Introduction
Gastroenteropancreatic neuroendocrine tumors
(GEP-NETs) are complicated and rare tumors arising from the
neuroendocrine system of the gut. The estimated annual incidence is
1–4 cases per 100,000 individuals and recent studies have revealed
an increasing incidence at several sites of the tumor (1–5).
GEP-NETs are traditionally classified according to their origin
from divisions of the gut (6).
However, the biological and clinical characteristics of the tumors
vary greatly between the subgroups. The 2010 WHO classification of
endocrine tumors of the gastroenteropancreatic tract has been used
more recently (7). According to the
classification, GEP-NETs are graded between highly differentiated
(G1) and poorly differentiated (G3). Intermediate grade (G2)
GEP-NETs have a moderately aggressive, but less predictable course,
while tumors of G1 grade are relatively indolent and those of G3
grade are extremely aggressive (8).
Therefore, there remains a requirement for new prognostic and
predictive factors in order to optimize treatments among the
patients of the intermediate grade.
Immunohistochemical markers have been used in a
number of tumors as effective predictors of malignant behavior. p16
and p21, the cyclin-dependent kinase inhibitors (CKIs), block
cellular proliferation and govern the G1/S cell cycle checkpoint
(9). These CKIs bind to cyclin
dependent kinases (CDK4 and/or CDK2) and thereby prevent activation
of the CDKs. Phosphorylation of the retinoblastoma protein, a key
step for cell cycle progression from G1 to S phase, does not occur
in the absence of activated CDKs (9). p16 and p21 expression has been studied
in a number of human tumors (10–13);
however, there are limited data on the expression of p16 and p21 in
GEP-NETs.
To further elucidate the molecular pathogenesis of
GEP-NETs and identify immunohistochemical markers for the
determination of patient outcomes, the expression of p16 and p21 in
a series of patients with GEP-NETs was tested.
Materials and methods
Patients
A total of 68 patients with GEP-NETs, undergoing
surgery at Henan Cancer Hospital (Zhengzhou, China) between 2000
and 2010, were investigated in this study. The median age at
diagnosis was 59 years (range, 28–86 years old). The patients were
not treated by chemotherapy or radiotherapy prior to surgery. All
patients were followed until mortality or until November 30, 2012.
No case was lost during follow-up and the patients were censored
following five follow-up years. The study was approved by the
ethics committee of Henan Cancer Hospital (Zhengzhou, China).
Written informed consent was obtained from the patients.
Ki-67 was restained and recounted to calculate the
Ki-67 index. The Ki-67 index was determined by assessing the
percentage of positively stained tumor cell nuclei. These
evaluations were made in the most viable areas of the stained tumor
sections that also demonstrated the highest degree of nuclear
labeling According to the standards of the 2010 WHO Classification
(7), tumors with a Ki-67 index of
<2% were categorized as G1, 3–20% were categorized as G2 and
≥20% as G3. In this study, 9 (13.2%) patients were G1, 37 (54.4%)
were G2 and 22 (32.4%) were G3.
Immunohistochemistry
Sections (5-μm thick) were cut and deparaffinized
with xylene and dehydrated in graded alcohols. Endogenous
peroxidase activity was blocked with 3% hydrogen peroxide in
methanol for 10 min. The sections were then treated with microwave
radiation for 10 min for antigen retrieval and to block intrinsic
antibody binding and then reacted with normal serum (mouse IgG) for
10 min at room temperature. The sections were subsequently
incubated with primary antibodies against p16 (clone 6H12), Ki67
(clone MIB-1) and p21 (clone DCS-60.2; Maixin Bio, Fuzhou, China)
overnight at 4°C, with appropriate negative and positive controls,
and reacted with the horseradish peroxidase-polymer anti-mouse
antibody (Maixin Bio) for 40 min. Diaminobenzidine
tetrahydrochloride was used as the final chromogen. Sections were
counterstained with Mayer’s hematoxylin and dehydrated in graded
alcohols before mounting.
