Introduction
Cancer stem cells (CSCs) are important as they are
capable of self-renewal, differentiation and the maintenance of
tumor growth and heterogeneity. Studies suggest that CSCs are not
only responsible for tumorigenesis, but that they also contribute
to tumor recurrence and, in certain cases, resistance to cancer
therapy (1). CSCs have been
isolated from several human tumors that express markers for
putative normal stem cells, including leukemia (2) and breast (3), brain (4), prostate (5) and ovarian (6,7)
cancers. However, this research has been impeded by the lack of
distinct molecular markers for CSCs.
The use of the Hoechst 33342 dye for identifying and
isolating CSCs as a side population (SP) overcomes the phenotypical
marker barrier and provides a functional marker (8). SP cells have been identified in
various cell lines that have been generated from hepatocellular
liver cancer (9), nasopharyngeal
carcinoma (10) and ovarian cancer
(11). These findings have
demonstrated that SP cells exhibit stem cell characteristics and
may be enriched as a stem cell population. Additionally, this
method may aid in identifying more effective CSC markers by
comparing the expression profiles of SP and non-side population
(NSP) cells, and it may ultimately play a crucial role in the
establishment of targeted cancer therapies.
Although this field is rapidly advancing, only a
small number of published studies have examined the role of SP
cells in human cervical cancer. Cervical cancer is the second most
common cancer, after breast cancer, for females worldwide and it is
also one of the most serious diseases that threaten female health.
Although traditional surgery, radiotherapy and combined treatments
have obtained good results for early-stage cervical squamous cell
carcinoma, the results from patients who have been treated for
cervical adenocarcinoma, which is an advanced and recurrent
cervical cancer, are not favorable. The five-year survival rate is
30–50% for patients with stage III cervical carcinoma and only
5–15% for patients with stage IV disease. The five-year survival
rate for patients with local recurrence and distant metastasis is
~10%. Thus, studying the CSCs in cervical cancers in order to
understand their role in metastasis, recurrence and resistance to
cancer therapy, is significant for identifying novel and more
specific therapeutic approaches. The HeLa cell line is a commonly
studied cervical adenocarcinoma cell line with a high capacity for
malignancy (12). The present study
aimed to identify the prevalence of SP cells in the HeLa cell line
and to evaluate the stem cell-like subpopulation of the HeLa cancer
cells. The subsequent results should aid in forming a foundation
for the design of future therapeutic strategies for cancer
patients.
Materials and methods
Cell culture
The HeLa human cervical adenocarcinoma cell line
infected with HPV18 was obtained from Professor Chen (Department of
Pathology, Peking Union Medical College Hospital, Chinese Academy
of Medical Sciences, Beijing, China) and maintained in the
Department of Gynecology and Obstetrics, Peking Union Medical
College Hospital, Chinese Academy of Medical Sciences. The cells
were cultured in DMEM media (Gibco, Carlsbad, CA, USA) supplemented
with 10% FBS (Gibco), 100 U/ml penicillin and 100 μg/ml
streptomycin (Gibco) at 37ºC in a 5% CO2 humidified
incubator.
Cell sorting
The cells that were in the logarithmic phase of
growth were analyzed by fluorescence-activating cell sorting (FACS;
FACS Diva Option; Becton Dickinson, Mountain View, CA, USA). The
cells were harvested using 0.25% trypsin (Gibco), washed twice with
PBS solution (Hyclone, Logan, UT, USA) and resuspended in DMEM at a
concentration of 1×106 cells/ml. The cells were then
incubated with 5 μg/ml Hoechst 33342 dye (Sigma-Aldrich, St. Louis,
MO, USA) for 120 min in the dark at 37ºC and mixed at 15 min
intervals. The cells were washed twice with pre-cooled PBS and
centrifuged at 1,000 × g at 4ºC. The cells were counterstained with
1 μg/ml propidium iodide (Sigma-Aldrich) in the dark and sorted by
FACS using a dual-wavelength analysis. Hoechst 33342 is extruded
from cells using a verapamil-sensitive ABC transporter, which is a
calcium channel antagonist. Therefore, a subset of the cells were
incubated with verapamil (50 μmol/l) for 30 min at 37ºC prior to
adding Hoechst 33342, in order to determine whether the verapamil
treatment was able to block the fluorescent efflux from the HeLa SP
cells. The SP and NSP cells were collected for further
experiments.
Cell proliferation
Freshly sorted SP and NSP cells were cultured at a
density of 400 cells per 35-mm culture dish in DMEM at 37ºC in a 5%
CO2 incubator. The growth of the cells was monitored and
images of the cells were captured at two, five, seven and eight
days.
In parallel, the freshly sorted SP and NSP cells
were seeded in 96-well plates at a density of 1,000 cells/well in
0.2 ml DMEM and cultured at 37ºC in a 5% CO2 incubator
to observe the growth rate. The culture medium was removed
following a 24-h incubation period and 0.2 ml 3-(4,5)-dimethylthiahiazo(-z-y1)-3,5-di-phenytetrazoliumromide
(MTT) solution (final concentration, 0.5 mg/ml; Sigma-Aldrich) was
added to four of the wells. The cells were incubated for 4 h and
the medium was replaced with 0.15 ml DMSO. The plates were shaken
for 10 min. A photometer (lQuant; Bio-TEK, Winooski, VT, USA) was
then used to measure the absorbance of the cells at 490 nm every 24
h for eight days. The experiments were repeated three times and a
cell growth curve was produced using the average values.
Colony-forming cell assays
The freshly sorted SP and NSP cells were counted,
plated in triplicate at a density of 250 cells per well in six-well
plates and cultured with DMEM for ~10 days. Following the expansion
of the majority of the cell clones to >50 cells, the cells were
washed twice with PBS, fixed in formaldehyde for 15 min and stained
with crystal violet for 20 min at room temperature. Subsequent to
washing the stain out, the numbers of colonies that contained
>50 cells were counted and the results were compared. The clone
formation efficiency (CFE) was the ratio of the clonal cell number
to the plated cell number. The results represent the average CFEs
of three experiments.
Long-term differentiation of SP and NSP
cells
The freshly sorted SP and NSP cells were cultured in
DMEM in a humidified 5% CO2 incubator at 37ºC. The
differentiation assay was performed at three weeks post-incubation,
when the cells reached the target cell number. The cultured SP and
NSP cells were stained with Hoechst 33342 and analyzed using FACS
to quantify the proportion of SP cells and to determine the
differentiation ability of the two subpopulations. Following the
second sorting, the SP cells were cultured in DMEM. At
approximately two weeks post-culture, the SP and NSP cells were
stained and analyzed using FACS for the third time to determine
whether the SP cells were enriched through repeated cell
sorting.
Tumor formation assay
Female five to six-week-old Balb/c mice were
purchased from the animal institute of the Chinese Academy of
Medical Science (CAMS, Beijing, China) and Peking Union Medical
College (PUMC, Beijing, China) and maintained in the barrier system
of a specific pathogen-free (SPF) environment. Approval for the
study was obtained from the animal care committee of CAMS and PUMC.
A total of 24 Balb/c mice were randomly divided into eight groups
containing three animals each. For the assay groups,
1×103, 1×104, 1×105 and
1×106 freshly sorted SP and NSP cells were suspended in
200 μl PBS and injected into the axillary fossa of the Balb/c mice.
