Introduction
Malignant tumors, including breast cancers (1,2),
usually exhibit a high rate of glycolytic activity compared with
normal tissues in the presence of oxygen, known as the Warburg
effect (3). This feature has been
applied in a clinical setting, including positron emission
tomography and computed tomography in oncology (4). Furthermore, the Warburg effect is
considered to be a negative prognostic indicator, which may allow
tumor cells to become invasive and develop a resistance to
radiation and chemotherapies (5).
There are numerous mechanisms of the Warburg effect.
Somatic mutations in mitochondrial DNA have been shown in a number
of tumors. The outcome of such mutations is suboptimal or
non-functional oxidative phosphorylation, meaning that cells must
accelerate glycolysis (6). The
second significant mechanism may involve hypoxia-inducible factor-1
(HIF-1), which is actively responsible for regulating the energy
production in hypoxic cells. HIF-1 has been shown to induce the
enzymes that are responsible for glycolysis (7,8) and to
decrease mitochondrial respiration (9). Glucose transporter (GLUT) also plays a
significant role in the Warburg effect. The transport of glucose is
the first rate-limiting step for glucose metabolism and is mediated
by facilitative GLUT proteins. An increase in glucose transport
within malignant cells has been associated with an increased and
deregulated expression of GLUT proteins, which provide adequate raw
materials for glycolysis (10).
Qualitative and quantitative changes in the regulatory glycolytic
enzymes, hexokinase (HK), phosphofructokinase-1 (PFK-1) and
pyruvate kinase (PK), are involved in the increase of the
glycolytic flux (11). Among these
glycolytic enzymes, PFK-1 has been more extensively studied than
the others, which is likely to be due to its various regulatory
mechanisms.
Human PFK-1 exists as three isoforms, PFK-M, PFK-L
and PFK-P, which undergo random tetramerization to produce various
homo- and heterotetrameric isoenzymes, which are distinguishable
from one another by their kinetic properties (12). Thus, various tissues exhibit
specific PFK-1 isoenzyme patterns that influence the glycolytic
efficiency (13). Zancan et
al reported the differences in PFK-1 isoenzyme patterns between
the mRNA levels of non-tumorigenic and tumorigenic breast cells.
PFK-L expression was identified to correlate with aggressiveness
and glycolytic efficiency in these cell lines (14). The cellular distribution of PFK-1
activity has also been shown to have a key role in the regulation
of metabolic activity. El-Bacha et al reported that the
majority of PFK-1 activity in human breast cancer tissues is
located in an actin-enriched fraction. Additionally, metastatic
tumors, when compared with non-metastatic tumors, showed a
significant increase in PFK-1 activity in this enriched fraction.
The altered cellular distribution of PFK-1 activity in human breast
cancer tissue may be associated with an increase in the glycolytic
flux, which in turn is strongly associated with the process of
carcinogenesis and tumor progression (11). Furthermore, Šmerc et al
demonstrated that the post-translational modification of PFK-M in
mammalian cancer cells consequently leads to the formation of
active shorter PFK-M fragments with altered kinetic parameters,
which may trigger the most significant change in the regulation of
glycolytic flux in cancer cells and may also have an impact on the
Warburg effect (15).
Despite a large number of studies to date, there
have been no investigations with regard to the differences in PFK-1
isoenzyme patterns between human breast cancer and paracancer
tissues. The present study compared the glycolytic efficiency and
isoenzyme patterns of PFK-1 at the protein level in human breast
cancer and paracancer tissues. It was found that the paracancer
tissues shared the same genetic information and a similar
microenvironment with the paired cancer tissues.
Materials and methods
Tissue procurement from patients
A total of 40 female patients, aged between 33–75
years, who were admitted to the Ren Min Hospital of Wuhan
University (Wuhan, China) were recruited for this study (Table I). Patients with metabolic diseases
such as diabetes mellitus and hyperthyroidism were excluded from
this study. A total of 40 pairs of human breast cancer and
paracancer tissues were obtained by dissection during surgery and
were immediately placed into ice-cold normal saline. The samples
were frozen in liquid nitrogen subsequent to being washed and
trimmed. The characteristics of the tumor tissues were determined
using contemporary histopathological examination of the
fresh-frozen samples taken from three to five sites. The sections
lying close to those that were tested histopathologically were
dissected as tumor tissues. Samples of paracancer tissues that were
not invaded by carcinoma were obtained from areas that were ~2 cm
away from the tumors in order to avoid contamination by the
disseminating cancer cells. The quality of these tissues was
evaluated by conventional histological examination for a final
histological diagnosis. Approval for this study was obtained from
the Ethical Committee of Ren Min Hospital, Wuhan University and
written informed consent was provided by the patients.