Four classes were used to score nuclear positively
stained tumor cells; none, <5, 5–50 and >50% of the cells.
Protein levels were classified as high for p16 when any nuclear
staining was identified in the tumor tissue and for p21 when ≥5% of
the tumor cells were positive, as outlined in previous studies
(14,15). All slides were evaluated the same
day by two pathologists to minimize the variability of the
results.
Statistical analyses
The associations between p16 and p21 protein
expression and clinicopathological variables were evaluated by
Fisher’s exact test. Survival rates were estimated by the
Kaplan-Meier method, starting from the time of diagnosis.
Prognostic analysis of p16 and p21 in GEP-NETs was performed by Cox
proportional hazards regression model. When analyzing the G2
subgroup, the colon and rectum were combined with the pancreas into
one group. p16 and p21 were always kept in the model and the
inclusion criteria of other variables were 0.10 on forward stepwise
regression. All statistical analyses were performed using SPSS
software (version 11.0; SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
The immunohistochemical results in GEP-NETs are
summarized in Table I. For p16, low
(no positive nuclei) and high (any positive nuclei) protein levels
were detected in 37 (54%) and 31 (46%) cases, respectively. Low
immunoreactivity (<5% positive nuclei) of p21 was found in 45
(66%) cases and high expression (≥5% positive nuclei) was observed
in 23 (34%) of the cases.
 | Table IImmunostaining results for p16 and
p21. |
Table I
Immunostaining results for p16 and
p21.
| Cases, n (%) |
|---|
|
|
|---|
| Expression | p16 | p21 |
|---|
| Negative | 37 (54) | 36 (53) |
| <5% | 2 (3) | 9 (13) |
| 5–50% | 23 (34) | 19 (28) |
| >50% | 6 (9) | 4 (6) |
No significant correlation was found between the
expression of p16 and p21 and clinicopathological variables,
including tumor origin, the classification, tumor size, functional
status, metastasis and localization of the metastases (Table II). Examples of immunohistochemical
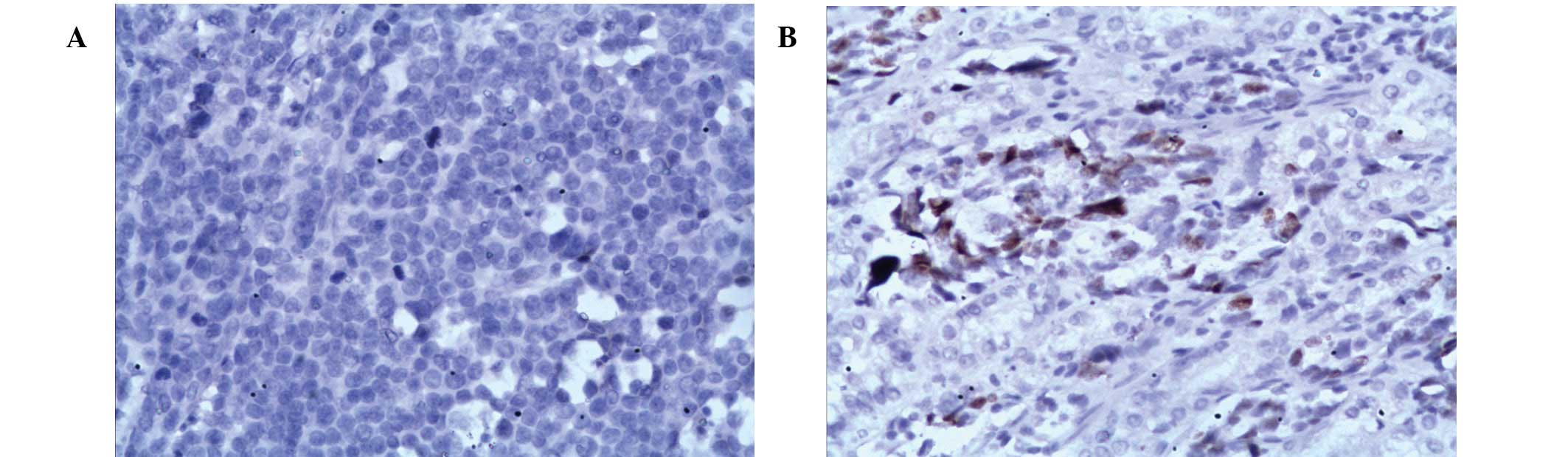
staining for low p16 (no positive nuclei staining) and high p21
(≥5% positive nuclei staining) are presented in Fig. 1.
