Introduction
Hepatocellular carcinoma (HCC) is a major health
problem worldwide. It is the fifth most common type of cancer in
males and the seventh most common in females, as well as the third
most common cause of cancer-related mortality (1–3).
Chronic hepatitis B (CHB) is the leading cause of HCC development
in Asia, although Japan has one of the lowest prevalence rates for
CHB among Asian countries (4). Each
year, more than 50 million people are infected with HBV worldwide
and more than 1 million deaths are attributed to HBV-related
complications, including liver cirrhosis and HCC (5,6).
In the majority of HCC patients, successful
treatment of HCC is followed by recurrence, leading to high
mortality rates (7). Thus, the
prediction of HCC recurrence and the performance of appropriate
therapy for HCC recurrence after initial treatment are essential
for the optimization of clinical outcomes (8).
Lamivudine (LAM) was the first nucleoside analog
(NA) introduced for the treatment of CHB. In several clinical
trials, it showed superior efficacy compared with the placebo in
terms of HBV DNA suppression, hepatitis B e antigen (HBeAg)
seroconversion and alanine aminotransferase (ALT) normalization
(9,10). However, a major limitation of LAM
therapy is the development of resistance, which occurs in up to 70%
of patients within 4 years of therapy (11). Adefovir (ADV) is not cross-resistant
with LAM and has been used for the treatment of CHB. However, in
two pivotal phase III clinical trials of ADV for patients with CHB,
among subjects who received a 10-mg dose once daily, only 21% of
HBeAg-positive patients and 51% of HBeAg-negative patients achieved
a serum HBV DNA level of <400 copies/ml at 48 weeks (12,13).
Entecavir (ETV) is a cyclopentyl guanosine analog
that has demonstrated superior virological, biochemical and
histological effects as compared with those of LAM and ADV in large
randomized controlled trials, and is now widely used as a first
choice NA with the purpose of improving clinical outcome in CHB
patients (14–18). For LAM-treated patients with no
viral breakthrough, switching therapy to ETV is also recommended
(19). In addition, it has been
shown recently that ETV is able to reduce the risk of HCC
occurrence and liver-related mortality in CHB patients (20,21).
However, to the best of our knowledge, predictive factors in
HBV-related HCC patients treated with ETV who have undergone
curative therapy remain unclear, and it is essential for clinicians
to examine these factors to optimize their clinical outcomes.
Therefore, the aims of the present study were to elucidate the
prognostic factors in patients with HBV-related HCC treated with
ETV who underwent curative therapy.
Patients and methods
Patients
A total of 131 treatment-naïve HBV-related HCC
patients received curative therapy at Osaka Red Cross Hospital
(Osaka, Japan) between January 2001 and November 2012. They were
all positive for HB surface antigen (HBsAg) and negative for
anti-HCV (HCV Ab). Curative therapy was defined as therapy
resulting in no apparent viable tumor on a dynamic computed
tomography (CT) performed within one month after initial treatment
for HCC. Following diagnosis of HCC, the most appropriate
therapeutic procedure was selected after discussions with surgeons
and physicians, according to the tumor characteristics and
underlying liver functional reserve of each patient. Of the
aforementioned 131 treatment-naïve HBV-related HCC patients, 32 did
not receive NA therapy, 69 received ETV monotherapy, 18 received
ADV add-on treatment having converted from LAM monotherapy due to
breakthrough hepatitis, 5 received ETV monotherapy having switched
from LAM monotherapy and 7 received LAM monotherapy (19,22,23).
Thus, a total of 74 HBV-related HCC patients treated with ETV were
analyzed in the present study. Predictive factors linked to overall
survival (OS) and recurrence-free survival (RFS) rates were
examined.
Written informed consent was obtained from all
patients prior to each therapy, and the study protocol complied
with all the provisions of the Declaration of Helsinki. This study
was approved by the Ethics Committee of Osaka Red Cross Hospital,
Japan, and the need for written informed consent was waived as the
data were analyzed retrospectively and anonymously. The present
study comprised a retrospective analysis of patient records
registered in our database, and all treatments were conducted in an
open-label manner.
HCC and liver cirrhosis (LC)
diagnosis
HCC was diagnosed using abdominal ultrasound and
dynamic CT scans (hyperattenuation during the arterial phase in all
or some part of the tumor and hypoattenuation in the portal-venous
phase), and/or magnetic resonance imaging (MRI), mainly based on
the recommendations of the American Association for the Study of
Liver Diseases (24). Arterial- and
portal-phase dynamic CT images were obtained at ~30 and 120 sec,
respectively, after the injection of the contrast material. HCC
stage was determined using the Liver Cancer Study Group of Japan
staging system (25). HCC was
confirmed pathologically only in patients who underwent surgery. LC
was determined by specimens at surgery, imaging modalities or
portal hypertension, such as esophageal varices and
splenomegaly.