 | Table ICommon features of the groups divided
by TNM classification according to the NCCN's guildlines of
2012. |
Table I
Common features of the groups divided
by TNM classification according to the NCCN's guildlines of
2012.
| TNM
classification | N | Mean age (years) | Hormonal
activity |
|---|
|
|---|
| Menopausal | Menstruating |
|---|
| I | 10 | 56 | 7 | 3 |
| II | 14 | 54 | 10 | 4 |
| III | 16 | 57 | 12 | 4 |
Glycolytic enzyme activities assay
All specimens had a wet weight of ~50 mg following
the addition of a 450-μl extraction buffer [1 M Tris (pH 7.5), 1%
Triton X-100, 5 M NaCl, 50 mM EDTA, 100 mM PMSF, 0.5 M NaF and 100
mM Na3VO4]. Homogenization was performed in a
Potter homogenizer (Beyotime Institute of Biology, Haimen, China)
to prepare 10% homogenate (m/v). The homogenate was centrifuged for
10 min at 20,000 × g. The supernatant was used for the enzyme
assays. All the procedures were carried out at 4°C.
As we were unable to perform the measurements
instantaneously, the stabilities of the individual enzymes varied
considerably in the homogenates, for this reason it was necessary
to construct an enzyme assay program in which the most labile
enzymes were measured first. Lactic acid (LA) content was measured
first. The activity of the remaining enzymes was measured in the
following order: PK, PFK-1, HK and then lactate dehydrogenase (LDH)
(16).
The assay kits for the determination of the LA
content (A019-2) and LDH (A020-1), HK (A077-1) and PK (A076-1)
activity were purchased from Nanjing Jiancheng Bioengineering
Institute (Nanjing, China). PFK-1 activity was assayed as described
previously (17) in a medium
containing 50 mM Tris-HCl (pH 7.4), 5 mM MgCl2, 5 mM
(NH4)2SO4, 1 mM fructose 6-P, 1 mM
ATP, 0.5 mM NADH, 2 mU/ml aldolase, 2 mU/ml triosephosphate
isomerase, 2 mU/ml α-glycerophosphate dehydrogenase and 100 μl
protein in a final volume of 1 ml. The reaction was initiated by
the addition of the protein and NADH oxidation was recorded by
measuring the decrease in absorbance at 340 nm using a
spectrophotometer at 37°C.
The soluble protein content was measured using an
assay kit purchased from Applygen Technologies Incorporation
(Beijing, China).
One unit of activity was defined as the amount of
enzyme that catalyzes the formation of 1 μmol of product per min in
standardized conditions. Specific activities were expressed as
units per gram of protein (U/gprot).
Western blot analysis
Due to the low protein concentration of the human
breast tissues in 10% homogenates, 20% (m/v) homogenates were
prepared. The supernatant was obtained as described previously and
boiled with 5X SDS-PAGE loading buffer at 100°C for 5 min. The
prepared samples were subjected to 10% SDS-PAGE and transferred to
polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA).
The membranes were then blocked in Tris-buffered saline, which
contained 0.1% Tween 20 (TBST) and 5% confining liquid (rabbit
serum or BSA) for 2 h at room temperature, followed by incubation
overnight in the TBST containing the primary antibody at 4°C.
Subsequent to being washed with TBST, the membranes were incubated
with HRP-conjugated rabbit anti-goat antibody (Jackson
ImmunoResearch, West Grove, PA, USA) or AP-conjugated mouse
anti-rabbit antibody (Pierce, Rockford, IL, USA) for 1 h at room
temperature. The antibodies that were used were anti-PFK-1 (catalog
no SC-31711), anti-PFK-M (catalog no. SC-67028), anti-PFK-L
(catalog no. SC-130226), anti-PFK-P (catalog no. SC-130227) and
anti-β-actin (catalog no. SC-130300) (Santa Cruz Biotechnology
Inc., Santa Cruz, CA, USA).