The three mice within each group were injected with a different
cell type and number of cells. The mice were monitored twice weekly
for the formation of palpable tumors and sacrificed at eight weeks
post-transplantation in order to assess tumor formation.
Radiation and drug sensitivity
assays
The SP and NSP cells were exposed to X-rays in order
to determine the differences in radiation sensitivity. A total of
100 freshly sorted SP and NSP cells were seeded per well in a
24-well plate with each cell type in six wells. Of the total wells,
three of each cell type were irradiated with 8 Gy of X-ray (1,000
cGy/min using a 12×6-cm irradiation field) the day after seeding,
and the cells were then cultured. The remaining three wells from
each cell group that were not exposed to X-rays were cultured as
matched controls under normal conditions. The cells were stained
with crystal violet at three weeks post-irradiation and the clone
number was determined, which reflected the ability of the cells to
survive irradiation.
In parallel, freshly sorted SP and NSP cells were
seeded in 96-well plates at a density of 1,000 cells/well in 0.2 ml
DMEM and cultured at 37ºC in a 5% CO2 incubator. The
culture medium was removed 24 h later and medium containing 12.5,
25, 50, 100 or 200 μg/ml cisplatin (8) was added. Each cisplatin concentration
was used in quadruplicate. Additionally, one group was used with a
concentration of 0 μg/ml as a blank control and three groups were
incubated in DMEM only. All the cells were cultured in 0.2 ml DMEM
at 37ºC in a 5% CO2 incubator for 24 h. The culture
medium was removed and 0.2 ml MTT was added to each well. The cells
were incubated for 4 h and the medium was replaced with 0.15 ml
DMSO. The plates were shaken for 10 min. The absorbance was
measured at 490 nm in a photometer within 30 min. The formula that
was used for the determination of the cell inhibition ratio is as
follows: Cell inhibition ratio = no-cisplatin control group A value
− (experimental group A value/no-cisplatin control group A
value).
CD133 and CD44 expression in SP and NSP
cells
HeLa cells and freshly sorted SP and NSP cells were
cultured in DMEM in a humidified 5% CO2 incubator at
37ºC. At approximately one week post-incubation, the cells were
treated with trypsin, washed and resuspended in 100 μl PBS
solution. PE-labeled CD133 antibodies and FITC-labeled CD44
antibodies (Cell Signaling Technology, Inc., Boston, MA, USA) were
added and the cells were incubated in the dark for 1 h. The cells
were washed twice in PBS and analyzed using FACS.
Statistical methods
Student’s t-test or a one-way ANOVA were used where
appropriate. The analyses used the SPSS 10.0 statistical software
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference. Data are expressed
as the mean ± SD from at least three independent experiments.
Results