 | Table IIPatient demographics and clinical
features. |
Table II
Patient demographics and clinical
features.
| | p16 expression | p21 expression |
|---|
| |
|
|
|---|
| Variable | Total, n | High (%) | Low (%) | P-valuea | High (%) | Low (%) | P-valuea |
|---|
| Gender | | | | 0.16 | | | 0.77 |
| Male | 51 | 26 (51) | 25 (49) | | 18 (35) | 33 (65) | |
| Female | 17 | 5 (29) | 12 (71) | | 5 (29) | 12 (71) | |
| Age, years | | | | 0.05 | | | 0.61 |
| <60 | 38 | 13 (34) | 25 (66) | | 14 (37) | 24 (63) | |
| ≥60 | 30 | 18 (60) | 12 (40) | | 9 (30) | 21 (70) | |
| Tumor origin | | | | 0.12 | | | 0.29 |
| Gastric | 51 | 27 (53) | 24 (47) | | 15 (29) | 36 (71) | |
| Colon and
rectum | 10 | 2 (20) | 8 (80) | | 4 (40) | 6 (60) | |
| Pancreas | 7 | 2 (29) | 5 (71) | | 4 (57) | 3 (43) | |
| WHO
classification | | | | 0.12 | | | 0.35 |
| G1 | 9 | 2 (22) | 7 (78) | | 5 (56) | 4 (44) | |
| G2 | 37 | 21 (57) | 16 (43) | | 11 (30) | 26 (70) | |
| G3 | 22 | 8 (36) | 14 (64) | | 7 (32) | 15 (68) | |
| Tumor size, cm | | | | 0.53 | | | 0.16 |
| <2 | 11 | 6 (55) | 5 (45) | | 6 (55) | 5 (45) | |
| >2 | 57 | 25 (44) | 32 (56) | | 17 (30) | 40 (70) | |
| Functional
status | | | | 1.00 | | | 1.00 |
| Nonfunctional | 51 | 23 (45) | 28 (55) | | 17 (33) | 34 (67) | |
| Functional | 17 | 8 (47) | 9 (53) | | 6 (35) | 11 (65) | |
| Metastasis | | | | 0.81 | | | 0.08 |
| Negative | 28 | 12 (43) | 16 (57) | | 13 (46) | 15 (54) | |
| Positive | 40 | 19 (47) | 21 (53) | | 10 (25) | 30 (75) | |
| Localization of
metastases | | | | 0.69 | | | 0.06 |
| Liver | 8 | 4 (50) | 4 (50) | | 4 (50) | 4 (50) | |
| Other | 27 | 10 (37) | 17 (63) | | 4 (15) | 23 (85) | |
The associations between clinicopathological and
immunohistochemical data and survival, in univariate and
multivariate analyses, are presented in Table III. The univariate analysis in all
cases showed a relative risk (RR) of succumbing to GEP-NETs of 1.4
(95% CI, 0.8–2.4; P=0.25) for low expression of p16 and an RR of
0.7 (95% CI, 0.4–1.2; P=0.16) for high expression of p21. In
multivariate analyses, WHO classification (P<0.01) and
metastasis (P=0.01) were the only parameters with statistical
significance.