Statistical analysis
The experimental data are presented as the mean ±
SD. The statistical comparisons were performed by the SNK test,
χ2 test or t-test, correspondingly, using SPSS 11.0
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Common patient features
The patients were divided into three groups
according to TNM classification (Table
I). No significant differences were identified with regard to
the mean age (SNK test; P=0.55, 0.86 and 0.43 in stage I vs. II, I
vs. III and II vs. III, respectively) or hormonal activity (P=0.96;
χ2, 0.090) of the patients in the various clinical
stages.
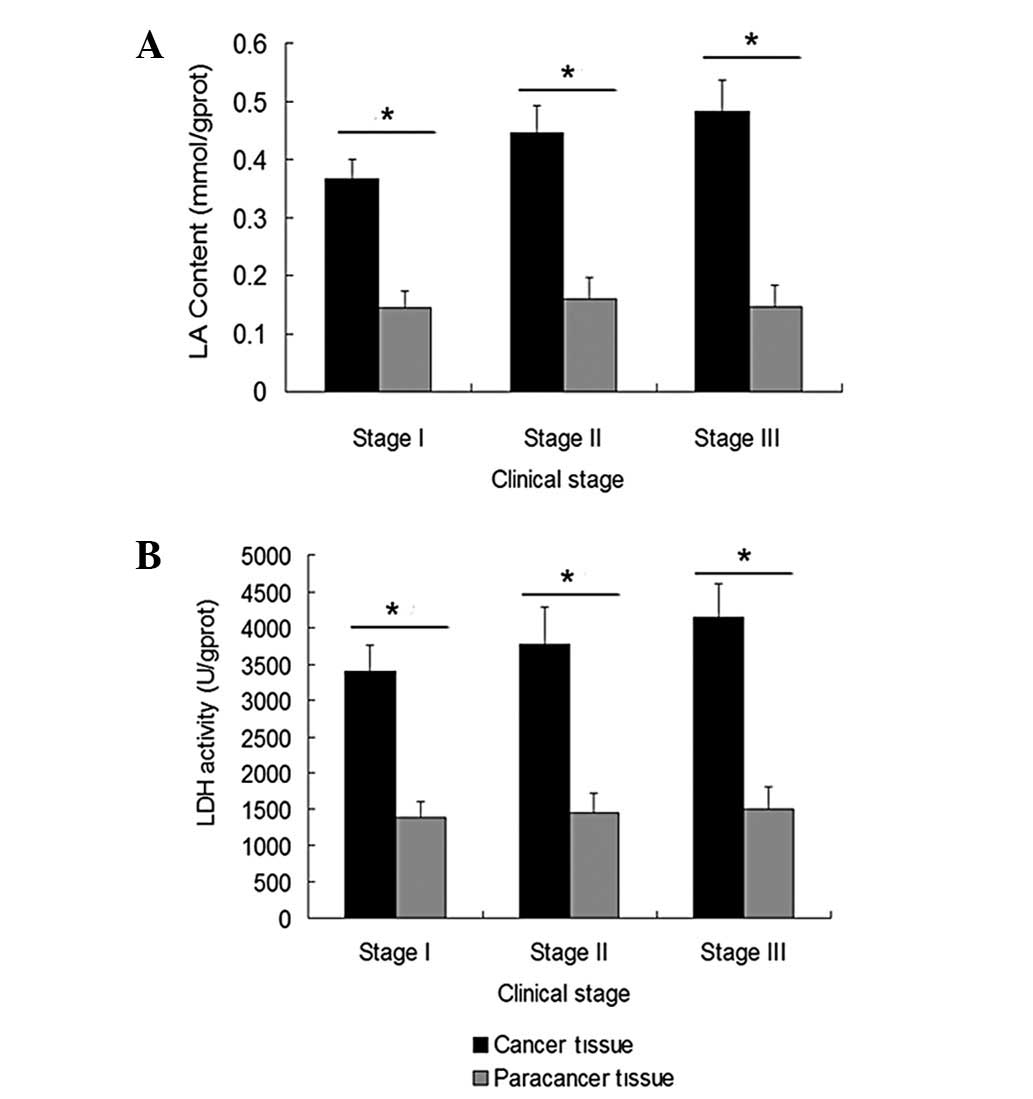
LA content and LDH activity are increased
in human breast cancer tissues
The LA content and LDH activity in human breast
cancer and paracancer tissues were detected in order to evaluate
the glycolytic efficiency. The LA content in the human breast
cancer and paracancer tissues of each clinical stage (I, II and
III) was 0.37±0.033 vs. 0.14±0.027 mmol/gprot (P=0.034), 0.45±0.047
vs. 0.16±0.036 mmol/gprot (P=0.027)and 0.48±0.052 vs. 0.15±0.036
mmol/gprot (P=0.017), respectively (Fig. 1A). The LDH activity in the human
breast cancer and paracancer tissues of each clinical stage was
3399±352 vs. 1375±239 U/gprot (P=0.043), 3770±511 vs. 1449±267
U/gprot (P=0.035) and 4153±452 vs. 1499±310 U/gprot (P=0.029),
respectively (Fig. 1B).