Serological studies
HBsAg, HCV Ab, HBeAg and HBeAb were detected using
commercial enzyme immunoassay kits (Architect, Dainabot, Tokyo,
Japan; Lumipulse; Fujirebio Inc, Tokyo, Japan). HBV DNA levels were
quantified using the COBAS® Amplicor HBV Monitor Test
(Roche Diagnostics, Tokyo, Japan), which has a dynamic range of
2.6–7.6 log copies/ml, or the COBAS TaqMan® HBV Test
(version 2.0; Roche Diagnostics), which has a dynamic range of over
2.1–9.0 log copies/ml.
Follow-up
Follow-up after each therapy consisted of periodic
blood tests and monitoring of tumor markers, including
α-fetoprotein (AFP) and des-γ-carboxy prothrombin (DCP), using
chemiluminescent enzyme immunoassays (Lumipulse PIVKA-II Eisai;
Eisai, Inc., Tokyo, Japan). Dynamic CT scans and/or MRI were
obtained every 2–4 months after each therapy. Chest CT, whole
abdominal CT, brain MRI and bone scintigraphy were performed when
extrahepatic HCC recurrence was suspected.
Statistical analysis
Data were analyzed using univariate and multivariate
analyses. Time to recurrence was defined as the interval between
each therapy and the first confirmed recurrence. For analysis of
RFS, follow-up ended at the time of first recurrence; other
patients were censored at their last follow-up visit or at the time
of death from any cause without recurrence. For analysis of OS,
follow-up ended at the time of death from any cause, and the
remaining patients were censored at the last follow-up visit. The
cumulative OS and RFS rates were calculated using the Kaplan-Meier
method and tested using the log-rank test. Factors with P<0.2 in
the univariate analysis were subjected to multivariate analysis
using the Cox proportional hazards model. These statistical methods
were used to estimate the interval from the initial treatment for
HCC. Data were analyzed using SPSS software (SPSS Inc., Chicago,
IL, USA) for Microsoft Windows. Data are expressed as the mean ±
standard deviation. Values of P<0.05 were considered to indicate
a statistically significant difference.
Results
Baseline characteristics
The baseline characteristics of the patients at
initial treatment for HBV-related HCC in the present study (n=74)
are shown in Table I. Patients
included 49 males and 25 females with a median age of 62 years. The
median observation period was 3.4 years (range, 0.2–11.5 years).
Surgical resection was performed in 30 patients (40.5%), and
percutaneous ablation therapy such as radiofrequency ablation (RFA)
and percutaneous ethanol injection (PEI) was performed in 44
patients (59.5%). Treatment procedure-related mortality was not
observed in any of the patients. NAs were being administered to 47
patients at the time of initial treatment for HCC, while the
remaining 27 patients had received NA therapy prior to initial
treatment. Thirty-five patients (47.3%) had a pre-treatment HBV DNA
level of >105 copies/ml and 20 patients (27.0%) had
HBeAg positivity at initial treatment for HCC.
 | Table IBaseline characteristics at initial
treatment (n=74). |
Table I
Baseline characteristics at initial
treatment (n=74).