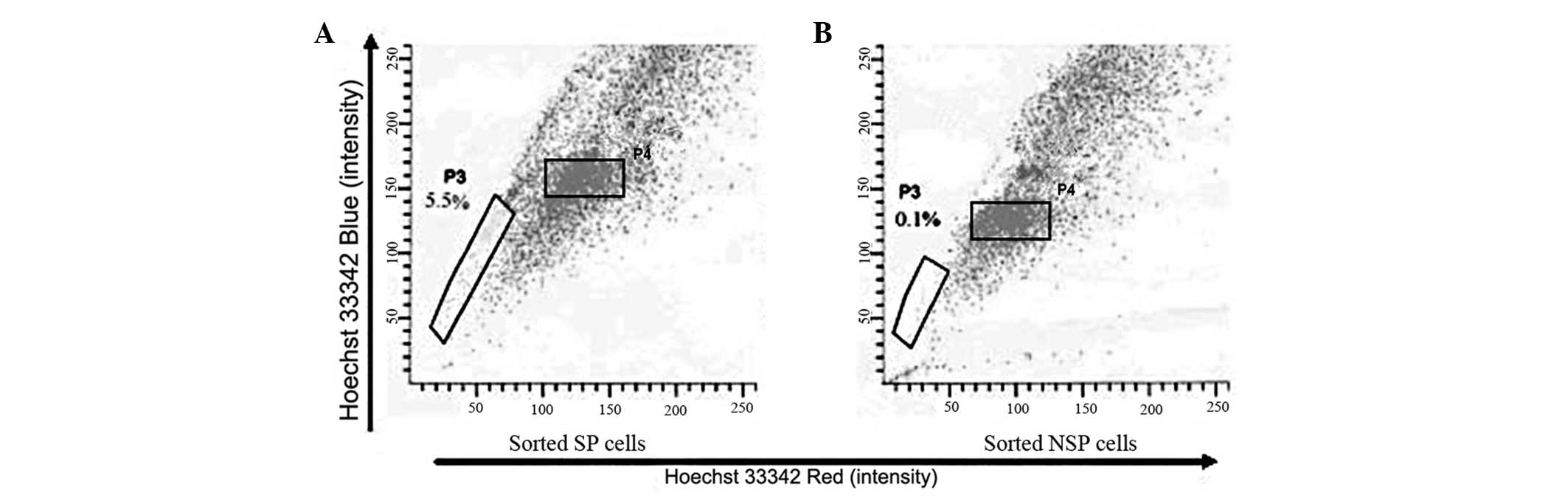
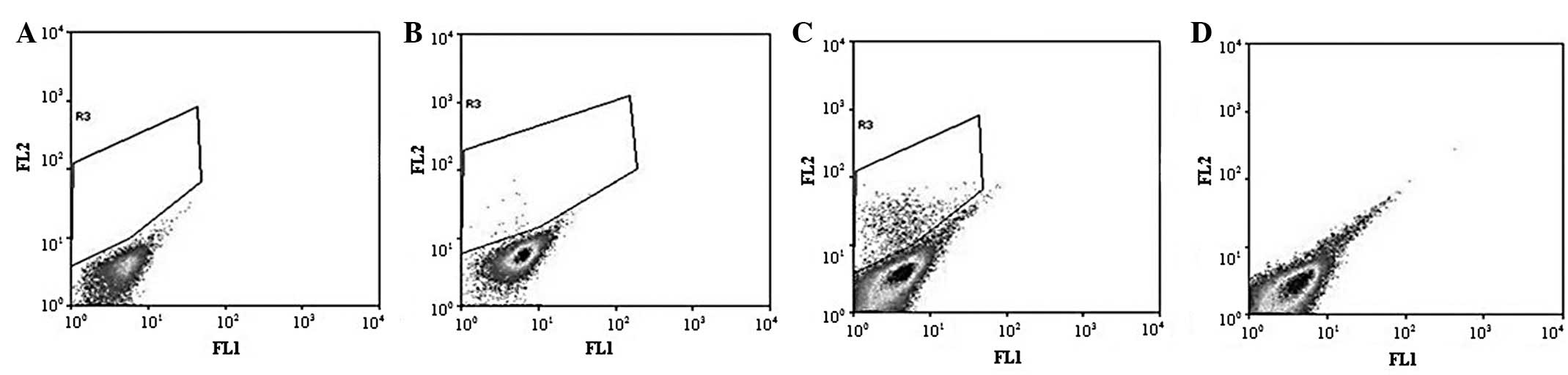
Cell sorting
Subsequent to excluding the dead cells and cellular
debris based on scatter signals and propidium iodide fluorescence,
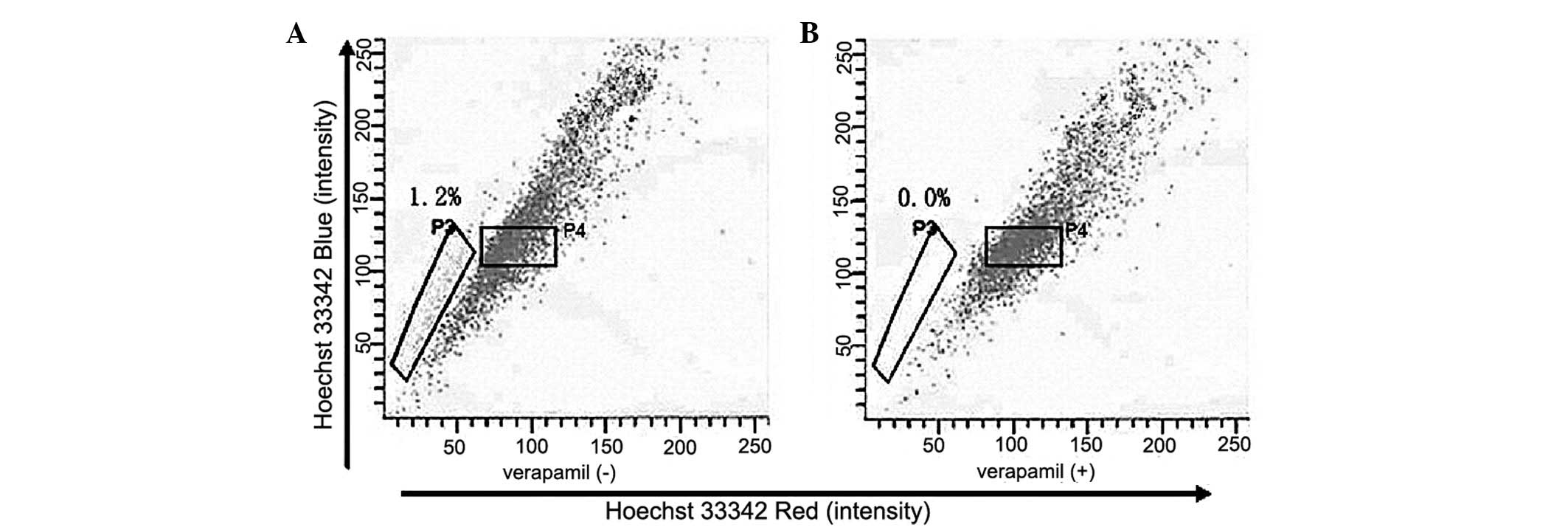
the HeLa cell line was sorted. The P3 gate identified the SP cells
that excreted Hoechst 33342 and the P4 gate identified the NSP
cells that were Hoechst 33342-positive (Fig. 1A). The SP cells accounted for ~1.2%
of the total cell number. Following pre-incubation with verapamil
for 30 min, the percentage of SP cells dropped to 0% of the total
cells (Fig. 1B), which is
consistent with studies that report Hoechst 33342 exclusion to be
verapamil-sensitive. The SP (P3) and NSP (P4) cells were collected
for subsequent experiments.
Cell proliferation
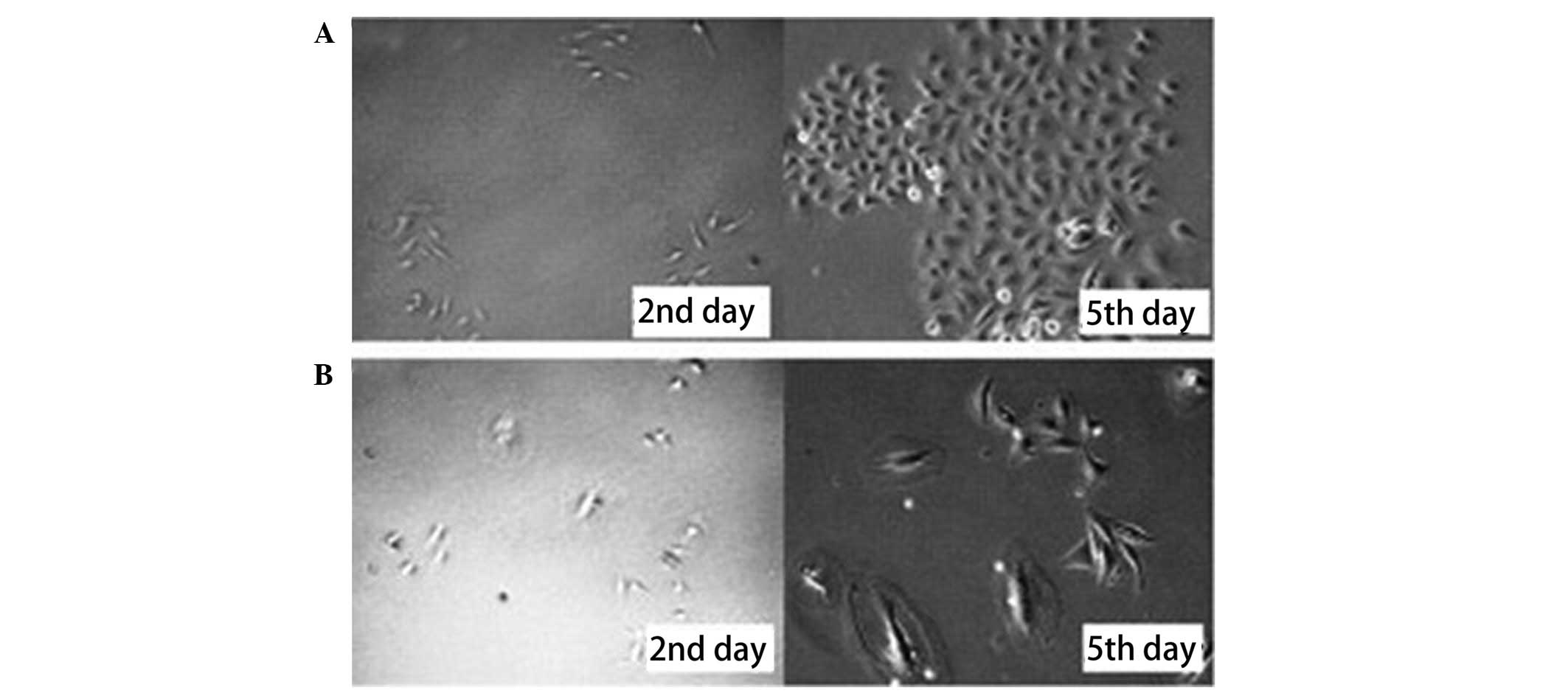
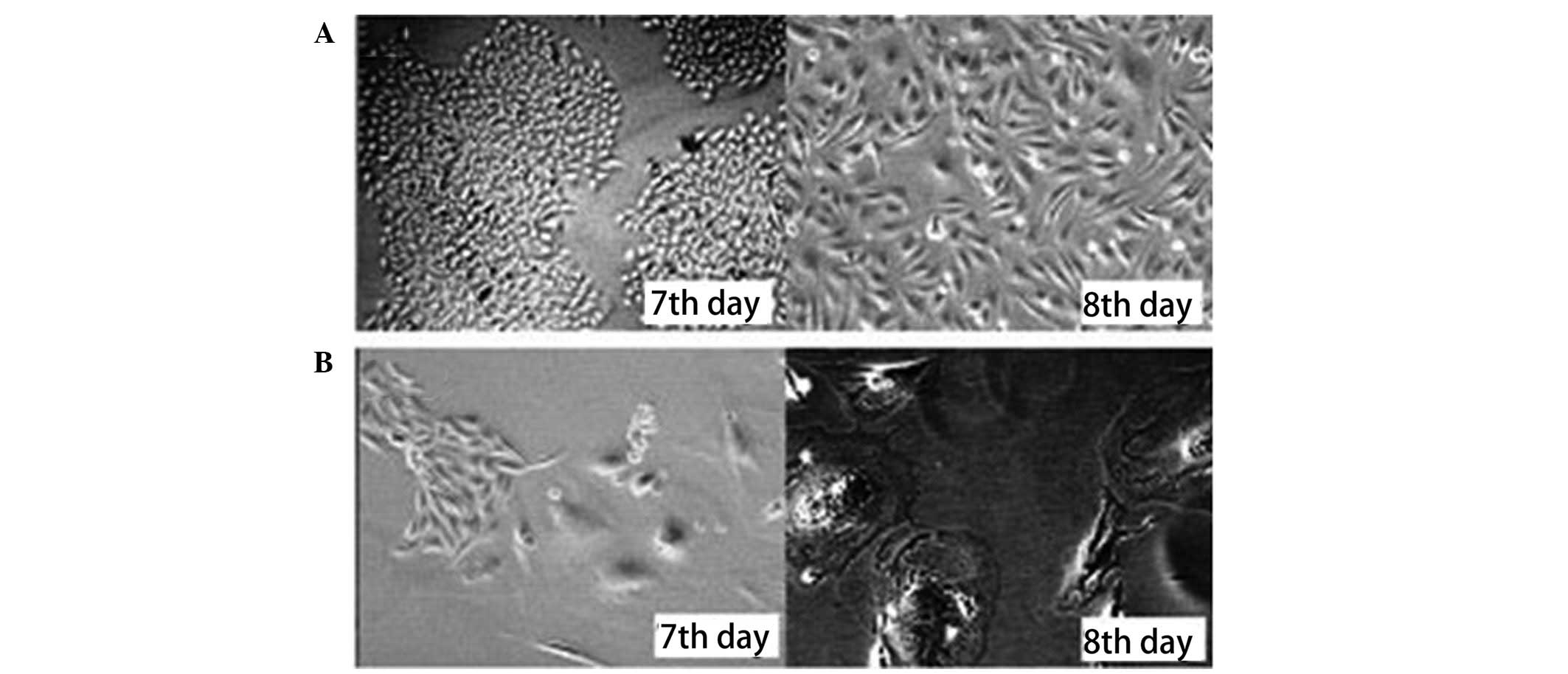
Freshly sorted SP and NSP cells were cultured in
DMEM at 37ºC in a 5% CO2 incubator. The growth of the
cells was observed and images of the cells were captured at two,
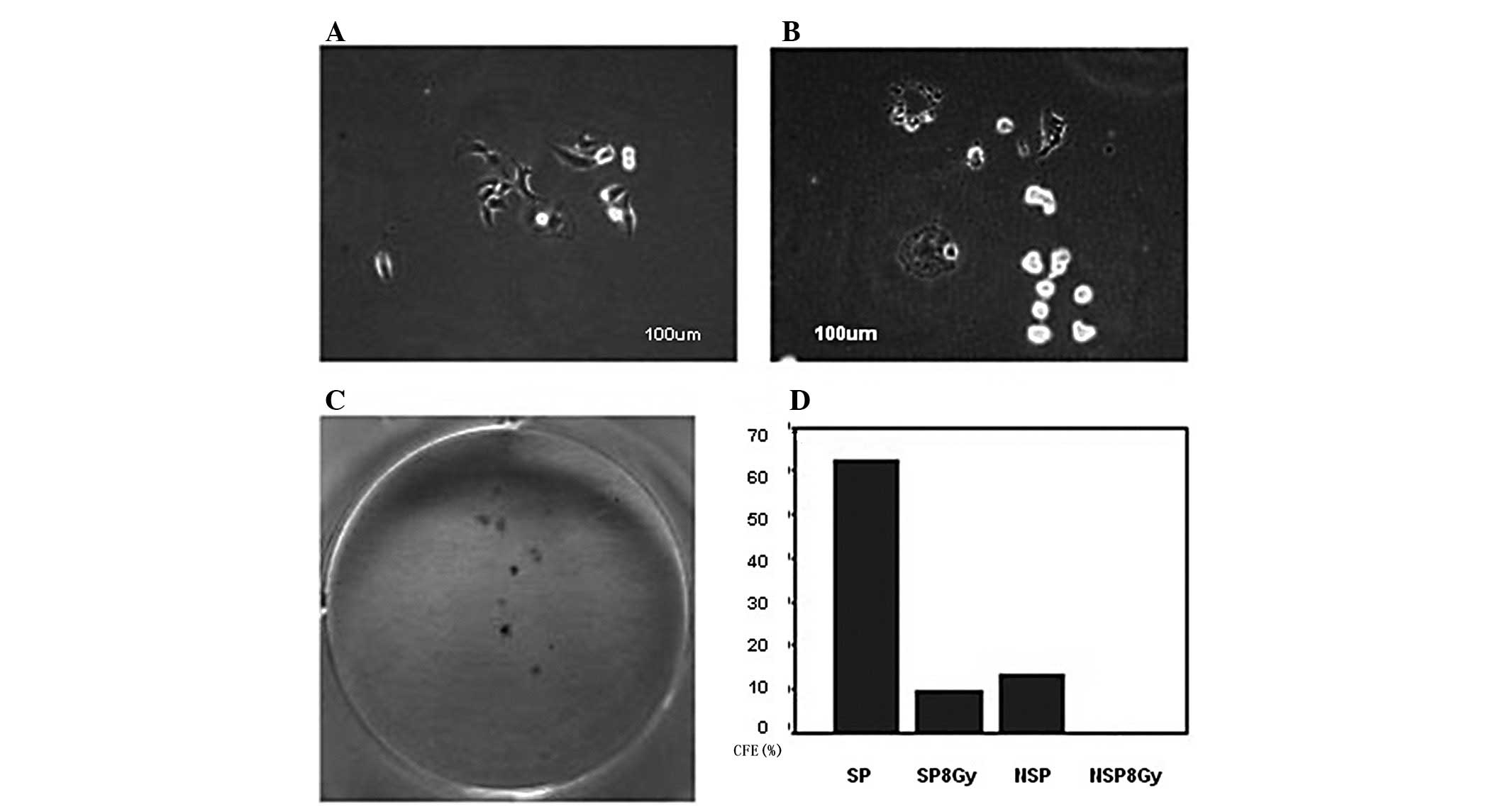
five, seven and eight days. Microscopic observation showed that the
SP cells proliferated, formed colonies and spread rapidly across
the culture plate. In contrast, the NSP cells grew slowly and only
a fraction formed colonies. The majority of these colonies appeared
swollen and disintegrated or divided several times and then
disintegrated (Figs. 2 and 3).