Furthermore, the LA content and LDH activity in the breast cancer
tissues increased with increasing clinical stage. Significant
differences were identified in the LA content and LDH activity
assays (P=0.042, 0.025 and 0.035 for LA and P=0.035, 0.020 and
0.031 for LDH in stage I vs. II, I vs. III and II vs. III,
respectively).
Activities of regulatory glycolytic
enzymes are higher in human breast cancer tissues
HK, PFK-1 and PK regulate the rate of glycolysis.
The activities of these enzymes were compared in order to identify
which of these enzymes were responsible for the different rates of
glycolysis between human breast cancer and paracancer tissues. The
HK activities in the human breast cancer and paracancer tissues of
each clinical stage (I, II and III) were 3.51±0.466 vs. 1.70±0.317
U/gprot (P=0.039), 4.34±0.422 vs. 1.55±0.260 U/gprot (P=0.022) and
4.68±0.518 vs. 1.47±0.389 U/gprot (P=0.011), respectively (Fig. 2A). The PFK-1 activities in the human
breast cancer and paracancer tissues of each clinical stage were
15.7±1.92 vs. 5.71±0.366 U/gprot (P=0.024), 18.6±1.48 vs.
5.39±0.459 U/gprot (P=0.018) and 20.2±1.94 vs. 5.48±0.612 U/gprot
(P=0.010), respectively (Fig. 2B).
The PK activities in the human breast cancer and paracancer tissues
of each clinical stage were 56.4±4.57 vs. 21.8±3.08 U/gprot
(P=0.032), 64.9±4.07 vs. 22.5±3.03 U/gprot (P=0.021) and 68.7±3.92
vs. 21.9±3.58 U/gprot (P=0.012), respectively (Fig. 2C). The HK, PFK-1 and PK activities
in the breast cancer tissues increased with increasing clinical
stage. Significant differences were identified in the HK, PFK-1 and
PK activity assays (P=0.041, 0.039 and 0.038 for HK, P=0.025, 0.018
and 0.027 for PFK-1 and P=0.033, 0.024 and 0.043, in stage I vs.
II, I vs. III and II vs. III, respectively).
Isoenzyme patterns of PFK-1 in human
breast cancer and paracancer tissues
The enzymic activities in the malignant tissues were
higher, particularly for PFK-1, as they were considered to be
regulatory enzymes of glycolysis. To investigate the correlation
between PFK-1 expression and glycolysis in breast tissues, the
total PFK-1 content and PFK-1 isoenzyme patterns were evaluated
using western blot analysis. The results are presented as scanned
images (Fig. 3A–C) and as relative
to β-actin (Fig. 3D). The ratios of
total PFK-1 to β-actin expression were 0.272±0.051 vs. 0.139±0.032
(P=0.033), 0.303±0.064 vs. 0.142±0.022 (P=0.029) and 0.370±0.040
vs. 0.136±0.030 (P=0.018), respectively, between the human breast
cancer and paracancer tissues of each clinical stage. The total
PFK-1 content increased with increasing clinical stage, and
significant differences were observed between stages I and II, I
and III and II and III (P=0.032, 0.011 and 0.025, respectively).