 | Table IIIRR of succumbing to GEP-NETs. |
Table III
RR of succumbing to GEP-NETs.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Variable | RR | 95% CI | P-value | RR | 95% CI | P-value |
|---|
| Gender | | | 0.13 | | | |
| Male | 1.0 | - | | | | |
| Female | 1.7 | 0.9–3.4 | | | | |
| Age, years | | | 0.20 | | | |
| <60 | 1.0 | - | | | | |
| ≥60 | 1.4 | 0.8–2.4 | | | | |
| Tumor origin | | | 0.05 | | | |
| Gastric | 1.0 | - | | | | |
| Colon and
rectum | 0.7 | 0.3–1.5 | | | | |
| Pancreas | 0.1 | 0–0.5 | | | | |
| WHO
Classification | | | <0.01 | | | <0.01 |
| G1 | 1.0 | - | | 1.0 | - | |
| G2 | 13.5 | 1.8–99.1 | | 15.1 | 1.9–122.5 | |
| G3 | 25.5 | 3.4–193.0 | | 26.8 | 3.4–213.0 | |
| Tumor size, cm | | | 0.08 | | | |
| <2 | 1.0 | - | | | | |
| >2 | 2.2 | 0.9–5.1 | | | | |
| Functional
status | | | 0.84 | | | |
| Nonfunctional | 1.0 | - | | | | |
| Functional | 0.9 | 0.5–1.8 | | | | |
| Metastasis | | | <0.01 | | | 0.01 |
| Negative | 1.0 | - | | 1.0 | - | |
| Positive | 2.5 | 1.4–4.4 | | 2.1 | 1.2–3.7 | |
| Localization of
metastases | | | 0.41 | | | |
| Liver | 1.0 | - | | | | |
| Other | 0.7 | 0.3–1.6 | | | | |
| p16 expression | | | 0.25 | | | 0.09 |
| High (+) | 1.0 | - | | 1.0 | - | |
| Low (−) | 1.4 | 0.8–2.4 | | 1.7 | 0.9–3.0 | |
| p21 expression | | | 0.16 | | | 0.12 |
| Low (<5%) | 1.0 | - | | 1.0 | - | |
| High (>5%) | 0.7 | 0.4–1.2 | | 0.6 | 0.3–1.1 | |
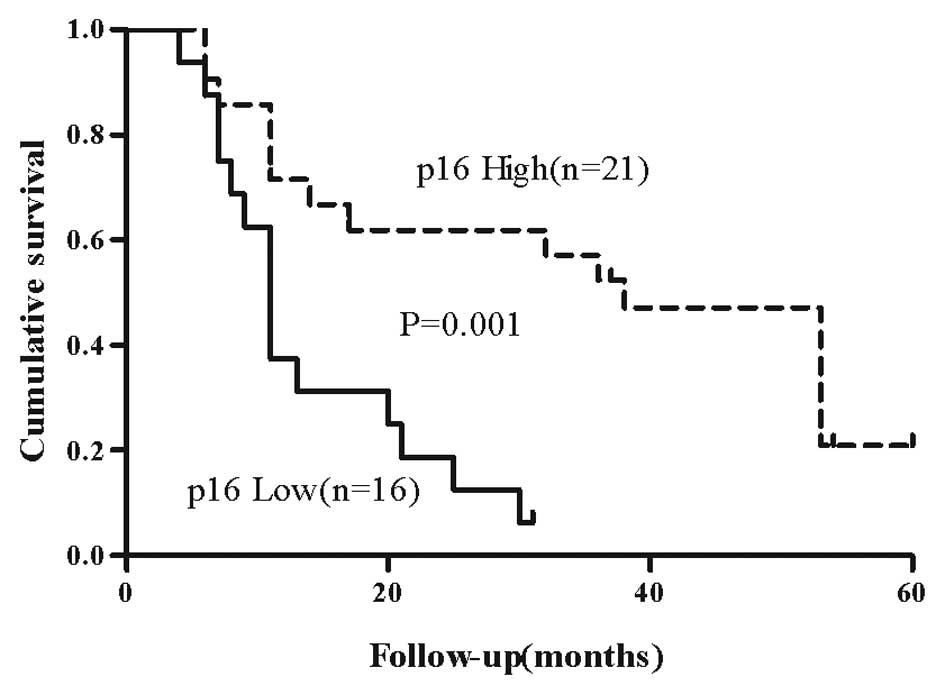
The results for p16 from the univariate analysis for
the patients classified as G2 are depicted in Fig. 2. In the survival analysis of the
cases classified as G2, low p16 indicated prognostic relevance with
an RR of 4.4 (95% CI, 1.8–10.6; P=0.001), revealing a poorer
prognosis for patients presenting with tumors expressing low levels
of p16 (Table IV).