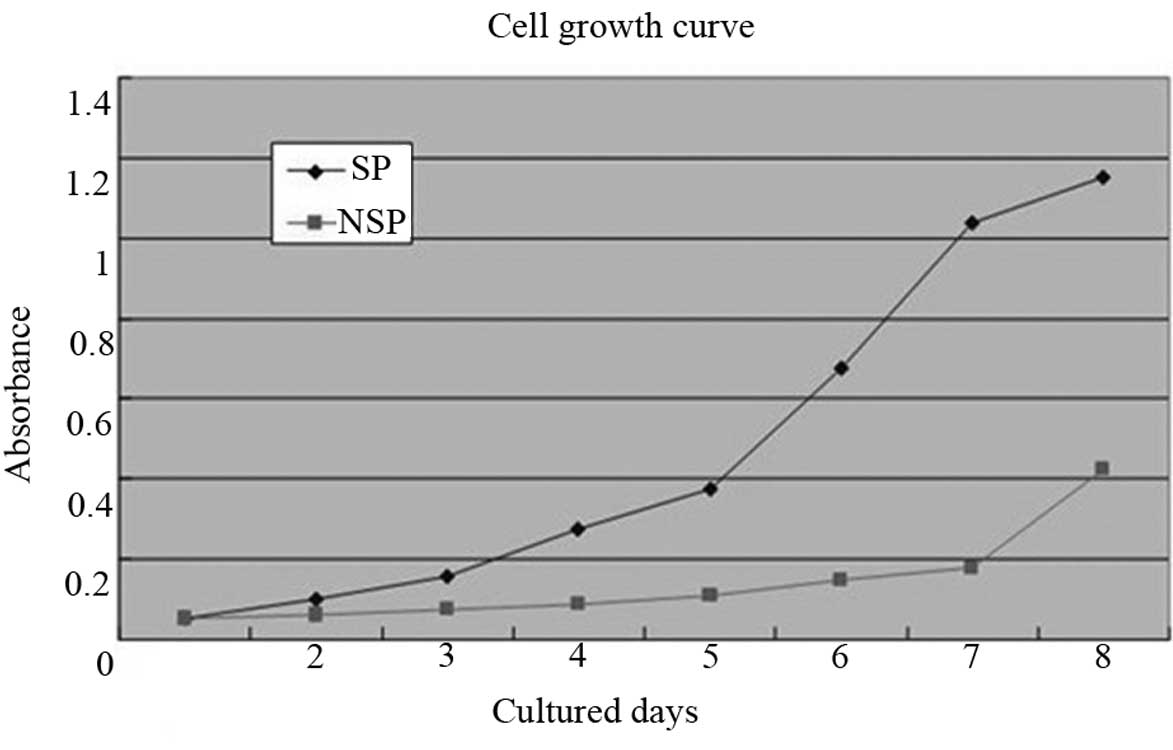
The MTT cell growth curves showed that the SP cells
entered a logarithmic growth phase within four days and a reached
plateau phase within eight days. However, the NSP cells grew
extremely slowly for the first seven days, following which, the
growth rate accelerated. The cellular growth rates were
significantly different (P<0.05) between the SP and NSP cells
(Fig. 4).
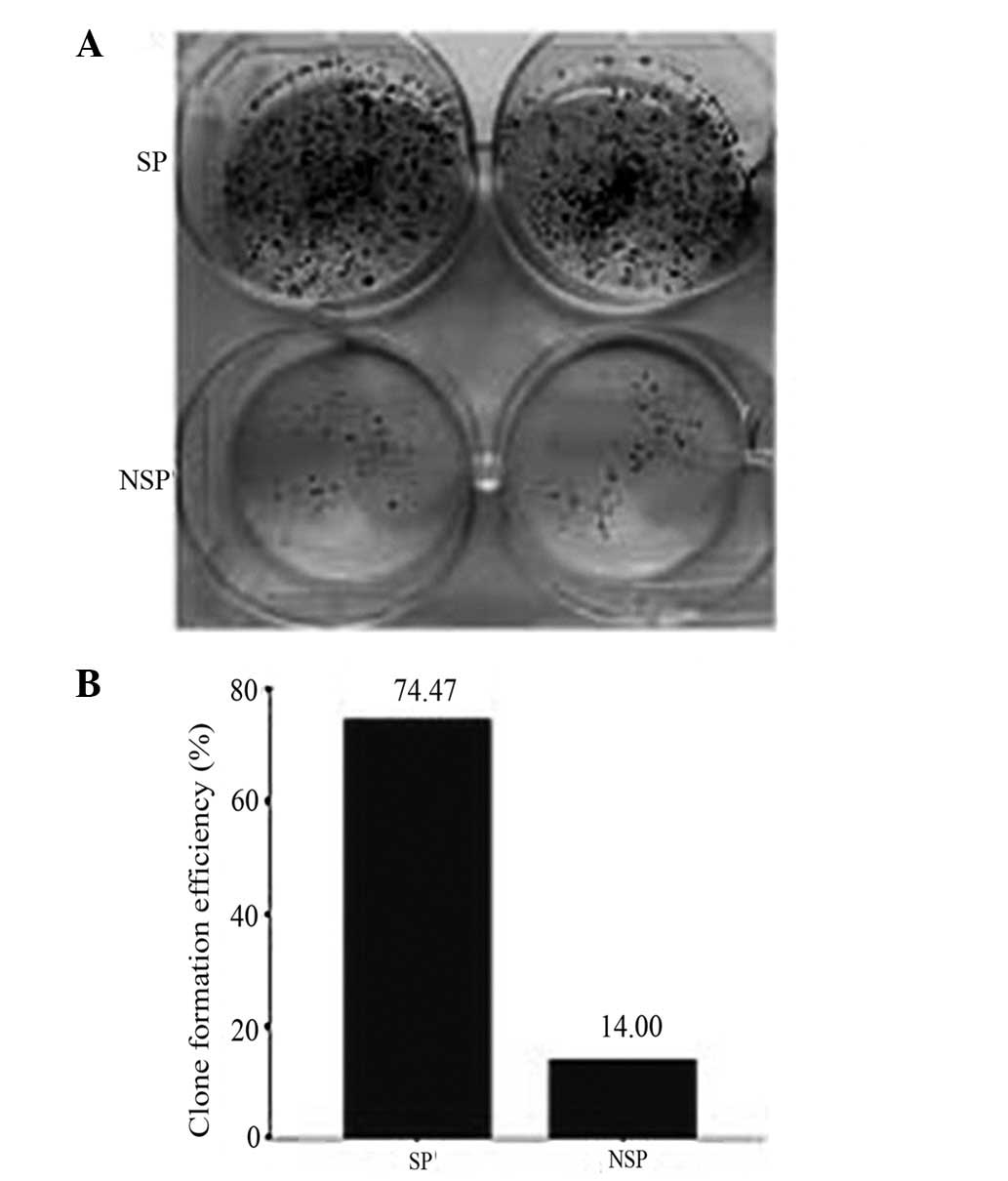
Colony-forming cell assays
The sorted SP and NSP cell clones expanded to >50
cells subsequent to being cultured in 6-well plates for ~10 days.
The CFE of the SP and NSP cells was 74.47±4.12 and 14.00±1.19%,
respectively, which represented a significant difference
(P<0.05; Fig. 5). Thus, the
ability of the SP cells to form clones was greater than that of the
NSP cells.