Furthermore, the isoenzyme patterns of PFK-1 were analyzed using
western blot analysis, and significant differences were identified
between the human breast cancer and paracancer tissues (Fig. 4). In the carcinomas, the percentages
of the M, L and P isoforms of PFK-1 were 22, 16 and 62% in stage I,
18, 14 and 68% in stage II, and 17, 11 and 72% in stage III,
respectively (Fig. 5A). By
contrast, in the paracancer tissues, the percentages of the M, L
and P isoforms of PFK-1 were 8, 70 and 22% in stage I, 3, 71 and
26% in stage II and 12, 64 and 24% in stage III, respectively
(Fig. 5B). PFK-P and PFK-L
accounted for the vast majority of the total PFK-1 content in the
human breast cancer and paracancer tissues, respectively. The
percentage of PFK-P increased with increasing clinical stage of the
carcinomas.
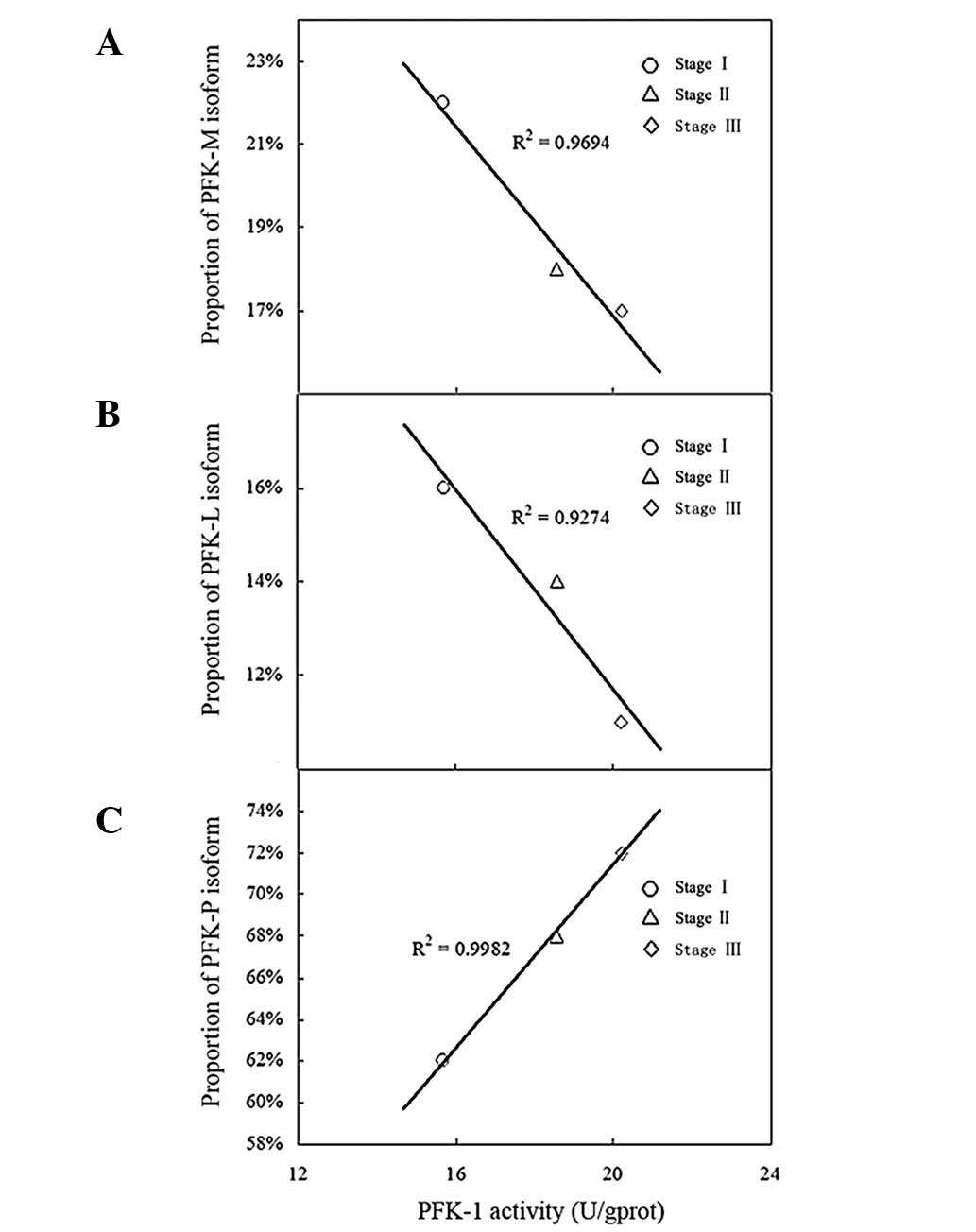
Correlation analysis between PFK-1
activity and isoenzyme patterns
The correlation between PFK-1 activity and isoenzyme
patterns was analyzed in the human breast cancer tissues of each
clinical stage (Fig. 6). The
statistics revealed that, with increasing pathological stages of
breast cancer, the expression of PFK-P was significantly positively
correlated with the activity of PFK-1 (R2=0.9982;
P=0.032). The expression of PFK-M (R2=0.9694; P=0.107)
and PFK-L (R2=0.9274; P=0.178) was negatively correlated
with the activity of PFK-1, but without statistical
significance.