| Variables at initial
therapy | No. or median
(range) |
|---|
| Age (years) | 62 (32–84) |
| Gender
(male/female) | 49/25 |
| HCC stage
(I/II/III) | 19/40/15 |
| Surgery/ablative
therapya | 30/44 |
| Maximum tumor size
(cm) | 2.3 (0.9–12.0) |
| Tumor number
(single/multiple) | 51/23 |
| Liver cirrhosis
(yes/no) | 41/33 |
| HBV DNA ≥
105 copies/ml (yes/no) | 35/39 |
| HBe antigen
(positive/negative) | 20/54 |
| AST (IU/l) | 37 (17–156) |
| ALT (IU/l) | 31 (8–209) |
| ALP (IU/l) | 308 (43–1446) |
| GGT (IU/l) | 45 (11–602) |
| Serum albumin
(g/dl) | 4.1 (3.0–4.7) |
| Total bilirubin
(mg/dl) | 0.8 (0.3–4.1) |
| Prothrombin time
(%) | 84 (52–129) |
| Platelets
(×104/mm3 ) | 12.1 (1.6–63.0) |
| AFP (ng/ml) | 18.4 (1.9–31720) |
| DCP (mAU/ml) | 28.5 (10–102190) |
| Diabetes mellitus
(yes/no) | 8/66 |
| Body mass index
(kg/m2) | 22.8 (16.7–36.6) |
Cumulative OS and RFS rates
The 1-, 3- and 5-year cumulative OS rates for all
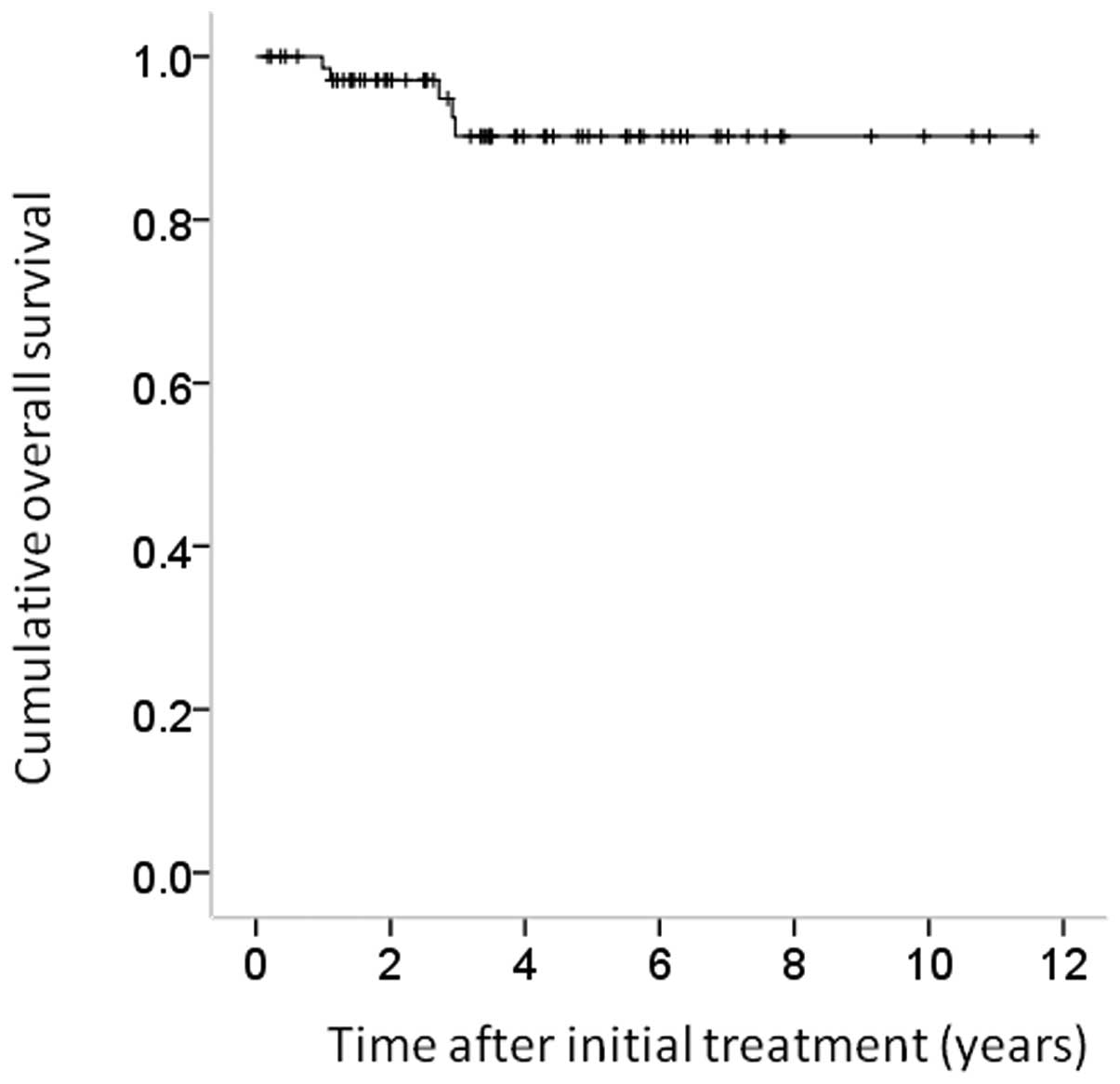
cases were 100, 89.8 and 89.8%, respectively (Fig. 1). The corresponding RFS rates for
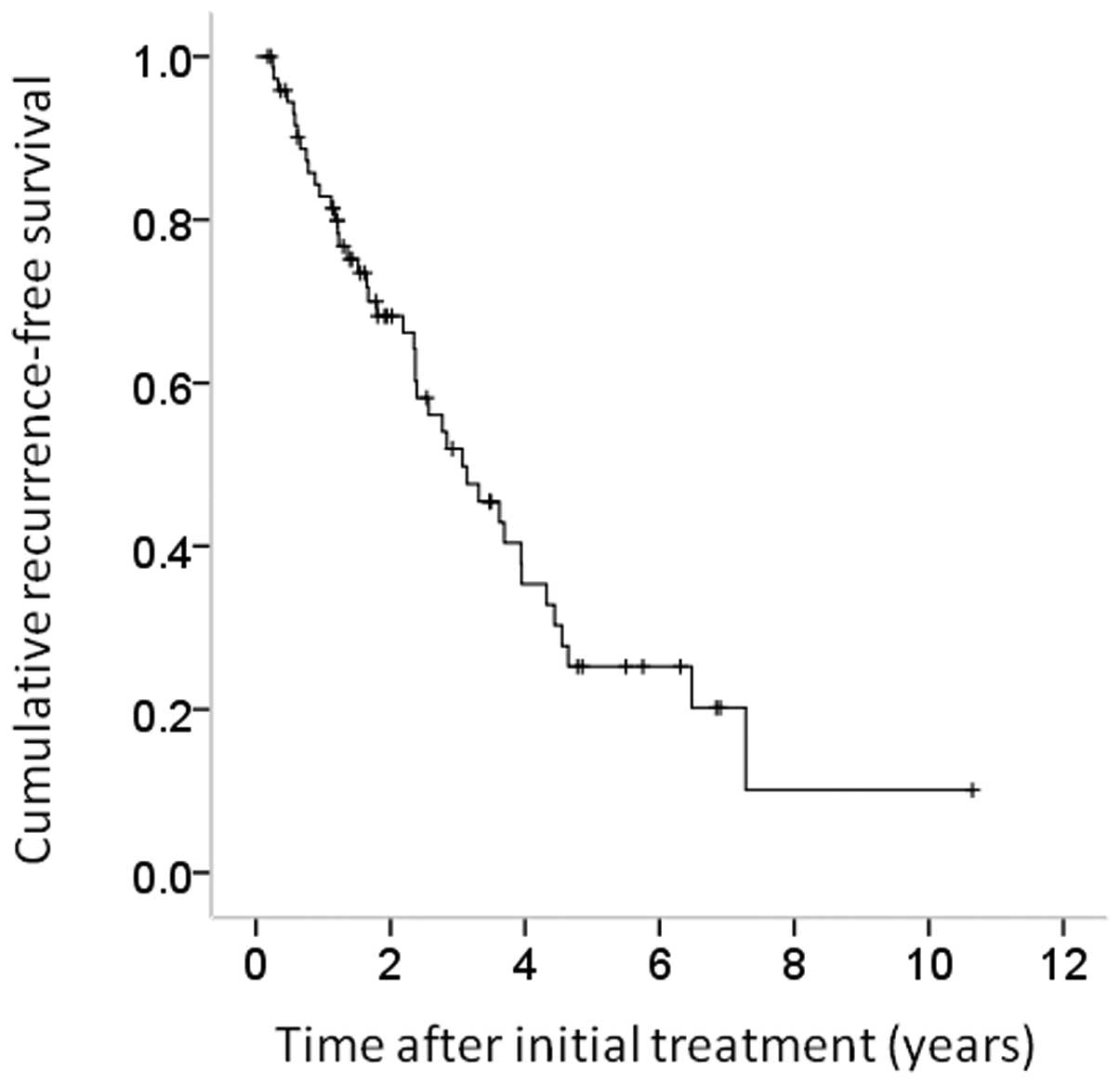
all cases were 82.8, 52.1 and 25.6%, respectively (Fig. 2).
Univariate and multivariate analyses of
factors contributing to OS
Univariate analysis identified HbeAg positivity
(P=0.003) as the only factor significantly associated with OS for
all cases (n=74) (Table II). The
hazard ratios (HRs) and 95% CIs calculated using multivariate
analysis for the five factors with P<0.2 in the univariate
analysis are detailed in Table II.