Long-term differentiation of SP and NSP
cells
Freshly sorted SP and NSP cells were cultured for
three weeks, stained with Hoechst 33342 and analyzed by FACS. The
percentage of SP cells that grew from the sorted SP cells was
~5.5%, whereas the percentage of SP cells that grew from the
sorted NSP cells was 0.1% (Fig.
6).
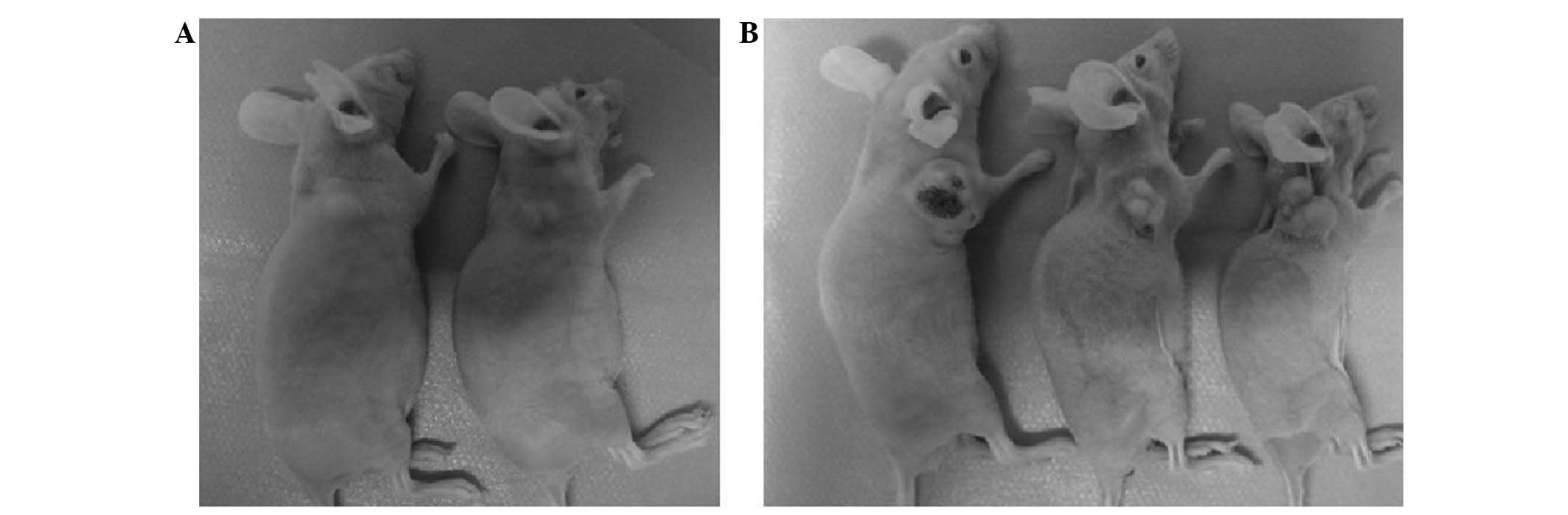
Tumor formation assay
Sorted SP and NSP cells were injected into the
Balb/c mice and allowed to grow for eight weeks. As few as
103 SP cells were sufficient for tumor formation in
these mice (2/3), whereas the mice that were injected with an equal
number of NSP cells produced no detectable tumors (0/3). Tumors
appeared following the injection of 104 SP cells (3/3).
In contrast, the injection of NSP cells failed to form tumors in
the Balb/c mice (0/3), with the exception of those in the group
that received the highest number (106) of NSP cells
(Table I; Fig. 7).
 | Table IIn vivo tumor formation ability
of the SP and NSP cells in Balb/c mice. |
Table I
In vivo tumor formation ability
of the SP and NSP cells in Balb/c mice.
| Quantity of
transplanted cells |
|---|
|
|
|---|
| Cell type,
n/total |
1×103 |
1×104 |
1×105 |
1×106 |
|---|
| SP cells | 2/3 | 3/3 | 3/3 | 3/3 |
| NSP cells | 0/3 | 0/3 | 0/3 | 2/3 |
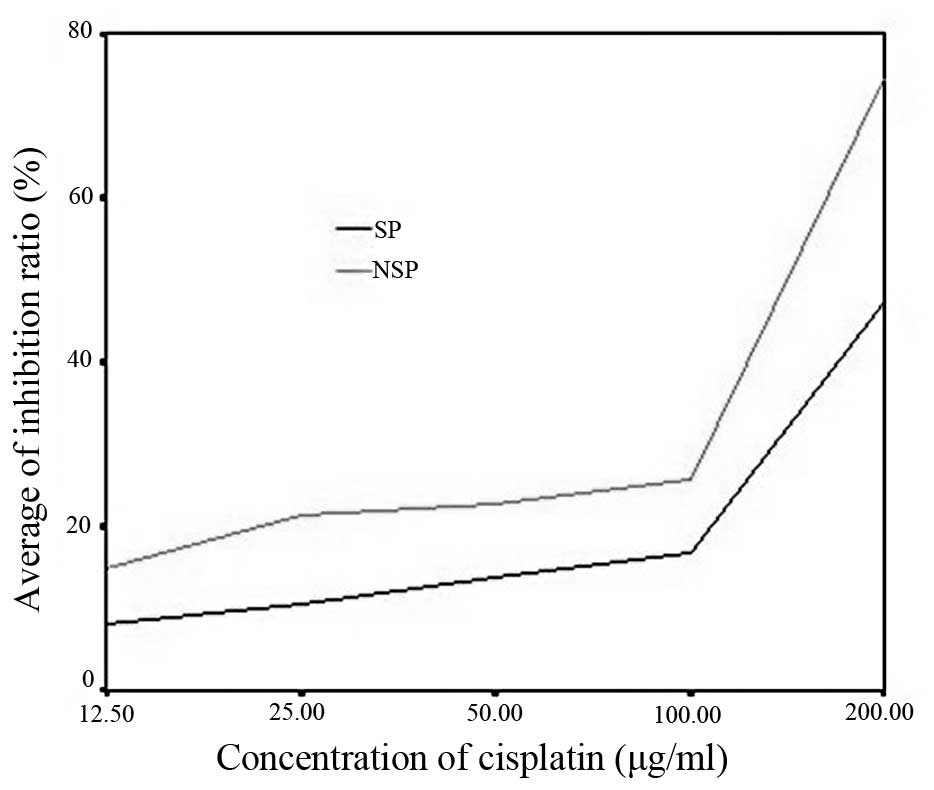
Chemotherapeutic drug sensitivity
assay
The cell inhibition ratio was calculated for the SP
and NSP cells following treatment with the various concentrations
of cisplatin (Table II). The
inhibition ratio curve (Fig. 8)
demonstrated that cisplatin suppressed the growth of the NSP cells
more than it suppressed the growth of the SP cells. Therefore, the
SP cells were more tolerant to cisplatin compared with the NSP
cells.
 | Table IICisplatin sensitivity of SP and NSP
cells. |
Table II
Cisplatin sensitivity of SP and NSP
cells.