Discussion
The most well-known energy metabolism alteration in
tumor cells is an increased glycolytic capacity in the presence of
a high O2 concentration. A persistent metabolism of
glucose to lactate, even in aerobic conditions, is an adaptation to
intermittent hypoxia in pre-malignant lesions (18). Early studies on breast cancer have
identified that the activities of all the glycolytic enzymes that
were tested from carcinomas were significantly higher than those of
non-malignant diseases (1).
Glycolytic enzyme activities were significantly higher in breast
cancer metastases compared with in primary tumors (19), and the transition of the breast
cancer towards the normal surrounding breast tissue showed a
decrease in glycolytic activity (20). However, the enhanced glycolytic rate
requires further investigation in order to be completely
understood.
Glycolytic enzyme activity has been reported to be
age-related. In albino Swiss mouse liver tissue, the activity of
PFK-1 was highest in 8- to 12-week-old mice, then gradually
decreased with age. The PFK-1 activity was maintained at a stable
level at 24 weeks old (21).
Furthermore, a study has reported that the brain PFK-1 activity in
the substantia nigra is lower in adult and aged mice (22). Indirect evidence has suggested that
estrogen may affect the expression of the PFK-M subtypes. It has
been identified that mouse PFK-M expression in the genioglossus
increases in a state of chronic hypoxia, and estrogen is able to
decrease PFK-M expression under hypoxic conditions (23). Although there is no direct evidence,
the influence of age and estrogen levels may have an effect on
breast cancer glycolysis. Therefore, the present study compared the
average age and menstrual status between patients with various
pathological stages of breast cancer. No significant differences
were identified between the various stages of cancer and the age
and menstrual status of the patients.
The glycolytic enzyme activity data presented in
this study confirmed the results of earlier observations. It is
well-known that LA is a product of the glycolysis process and LDH
catalyzes the process of LA production (24). The present study evaluated the
glycolytic rate by measuring the LA content and LDH activity. A
significantly higher LA content and LDH activity was observed in
the carcinomas compared with the paracancer tissues. Furthermore,
the LA content and LDH activity in the breast cancer tissues
increased with increasing clinical stage. The activities of the
glycolytic regulatory enzymes, HK, PFK-1 and PK, in the human
breast cancer tissues were all significantly higher than those in
the paracancer tissues of each clinical stage. The enzymatic
activities also progressed with the clinical stages.