 | Table IVRR of mortality in the G2 group of
GEP-NETs (n=37). |
Table IV
RR of mortality in the G2 group of
GEP-NETs (n=37).
| Univariate
analysis |
|---|
|
|
|---|
| Variable | RR | 95% CI | P-value |
|---|
| Gender | | | 0.91 |
| Male | 1.0 | - | |
| Female | 1.0 | 0.5–2.3 | |
| Age, years | | | 0.78 |
| <60 | 1.0 | - | |
| ≥60 | 0.9 | 0.4–1.8 | |
| Tumor origin | | | 0.63 |
| Gastric | 1.0 | - | |
| Colon, rectum and
pancreas | 0.8 | 0.3–2.1 | |
| Tumor size, cm | | | 0.48 |
| <2 | 1.0 | - | |
| >2 | 1.5 | 0.5–5.1 | |
| Functional
status | | | 0.30 |
| Nonfunctional | 1.0 | - | |
| Functional | 1.5 | 0.7–3.1 | |
| Metastasis | | | 0.14 |
| Negative | 1.0 | - | |
| Positive | 1.8 | 0.8–3.8 | |
| Localization of the
metastases | | | 0.85 |
| Liver | 1.0 | - | |
| Other | 0.9 | 0.3–2.4 | |
| p16 expression | | | <0.01 |
| High (+) | 1.0 | - | |
| Low (−) | 4.4 | 1.8–10.6 | |
| p21 expression | | | 0.99 |
| Low (<5%) | 1.0 | - | |
| High (>5%) | 1.0 | 0.5–2.2 | |
Discussion
The diagnostic and prognostic role of p16 and p21 in
human tumors has been evaluated for a number of years. However,
there are few studies regarding the expression status of p16 and
p21 in GEP-NETs. In this study, 68 patients with GEP-NETs were
analyzed and p16 was found to represent a valuable prognostic
marker for survival.
The p16ink4a tumor suppressor protein is
encoded by the CDKN2A gene and functions as an inhibitor of CDK4
and CDK6. Inactivation of the CDKN2A gene contributes to the bypass
of a mid-late G1 restriction point (R point) and is associated with
progression to malignant disease. Inactivation of the
p16ink4a gene by deletion, methylation and point
mutation has been found in ~50% of all human tumors (16–19).
In GEP-NETs, inactivation of the CDKN2A gene appears to confer a
more malignant prognosis (20).
However, loss or reduction of p16ink4a
transcription or staining without marked inactivation of the CDKN2A
gene has also been reported (21).
Arnold et al found that p16 expression was lost in 49/118
(42%) of GEP-NETs and there were no promoter methylation of the
gene (14). Therefore, an
additional mechanism may contribute to GEP-NETs that retain the
wild-type CDKN2A gene, potentially underestimating the percentage
of p16ink4a inactivation.
In the present study, p16 expression was lost in
37/68 (54%) of cases. Among the G2 subgroup, a low level of p16
expression was shown to be associated with decreased overall
survival.
The cyclin-dependent kinase inhibitor p21
(Waf1/Cip1) is considered as a negative regulator of the cell cycle
and a tumor suppressor. Previously, Kawahara et al found
that overexpression of p21 correlates with malignant behavior in
GEP-NETs patients (15). In the
present study, high p21 expression was found in 23/68 (34%) of the
68 patients. However, no prognostic significance for p21 was
identified.