| Cisplatin
concentration (μg/ml) | Inhibition
ratio | P-value |
|---|
|
|---|
| SP cells | NSP cells |
|---|
| 12.5 | 8.22±2.54 | 14.86±0.65 | <0.05 |
| 25.0 | 10.58±3.84 | 21.28±4.52 | <0.05 |
| 50.0 | 13.74±3.19 | 22.61±2.59 | <0.05 |
| 100.0 | 16.67±2.17 | 25.76±4.75 | <0.05 |
| 200.0 | 47.30±3.66 | 74.51±1.88 | <0.05 |
Radiotherapy sensitivity assay
The NSP cells were exposed to X-rays and cultured
for two days. Only a small number of the exposed NSP cells
survived, which then gradually became swollen and ultimately
underwent apoptosis (Fig. 9B),
rather than continuing to divide. A fraction of the SP cells that
were exposed to X-rays died. However, the living SP cells grew
slowly and formed clones within three weeks (Fig. 9A and C). The CFE of the SP cells
exposed to X-rays was 9.75±2.21%, which was significantly less than
that of the unexposed SP cells (63.44±2.36%; P<0.05), but
significantly greater than the CFE of the NSP cells that were
exposed to 8 Gy X-rays (0%; P<0.05). The CFE of the NSP cells
that were exposed to 8 Gy X-rays was significantly lower than that
of the NSP cells that were not exposed to X-rays (12.28±1.60%;
P<0.05; Fig. 9D).
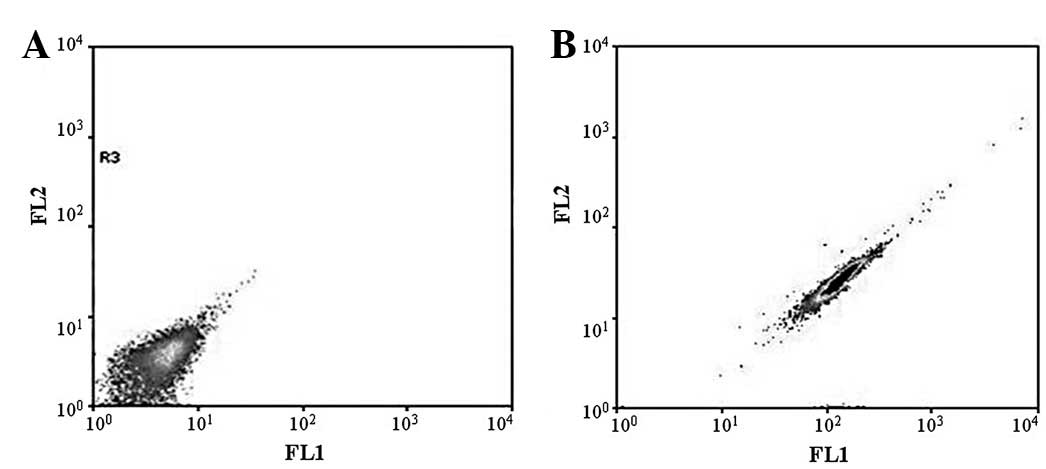
CD133 expression in SP and NSP cells
A small number of the HeLa cells expressed CD133
(~0.16% of the total cell number). No CD133 expression was detected
in the NSP cells. By contrast, the fraction of CD133-positive SP
cells was elevated to 3.64% (Fig.
10).
CD44 expression in SP and NSP cells
CD44 expression was positive in all the HeLa cells.
There was no significant difference in CD44 expression between the
SP and NSP cells (Fig. 11).
Discussion
SP cells were first isolated from murine bone marrow
by Goodell et al in 1996 (8). This small subset of cells were
identified to express a surface marker of hematopoietic stem cells
(HSCs) and were also able to rebuild the hematopoietic system of
the bone marrow. Since the initial application of the SP technique
in bone marrow HSCs, the method has been adapted to identify
putative stem cells and progenitor cells in multiple tissues and
organs, including umbilical cord blood (13), skeletal muscle (14) and mammary glands (15). SP cells possess certain intrinsic
stem cell properties, as they have a long life cycle, are mostly in
a relative resting state during telophase, have a high ability for
self-renewal and are tolerant to chemotherapeutic drugs. In 2004,
Kondo et al(12) first
isolated SP cells from the C6 glioma cell line and suggested that
the cells displayed stem cell-like characteristics. SP cells have
been identified in a large variety of neoplasms. The small and rare
subset of SP cells have an increased capacity to propagate tumor
growth, self-renew, initiate tumor formation when xenografted into
NOD/SCID mice and are particularly resistant to chemotherapeutic
agents. These characteristics are shared with tumor stem cells
(12,16,17).
In the present study, the SP cells were successfully
sorted from the HeLa cervical adenocarcinoma cell line using FACS.
The HeLa cell line contained ~1.2% SP cells, the characteristics of
which were investigated. An MTT assay revealed that the rate of
propagation and the colony formation capacity of the SP cells were
significantly higher than that of the NSP cells in the HeLa cell
line. These findings indicate that the SP cells may play a
significant role in the proliferation and renewal of HeLa cells.
Whereas NSP cells are mature cells that eventually undergo
apoptosis, the SP cells contribute to maintaining the number of
HeLa cells. This finding is consistent with other studies (12,16,17)
and suggests that the SP cells from the HeLa cell line have a
strong capacity for propagation and colony formation. In the tumor
formation assay of the present study, as few as 103 SP
cells were sufficient for tumor formation in the Balb/c mice. The
tumorigenic ability of the SP cells was 1,000 times higher than
that of the NSP cells. Therefore, the SP cells were significantly
more tumorigenic than the NSP cells. One of the most crucial
functions of stem cells is self-renewal. In the present study, the
SP cells showed a higher survival ability compared with the NSP
cells. Stem cells grow through two mechanisms, symmetrical division
and an asymmetrical second division (18–21).
The present data revealed that the percentage of the SP cells
within the sorted HeLa cells was ~5.5% and that the remaining cells
were NSP cells, indicating that SP cells in culture are able to
self-renew and generate SP and NSP progeny. However, the NSP cells
that are produced are mature cells, which are terminally
differentiated. Under the same culture conditions, the cells
produce NSP cells only. In contrast to the SP cells, NSP cells
undergo apoptosis following several generations of division.
According to the CSC theory, a small subpopulation
of cancer cells may have the capacity to tolerate the aggressive
insults of radiotherapy and chemotherapy. These cells may be
responsible for cancer reoccurrence. CSCs may be able to
re-establish cancer even if the majority of the cancer cells are
killed (22). In the present study,
the examination of the resistance to radiotherapy and chemotherapy
demonstrated that none of the NSP cells were able to survive for
more than a few days following exposure to 8 Gy X-rays. Although
the CFE of the SP cells that were exposed to X-rays was lower than
that of the unexposed SP cells, the exposed SP cells demonstrated a
significantly higher CFE than the exposed NSP cells. Thus, SP cells
are more resistant to radiotherapy than NSP cells and have the
characteristics of CSCs. An accepted hypothesis of chemoresistance
proposes that standard therapies fail to target tumor progenitors
that express normal stem cell phenotypes (23). In the present study, SP cells were
identified to be more resistant to cisplatin, indicating a role for
these cells in chemoresistance in cervical cancer. It has been
reported that high levels of the ABC transporter protein, ABCG2,
enhance the efflux capacity of SP cells for Hoechst 33342 dye and
for lipophilic anti-cancer drugs, including those used for cervical
cancer. If the ABC transporter is blocked, cells become sensitive
to drugs (24). In the present
study, western blot analyses showed that although ABCG2 was
overexpressed in the SP cells, the NSP cells hardly expressed the
protein (data not shown). This finding demonstrates that the
mechanism underlying chemoresistance is likely to involve the
overexpression of ABCG2. Evidence obtained in the present study has
demonstrated that the SP cells that were sorted from the HeLa cell
line present a strong capacity for cell proliferation, tumor
formation, self-renewal, differentiation and resistance to
radiotherapy and chemotherapy, which are typical CSC
characteristics.