The present study also detected the expression of
total PFK-1 using western blot analysis. Due to the superior
activity of PFK-1 in the cancer tissues, the results revealed that
the total PFK-1 level in the human breast cancer tissues of
superior clinical stages was higher. In order to further understand
the mechanism of the increased glycolysis rate, the PFK-1 isoenzyme
patterns were analyzed between the human breast cancer and
paracancer tissues. Notably, a significant difference was
identified. The human breast cancer and paracancer tissues mainly
expressed PFK-P and PFK-L, respectively, which is concordant with
the results of other studies. Sánchez-Martínez and Aragon observed
that the presence of an ascites tumor of mammary origin
predominantly contained PFK-P, whereas the PFK-L isoform was more
abundant in the mammary gland. The proportions of the PFK-P, PFK-L
and PFK-M isoforms in the ascites were 50, 32 and 18%,
respectively; whereas those in the murine mammary gland were 2, 65
and 33%, respectively (25). A
study determined the major isoform of PFK-1 in breast cancer cells
using western blot analysis. PFK-P was identified to be the major
isoform in breast cancer cells, including MCF-7, MDA-MB-231, BT-474
and SK-BR-3 cells; whereas PFK-L was the major isoform in MCF10A
cells, which is a non-tumorigenic breast cell line. The study shows
that PFK-P plays a crucial role in the glycolytic activities and
proliferation of breast cancer cells (26). However, the results of the study by
Zancan et al(14) are
inconsistent with these findings. The mRNA level of PFK-1 isoforms
were also detected in three cell lines, MCF10A, MCF-7 and
MDA-MB-231. The results revealed that the glycolytic efficiency in
breast cancer cells depended primarily on the preferential
expression of PFK-L over the PFK-M and PFK-P isoforms (14). The differences may be associated
with the various aspects of PFK-1 expression and the different
experimental conditions that were used, which may also indicate the
complexity of the post-transcriptional regulation of PFK-1.
As previously described, the subunit composition has
been shown to promote kinetic and regulatory differences among the
isoenzyme pools that affect the affinity for fructose-6-P and for
certain effectors, including ATP, AMP or
fructose-2,6-P2, and that were suggested to contribute
to the characteristics of the glycolytic operation in particular
tissues. PFK-M is inhibited by allosteric inhibitors, including
citrate and ATP, whereas PFK-L and PFK-P are less sensitive to the
inhibitory effect of these allosteric effectors and are more
sensitive to fructose 2,6-bisphosphate, which is a potent activator
(26). PFK-P may contribute to
maintaining a high glycolytic status at an increased citrate level
and sufficient ATP concentration. Although PFK-L accounts for a
large proportion of the total PFK-1 levels in human breast
paracancer tissues, the total PFK-1 content in paracancer tissues
is markedly lower than THAT in cancer tissues. Therefore, the PFK-1
activity is higher in cancer tissues.
The present study detected the PFK-1 isoenzyme
patterns in human breast cancer and paracancer tissues and
identified that during the development of breast cancer, the
enhancement of glycolytic activity depends primarily on the
conversion of PFK-1, from PFK-L to PFK-P.
Acknowledgements
The authors would like to thank Professor Changhua
Wang. This study was supported by the 11th Five Years Key Programs
for Science and Technology Development of China Hubei Province (no.
505-1).
Abbreviations:
|
LA
|
lactic acid
|
|
LDH
|
lactate dehydrogenase
|
|
HK
|
hexokinase
|
|
PFK-1
|
phosphofructokinase-1
|
|
PK
|
pyruvate kinase
|
References
|
1
|
Szutowicz A, Kwiatkowski J and Angielski
S: Lipogenetic and glycolytic enzyme activities in carcinoma and
nonmalignant diseases of the human breast. Br J Cancer. 39:681–687.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hennipman A, Smits J, van Oirschot B, van
Houwelingen JC, Rijksen G, Neyt JP, Van Unnik JA and Staal GE:
Glycolytic enzymes in breast cancer, benign breast disease and
normal breast tissue. Tumour Biol. 8:251–263. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Drake B and Cook GJ: Positron emission
tomography computed tomography in oncology. Br J Hosp Med (Lond).
72:631–637. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Robey IF, Stephen RM, Brown KS, Baggett
BK, Gatenby RA and Gillies RJ: Regulation of the Warburg effect in
early-passage breast cancer cells. Neoplasia. 10:745–756.
2008.PubMed/NCBI
|
|
6
|
Gottlieb E and Tomlinson IP: Mitochondrial
tumour suppressors: a genetic and biochemical update. Nat Rev
Cancer. 5:857–866. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guppy M, Leedman P, Zu X and Russell V:
Contribution by different fuels and metabolic pathways to the total
ATP turnover of proliferating MCF-7 breast cancer cells. Biochem J.
364:309–315. 2002.PubMed/NCBI
|
|
8
|
Dalgard CL, Lu H, Mohyeldin A and Verma A:
Endogenous 2-oxoacids differentially regulate expression of oxygen
sensors. Biochem J. 380:419–424. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Papandreou I, Cairns RA, Fontana L, Lim AL
and Denko NC: HIF-1 mediates adaptation to hypoxia by actively
downregulating mitochondrial oxygen consumption. Cell Metab.