Only HBeAg positivity (P=0.020) was revealed to be a significant
predictor of OS in the multivariate analysis.
 | Table IIUnivariate and multivariate analysis
contributing to overall survival. |
Table II
Univariate and multivariate analysis
contributing to overall survival.
| | Univariate
analysis | Multivariate
analysis |
|---|
| |
|
|
|---|
| Variables at initial
treatment | No. | P-valuea | Hazard ratio (95%
CI) | P-valueb |
|---|
| Gender (male vs.
female) | 49/25 | 0.572 | | |
| Age (years) (≥60
vs. <60) | 43/31 | 0.910 | | |
| HCC stage (I or II
vs. III) | 59/15 | 0.131 | 0.143
(0.009–2.327) | 0.172 |
| Maximum tumor size
(cm) (≥2.5 vs. <2.5) | 32/42 | 0.927 | | |
| Tumor number
(single vs. multiple) | 23/51 | 0.096 | 0.777
(0.078–7.776) | 0.830 |
| Liver cirrhosis
(yes vs. no) | 41/33 | 0.295 | | |
| HBe antigen
(positive vs. negative) | 20/54 | 0.003 | 0.058
(0.005–0.645) | 0.020 |
| HBV DNA
(≥105 copies/ml vs. <105 copies/ml) | 35/39 | 0.827 | | |
| AST (IU/l) (≥40 vs.
<40) | 34/40 | 0.518 | | |
| ALT (IU/l) (≥40 vs.
<40) | 33/41 | 0.170 | 0.305
(0.030–3.125) | 0.317 |
| ALP (IU/l) (≥300
vs. <300) | 40/34 | 0.795 | | |
| GGT (IU/l) (≥50 vs.
<50) | 35/39 | 0.607 | | |
| Serum albumin
(g/dl) (≥4.2 vs. <4.2) | 33/41 | 0.785 | | |
| Total bilirubin
(mg/dl) (≥1.0 vs. <1.0) | 27/47 | 0.686 | | |
| Platelet count
(×104/mm3) (≥12 vs. <12) | 37/37 | 0.716 | | |
| Prothrombin time
(%) (≥80 vs. <80) | 42/32 | 0.387 | | |
| Serum AFP (ng/ml)
(≥20 vs.<20) | 37/37 | 0.555 | | |
| DCP (mAU/ml) (≥30
vs. <30) | 36/38 | 0.719 | | |
| Diabetes mellitus
(yes vs. no) | 8/66 | 0.560 | | |
| Body mass index ≥23
kg/m2 (yes vs. no) | 36/38 | 0.183 | 0.100
(0.007–1.485) | 0.094 |
Univariate and multivariate analyses of
factors contributing to RFS
Univariate analysis identified the following factors
as significantly associated with RFS for all cases (n=74): Presence
of LC (P=0.017), HBeAg positivity (P<0.001), serum albumin ≥4.2
g/dl (P=0.003) and presence of diabetes mellitus (P=0.028)
(Table III). The HRs and 95% CIs
calculated using multivariate analysis for the seven factors with
P<0.2 in the univariate analysis are detailed in Table III. HCC stage (P=0.021), HBeAg
positivity (P<0.001) and γ-glutamyl transpeptidase (GGT) ≥50
IU/l (P=0.009) were found to be significant prognostic factors
linked to RFS.
 | Table IIIUnivariate and multivariate analysis
contributing to recurrence-free survival. |
Table III
Univariate and multivariate analysis
contributing to recurrence-free survival.
| | Univariate
analysis | Multivariate
analysis |
|---|
| |
|
|
|---|
| Variables at
initial treatment | No. | P-valuea | Hazard ratio (95%
CI) | P-valueb |
|---|
| Gender (male vs.
female) | 49/25 | 0.847 | | |
| Age (years) (≥60
vs. <60) | 43/31 | 0.598 | | |
| HCC stage (I or II
vs. III) | 59/15 | 0.154 | 0.359
(0.150–0.859) | 0.021 |
| Maximum tumor size
(cm) (≥2.5 vs. <2.5) | 32/42 | 0.539 | | |
| Tumor number
(single vs. multiple) | 23/51 | 0.283 | | |
| Liver cirrhosis
(yes vs. no) | 41/33 | 0.017 | 0.394
(0.135–1.148) | 0.088 |
| HBe antigen
(positive vs. negative) | 20/54 | <0.001 | 0.202
(0.088–0.463) | <0.001 |
| HBV DNA
(≥105 copies/ml vs. <105 copies/ml) | 35/39 | 0.853 | | |
| AST (IU/l) (≥40 vs.
<40) | 34/40 | 0.482 | | |
| ALT (IU/l) (≥40 vs.
<40) | 33/41 | 0.644 | | |
| ALP (IU/l) (≥300
vs. <300) | 40/34 | 0.237 | | |
| GGT (IU/l) (≥50 vs.