CD133 is a membrane glycoprotein that has generated
much interest in the stem cell field. The glycoprotein was
originally discovered in hematopoietic stem or progenitor cells and
CD133 expression has been confirmed in CSCs from the colon
(25), pancreas (26), liver (27) and larynx (28). CD133 is therefore considered to be a
marker for CSCs. In the present study, CD133 was expressed by
~3.64% of the SP cells, but none of the NSP cells. Although the
ratio of CD133+ SP cells is low, CD133 may be considered
as a surface marker for cervical adenocarcinoma stem cells. Thus,
SP cells are enriched with tumorigenic stem-like cancer cells, but
are not equal to CSCs. Furthermore, SP cells differentiate into NSP
progeny during long-term culture. CSCs expressing CD133 that have
been isolated from gliomas are more resistant to radiotherapy than
CD133− tumor cells (29). The role of CD133 expression in SP
cells from the HeLa cell line, particularly as a biomarker for
cervical cancer, requires further investigation.
CD44 is considered to be a biomarker for breast
(3) and ovarian (30) CSCs. In the present study, all HeLa
cells were observed to express CD44, as determined by flow
cytometry. CD44 is therefore not considered to be a biomarker for
cervical adenocarcinoma stem cells.
In summary, the SP cells that were isolated from the
HeLa cell line demonstrated enhanced self-renewal, a high
proliferation potential in vitro, a strong ability to form
tumors in vivo and a high resistance to radiotherapy and
chemotherapy, which are all properties of CSCs. In cervical cancer,
as in other cancers, the characterization of CSCs may allow the
development of new treatments that are specifically targeted
against this critical population of SP cells, particularly against
their ability to self-renew, which may result in more effective
therapies.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (NSFC; no. 30973194).
References
|
1
|
Reya T, Morrison SJ, Clarke SF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
et al: Prospective identification of tumorigenic breast cancer
cells. Proc Natl Acad Sci USA. 100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singh SK, Clarke ID, Terasaki M, et al:
Identification of a cancer stem cell in human brain tumors. Cancer
Res. 63:5821–5828. 2003.PubMed/NCBI
|
|
5
|
Patrawala L, Calhoun T,
Schneider-Broussard R, et al: Highly purified CD44+ prostate cancer
cells from xenograft human tumors are enriched in tumorigenic and
metastatic progenitor cells. Oncogene. 25:1696–1708. 2006.
|
|
6
|
Baba T, Convery PA, Matsumura N, et al:
Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian
cancer cells. Oncogene. 28:209–218. 2009.
|
|
7
|
Zhang S, Balch C, Chan MW, et al:
Identification and characterization of ovarian cancer-initiating
cells from primary human tumors. Cancer Res. 68:4311–4320. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goodell MA, Brose K, Paradis G, et al:
Isolation and functional properties of murine hematopoietic stem
cells that are replicating in vivo. J Exp Med. 183:1797–1806. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hayashi S, Fujita K, Matsumoto S, et al:
Isolation and identification of cancer stem cells from a side
population of a human hepatoblastoma cell line, HuH-6 clone-5.
Pediatr Surg Int. 27:9–16. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J, Guo LP, Chen LZ, et al:
Identification of cancer stem cell-like side population cells in
human nasopharyngeal carcinoma cell line. Cancer Res. 67:3716–3724.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao Q, Geng L, Kvalheim G, et al:
Identification of cancer stem-like side population cells in ovarian
cancer cell line OVCAR-3. Ultrastruct Pathol. 33:175–181. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kondo T, Setoguchi T and Taga T:
Persistence of a small subpopulation of cancer stem-like cells in
the C6 glioma cell line. Proc Natl Acad Sci USA. 101:781–786. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Storms RW, Goodell MA, Fisher A, et al:
Hoechst dye efflux reveals a novel CD7(+)CD34(−) lymphoid
progenitor in human umbilical cord blood. Blood. 96:2125–2133.
2000.PubMed/NCBI
|
|
14
|
Iwatani H, Ito T, Imai E, et al:
Hematopoietic and nonhematopoietic potentials of Hoechst(low)/side
population cells isolated from adult rat kidney. Kidney Int.
65:1604–1614. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clayton H, Titley I and Vivanco Md: Growth
and differentiation of progenitor/stem cells derived from the human
mammary gland. Exp Cell Res. 297:444–460. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ho MM, Ng AV, Lam S and Hung JY: Side
population in human lung cancer cell lines and tumors is enriched
with stem-like cancer cells. Cancer Res. 67:4827–4833. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Guo LP, Chen LZ, et al:
Identification of cancer stem cell-like side population cells in
human nasopharyngeal carcinoma cell line. Cancer Res. 67:3716–3724.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morrison SJ and Kimble J: Asymmetric and
symmetric stem-cell divisions in development and cancer. Nature.
441:1068–1074. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Clevers H: Stem cells, asymmetric division
and cancer. Nat Genet. 37:1027–1028. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fürthauer M and González-Gaitan M:
Endocytosis, asymmetric cell division, stem cells and cancer: unus
pro omnibus, omnes pro uno. Mol Oncol. 3:339–353. 2009.PubMed/NCBI
|
|
21
|
Neumüller RA and Knoblich JA: Dividing
cellular asymmetry: asymmetric cell division and its implications
for stem cells and cancer. Genes Dev. 23:2675–2699. 2009.PubMed/NCBI
|
|
22
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar
|
|
23
|
Zhou S, Schuetz JD, Bunting KD, et al: The
ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem
cells and is a molecular determinant of the side-population
phenotype. Nat Med. 7:1028–1034. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Balabanov S, Gontarewicz A, Keller G, et
al: Abcg2 overexpression represents a novel mechanism for acquired
resistance to the multi-kinase inhibitor Danusertib in
BCR-ABL-positive cells in vitro. PLoS One. 6:e191642011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
et al: Identification and expansion of human
colon-cancer-initiating cells. Nature. 445:111–115. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hermann PC, Huber SL, Herrler T, et al:
Distinct populations of cancer stem cells determine tumor growth
and metastatic activity in human pancreatic cancer. Cell Stem Cell.
1:313–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang ZF, Ho DW, Ng MN, et al: Significance
of CD90+ cancer stem cells in human liver cancer. Cancer Cell.
13:153–166. 2008.
|
|
28
|
Zhou L, Wei X, Cheng L, et al: CD133, one
of the markers of cancer stem cells in Hep-2 cell line.
Laryngoscope. 117:455–460. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bao S, Wu Q, McLendon RE, et al: Glioma
stem cells promote radioresistance by preferential activation of
the DNA damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang S, Balch C, Chan MW, et al:
Identification and characterization of ovarian cancer-initiating
cells from primary human tumors. Cancer Res. 68:4311–4320. 2008.
View Article : Google Scholar : PubMed/NCBI
|