. Ther Deliv. 1:335–344. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hsiao WL and Liu L: The role of
traditional Chinese herbal medicines in cancer therapy - from TCM
theory to mechanistic insights. Planta Med. 76:1118–1131. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen ZF and Liang H: Progresses in TCM
metal-based antitumour agents. Anticancer Agents Med Chem.
10:412–423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Darnell JE Jr: STATs and gene regulation.
Science. 277:1630–1635. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zushi S, Shinomura Y, Kiyohara T, et al:
STAT3 mediates the survival signal in oncogenic ras-transfected
intestinal epithelial cells. Int J Cancer. 78:326–330. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Masuda M, Suzui M, Yasumatu R, et al:
Constitutive activation of signal transducers and activators of
transcription 3 correlates with cyclin D1 overexpression and may
provide a novel prognostic marker in head and neck squamous cell
carcinoma. Cancer Res. 62:3351–3355. 2002.
|