<50) | 35/39 | 0.160 | 0.340
(0.152–0.760) | 0.009 |
| Serum albumin
(g/dl) (≥4.2 vs. <4.2) | 33/41 | 0.003 | 1.642
(0.712–3.787) | 0.245 |
| Total bilirubin
(mg/dl) (≥1.0 vs. <1.0) | 27/47 | 0.43 | | |
| Platelet count
(×104/mm3) (≥12 vs. <12) | 37/37 | 0.148 | 0.525
(0.208–1.322) | 0.172 |
| Prothrombin time
(%) (≥80 vs. <80) | 42/32 | 0.295 | | |
| Serum AFP (ng/ml)
(>≥20 vs. <20) | 37/37 | 0.503 | | |
| DCP (mAU/ml) (≥30
vs. <30) | 36/38 | 0.344 | | |
| Diabetes mellitus
(yes vs. no) | 8/66 | 0.028 | 0.987
(0.386–2.523) | 0.978 |
| Body mass index ≥23
kg/m2 (yes vs. no) | 36/38 | 0.205 | | |
HBeAg seroconversion, HBeAg loss and
HBsAg loss
In the present study, 20 patients had HBeAg
positivity at initial treatment for HCC. Of these patients, HBeAg
seroconversion was observed in nine patients (45.0%) during the
observation period, and HBeAg loss without HBeAg seroconversion was
observed in one patient (5.0%). None of the patients experienced
HBsAg loss during the observation period.
Effect of ETV therapy on the reduction of
HBV DNA viral load and ETV-related serious adverse events
(SAEs)
In this study, 73 patients (98.6%) achieved an HBV
DNA level of <400 copies/ml during the follow-up period. No
viral breakthrough hepatitis, as defined by 1 log increase from
nadir, was observed during ETV therapy. No ETV-related SAEs were
observed.
Causes of death
In the present study, five patients (6.8%) died
during the follow-up period. The causes of death were HCC
recurrence in four patients and miscellaneous causes in one
patient.
HCC recurrence
In the present study, 42 patients (56.8%) exhibited
HCC recurrence during the follow-up period. The patterns of HCC
recurrence after initial treatment were: Single HCC recurrence in
the liver in 25 patients, multiple HCC recurrences in the liver in
14 patients, multiple HCC recurrences in the liver with lung
metastases in one patient, multiple HCC recurrences in the liver
with bone metastases in one patient and single lymph node
metastasis in one patient. Treatment methods for the first HCC
recurrence were: Surgical resection in three patients, percutaneous
ablation therapy in 31 patients, transcatheter arterial
chemoembolization in six patients and systemic chemotherapy in two
patients.
Discussion
To the best of our knowledge, there have been no
studies regarding predictive factors in HBV-related HCC patients
treated with ETV who have undergone curative therapy, despite the
fact that ETV is now a first-line NA therapy for patients with CHB
due to the superior efficacy of HBV DNA suppression, ALT
normalization and histological improvement compared with LAM and
ADV treatment (14–18). In the present era of NA treatment
for CHB patients, the identification of predictors in HBV-related
HCC patients treated with ETV is essential for improved prognosis.
Hence, we conducted this retrospective analysis.
In the multivariate analysis, HBeAg positivity was
the only independent predictor of OS. Although several studies have
reported that liver function-related factors (such as serum albumin
level) and tumor-related factors (such as HCC stage, maximum tumor
size and tumor markers) are closely associated with OS in patients
with HBV-related HCC, the majority of these studies did not use ETV
as an antiviral therapy (26–28).
In the present era of ETV use, HBV viral status rather than liver
function or tumor-related factors may influence OS in patients with
HBV-related HCC.
In the present study, HBeAg positivity was also
significantly associated with RFS in the multivariate analysis. Sun
et al(29) reported that
HBeAg is associated with a higher risk of early recurrence and
poorer survival in patients following curative resection of small
HCC. The results of the present study were similar to their
findings; in HBV-related HCC patients with HBeAg positivity,
careful observation for HCC recurrence is required after curative
therapy. Notably, serum GGT level was a significant factor
contributing to RFS in the multivariate analysis in this study.
Several studies have reported that a high level of GGT is related
to a higher incidence of HCC development and recurrence (30,31).
In HBV-related HCC patients with higher GGT levels at initial
treatment, close observation for HCC recurrence is also required
after curative therapy. HCC stage is also an independent predictor
linked to RFS. Even in HCC patients who have undergone curative
therapy, clinicians should be aware of tumor-related factors.