3:187–197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Macheda ML, Rogers S and Best JD:
Molecular and cellular regulation of glucose transporter (GLUT)
proteins in cancer. J Cell Physiol. 202:654–662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
El-Bacha T, de Freitas MS and Sola-Penna
M: Cellular distribution of phosphofructokinase activity and
implications to metabolic regulation in human breast cancer. Mol
Genet Metab. 79:294–299. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vora S, Halper JP and Knowles DM:
Alterations in the activity and isozymic profile of human
phosphofructokinase during malignant transformation in vivo and in
vitro: transformation- and progression-linked discriminants of
malignancy. Cancer Res. 45:2993–3001. 1985.
|
|
13
|
Dunaway GA: A review of animal
phosphofructokinase isozymes with an emphasis on their
physiological role. Mol Cell Biochem. 52:75–91. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zancan P, Sola-Penna M, Furtado CM and Da
Silva D: Differential expression of phosphofructokinase-1 isoforms
correlates with the glycolytic efficiency of breast cancer cells.
Mol Genet Metab. 100:372–378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Šmerc A, Sodja E and Legiša M:
Posttranslational modification of 6-phosphofructo-1-kinase as an
important feature of cancer metabolism. PLoS One.
6:e196452011.PubMed/NCBI
|
|
16
|
Shonk CE and Boxer GE: Enzyme patterns in
human tissues. I. Methods for the determination of glycolytic
enzymes. Cancer Res. 24:709–721. 1964.PubMed/NCBI
|
|
17
|
Coelho WS, Costa KC and Sola-Penna M:
Serotonin stimulates mouse skeletal muscle 6-phosphofructo-1-kinase
through tyrosine-phosphorylation of the enzyme altering its
intracellular localization. Mol Genet Metab. 92:364–370. 2007.
View Article : Google Scholar
|
|
18
|
Gatenby RA and Gillies RJ: Why do cancers
have high aerobic glycolysis? Nat Rev Cancer. 4:891–899. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hennipman A, van Oirschot BA, Smits J,
Rijksen G and Staal GE: Glycolytic enzyme activities in breast
cancer metastases. Tumour Biol. 9:241–248. 1988. View Article : Google Scholar
|
|
20
|
Hennipman A, van Oirschot BA, Smits J,
Rijksen G and Staal GE: Heterogeneity of glycolytic enzyme activity
and isozyme composition of pyruvate kinase in breast cancer. Tumour
Biol. 9:178–189. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hron WT and Menahan LA: Age-related
changes in activities of hepatic phosphofructokinase, pyruvate
kinase and pyruvate dehydrogenase in liver and adipose tissue of
the swiss albino mouse. Enzyme. 30:83–88. 1983.
|
|
22
|
Steffen V, Gordillo E, Castano A, Caño J
and Machado A: Age-dependent changes in the activity and
isoenzymatic pattern of the phosphofructokinase in different areas
of the central nervous systems. Neurosci Lett. 125:15–18. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jia SS and Liu YH: Effects of estrogen on
the expression of phosphofructokinase muscle-specific isoform in
genioglossus of chronic intermittent hypoxia rats. Zhonghua Kou
Qiang Yi Xue Za Zhi. 45:627–630. 2010.(In Chinese).
|
|
24
|
Augoff K and Grabowski K: Significance of
lactate dehydrogenase measurements in diagnosis of malignancies.
Pol Merkur Lekarski. 17:644–647. 2004.(In Polish).
|
|
25
|
Sánchez-Martínez C and Aragon JJ: Analysis
of phosphofructokinase subunits and isozymes in ascites tumor cells
and its original tissue, murine mammary gland. FEBS Lett.
409:86–90. 1997.PubMed/NCBI
|
|
26
|
Moon JS, Kim HE, Koh E, Park SH, Jin WJ,
Park BW, Park SW and Kim KS: Krüppel-like factor 4 (KLF4) activates
the transcription of the gene for the platelet isoform of
phosphofructokinase (PFKP) in breast cancer. J Biol Chem.
286:23808–23816. 2011.
|