Resistance to NAs is a major issue affecting
long-term NA therapy. However, in our results, 73 patients (98.6%)
achieved an HBV DNA level of <400 copies/ml during the follow-up
period, and no viral breakthrough hepatitis, as defined by 1 log
increase from nadir, was observed during ETV therapy with the
median follow-up period of 3.4 years. No ETV-related SAEs were
identified. In addition, no patients succumbed to liver failure in
the present study. Our results indicate that ETV therapy for
patients with HBV-related HCC had a strong antiviral effect,
maintained liver function, had a high genetic barrier to resistance
and was a well-tolerated therapy as previously reported (14–16,18–21,28,32).
Higher HBV viral load was not a significant factor
in terms of OS and RFS, although several studies have demonstrated
that pretreatment HBV viral load was an independent predictor
linked to clinical outcomes (33–35). A
possible reason for this is that in the majority of patients with
high HBV viral load in our study, the HBV viral load was reduced to
lower HBV DNA level by ETV therapy, resulting in improved OS and
RFS.
The present study had certain limitations. First,
this is a retrospective study with a heterogeneous patient
population. Second, the number of patients in our study was small
for survival analysis. Further prospective studies with a
sufficient sample size will thus be required in the future.
However, the results of this study demonstrate that HBeAg
positivity at initial treatment for HBV-related HCC was a
significant predictor of OS and RFS following curative therapy.
In conclusion, in the present era of ETV as a
first-line therapy for CHB, HBeAg positivity may be a useful
predictor of survival in HBV-related HCC patients after curative
therapy.
Acknowledgements
The authors would like to thank Haruko Takada for
data collection.
References
|
1
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Lope CR, Tremosini S, Forner A, et al:
Management of HCC. J Hepatol. 56(Suppl 1): S75–S87. 2012.
|
|
3
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chan HL and Sung JJ: Hepatocellular
carcinoma and hepatitis B virus. Semin Liver Dis. 26:153–161. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perz JF, Armstrong GL, Farrington LA,
Hutin YJ and Bell BP: The contributions of hepatitis B virus and
hepatitis C virus infections to cirrhosis and primary liver cancer
worldwide. J Hepatol. 45:529–538. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Belongia EA, Costa J, Gareen IF, et al:
NIH consensus development statement on management of hepatitis B.
NIH Consens State Sci Statements. 25:1–29. 2008.PubMed/NCBI
|
|
7
|
Nishikawa H, Arimoto A, Wakasa T, Kita R,
Kimura T and Osaki Y: Effect of transcatheter arterial
chemoembolization prior to surgical resection for hepatocellular
carcinoma. Int J Oncol. 42:151–160. 2013.
|
|
8
|
Lencioni R: Loco-regional treatment of
hepatocellular carcinoma. Hepatology. 52:762–773. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lai CL, Chien RN, Leung NW, et al: A
one-year trial of lamivudine for chronic hepatitis B. Asia
Hepatitis Lamivudine Study Group. N Engl J Med. 339:61–68.
1998.PubMed/NCBI
|
|
10
|
Dienstag JL, Schiff ER, Wright TL, et al:
Lamivudine as initial treatment for chronic hepatitis B in the
United States. N Engl J Med. 341:1256–1263. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lok AS, Lai CL, Leung N, et al: Long-term
safety of lamivudine treatment in patients with chronic hepatitis
B. Gastroenterology. 125:1714–1722. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marcellin P, Chang TT, Lim SG, et al:
Adefovir dipivoxil for the treatment of hepatitis B e
antigen-positive chronic hepatitis B. N Engl J Med. 348:808–816.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hadziyannis SJ, Tassopoulos NC, Heathcote
EJ, et al: Adefovir Dipivoxil 438 Study Group: Adefovir dipivoxil
for the treatment of hepatitis B e antigen-negative chronic
hepatitis B. N Engl J Med. 348:800–807. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang TT, Gish RG, de Man R, et al: BEHoLD
AI463022 Study Group: A comparison of entecavir and lamivudine for
HBeAg-positive chronic hepatitis B. N Engl J Med. 354:1001–1110.
2006. View Article : Google Scholar
|
|
15
|
Lai CL, Shouval D, Lok AS, et al: BEHoLD
AI463027 Study Group: Entecavir versus lamivudine for patients with
HBeAg-negative chronic hepatitis B. N Engl J Med. 354:1011–1120.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zoulim F: Hepatitis B virus resistance to
antiviral drugs: where are we going? Liver Int. 31(Suppl 1):
S111–S116. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leung N, Peng CY, Hann HW, et al: Early
hepatitis B virus DNA reduction in hepatitis B e antigen-positive
patients with chronic hepatitis B: A randomized international study
of entecavir versus adefovir. Hepatology. 49:72–79. 2009.
View Article : Google Scholar
|
|
18
|
Chang TT, Lai CL, Kew Yoon S, et al:
Entecavir treatment for up to 5 years in patients with hepatitis B
e antigen-positive chronic hepatitis B. Hepatology. 51:422–430.
2010.PubMed/NCBI
|
|
19
|
Ide T, Sata M, Chayama K, et al:
Evaluation of long-term entecavir treatment in stable chronic
hepatitis B patients switched from lamivudine therapy. Hepatol Int.
4:594–600. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wong GL, Chan HL, Mak CH, et al: Entecavir
treatment reduces hepatic events and deaths in chronic hepatitis B
patients with liver cirrhosis. Hepatology. Feb 6–2013.(Epub ahead
of print). View Article : Google Scholar
|
|
21
|
Hosaka T, Suzuki F, Kobayashi M, et al:
Long-term entecavir treatment reduces hepatocellular carcinoma
incidence in patients with hepatitis B virus infection. Hepatology.
58:98–107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shakado S, Watanabe H, Tanaka T, et al:
Combination therapy of lamivudine and adefovir in Japanese patients
with chronic hepatitis B. Hepatol Int. 2:361–369. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ohishi W and Chayama K: Treatment of
chronic hepatitis B with nucleos(t)ide analogues. Hepatol Res.
42:219–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bruix J and Sherman M: Practice Guidelines
Committee, American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar
|
|
25
|
No authors listed. The general rules for
the clinical and pathological study of primary liver cancer. Liver
Cancer Study Group of Japan. Jpn J Surg. 19:98–129. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lo CM, Liu CL, Chan SC, et al: A
randomized, controlled trial of postoperative adjuvant interferon
therapy after resection of hepatocellular carcinoma. Ann Surg.
245:831–842. 2007. View Article : Google Scholar
|
|
27
|
Chan AC, Chok KS, Yuen WK, et al: Impact
of antiviral therapy on the survival of patients after major
hepatectomy for hepatitis B virus-related hepatocellular carcinoma.
Arch Surg. 146:675–681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Du Y, Su T, Ding Y and Cao G: Effects of
antiviral therapy on the recurrence of hepatocellular carcinoma
after curative resection or liver transplantation. Hepat Mon.
12:e60312012.PubMed/NCBI
|
|
29
|
Sun HC, Zhang W, Qin LX, et al: Positive
serum hepatitis B e antigen is associated with higher risk of early
recurrence and poorer survival in patients after curative resection
of hepatitis B-related hepatocellular carcinoma. J Hepatol.
47:684–690. 2007. View Article : Google Scholar
|
|
30
|
Yao D, Jiang D, Huang Z, et al: Abnormal
expression of hepatoma specific gamma-glutamyl transferase and
alteration of gamma-glutamyl transferase gene methylation status in
patients with hepatocellular carcinoma. Cancer. 88:761–769. 2000.
View Article : Google Scholar
|
|
31
|
Zhang JB, Chen Y, Zhang B, et al:
Prognostic significance of serum gamma-glutamyl transferase in
patients with intermediate hepatocellular carcinoma treated with
transcatheter arterial chemoembolization. Eur J Gastroenterol
Hepatol. 23:787–793. 2011. View Article : Google Scholar
|
|
32
|
Ono A, Suzuki F, Kawamura Y, et al:
Long-term continuous entecavir therapy in nucleos(t)ide-naïve
chronic hepatitis B patients. J Hepatol. 57:508–514.
2012.PubMed/NCBI
|
|
33
|
Huang G, Yang Y, Shen F, et al: Early
viral suppression predicts good postoperative survivals in patients
with hepatocellular carcinoma with a high baseline HBV-DNA load.
Ann Surg Oncol. 20:1482–1490. 2013. View Article : Google Scholar
|
|
34
|
Chen L, Zhang Q, Chang W, Du Y, Zhang H
and Cao G: Viral and host inflammation-related factors that can
predict the prognosis of hepatocellular carcinoma. Eur J Cancer.
48:1977–1987. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xia F, Lai EC, Lau WY, et al: High serum
hyaluronic acid and HBV viral load are main prognostic factors of
local recurrence after complete radiofrequency ablation of
hepatitis B-related small hepatocellular carcinoma. Ann Surg Oncol.
19:1284–1291. 2012. View Article : Google Scholar
|