In conclusion, the current study demonstrates a
prognostic relevance for p16. Low expression of p16 was found to
correlate with a shorter overall survival in patients graded as the
G2 subgroup. Results of the present study indicate the value of the
incorporation of immunohistochemical expression of p16 into a new
classification to individualize therapeutic strategies within this
subgroup in the future.
References
|
1
|
Gastrointestinal Pathology Study Group of
Korean Society of Pathologists. Cho MY, Kim JM, Sohn JH, et al:
Current trends of the incidence and pathological diagnosis of
gastroenteropancreatic neuroendocrine tumors (GEP-NETs) in Korea
2000–2009: multicenter study. Cancer Res Treat. 44:157–165.
2012.PubMed/NCBI
|
|
2
|
Quaedvlieg PF, Visser O, Lamers CB,
Janssen-Heijen ML and Taal BG: Epidemiology and survival in
patients with carcinoid disease in The Netherlands. An
epidemiological study with 2391 patients. Ann Oncol. 12:1295–1300.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Modlin IM, Lye KD and Kidd M: A 5-decade
analysis of 13,715 carcinoid tumors. Cancer. 97:934–959. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lepage C, Bouvier AM, Phelip JM, Hatem C,
Vernet C and Faivre J: Incidence and management of malignant
digestive endocrine tumours in a well defined French population.
Gut. 53:549–553. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hemminki K and Li X: Incidence trends and
risk factors of carcinoid tumors: a nationwide epidemiologic study
from Sweden. Cancer. 92:2204–2210. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Williams ED and Sandler M: The
classification of carcinoid tum ours. Lancet. 1:238–239. 1963.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rindi G, Klimstra DS, Arnold R and Kloppel
G: Nomenclature and classification of neuroendocrine neoplasms of
the digestive system. WHO Classification of Tumours of the
Digestive System. Bosman FT, Carneiro F, Hruban RH and Theise ND:
IARC Press; Lyon: pp. 13–14. 2010
|
|
8
|
Klimstra DS, Modlin IR, Coppola D, Lloyd
RV and Suster S: The pathologic classification of neuroendocrine
tumors: a review of nomenclature, grading, and staging systems.
Pancreas. 39:707–712. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ekholm SV and Reed SI: Regulation of G(1)
cyclin-dependent kinases in the mammalian cell cycle. Curr Opin
Cell Biol. 12:676–684. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Witkiewicz AK, Knudsen KE, Dicker AP and
Knudsen ES: The meaning of p16(ink4a) expression in tumors:
functional significance, clinical associations and future
developments. Cell Cycle. 10:2497–2503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aaltomaa S, Lipponen P, Eskelinen M,
Ala-Opas M and Kosma VM: Prognostic value and expression of
p21(waf1/cip1) protein in prostate cancer. Prostate. 39:8–15. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiangming C, Hokita S, Natsugoe S, et al:
p21 expression is a prognostic factor in patients with p53-negative
gastric cancer. Cancer Lett. 148:181–188. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Elledge RM and Allred DC: Prognostic and
predictive value of p53 and p21 in breast cancer. Breast Cancer Res
Treat. 52:79–98. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arnold CN, Sosnowski A, Schmitt-Graff A,
Arnold R and Blum HE: Analysis of molecular pathways in sporadic
neuroendocrine tumors of the gastro-entero-pancreatic system. Int J
Cancer. 120:2157–2164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawahara M, Kammori M, Kanauchi H, et al:
Immunohistochemical prognostic indicators of gastrointestinal
carcinoid tumours. Eur J Surg Oncol. 28:140–146. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ortega S, Malumbres M and Barbacid M:
Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim
Biophys Acta. 1602:73–87. 2002.PubMed/NCBI
|
|
17
|
Gonzalez S and Serrano M: A new mechanism
of inactivation of the INK4/ARF locus. Cell Cycle. 5:1382–1384.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Esteller M, Corn PG, Baylin SB and Herman
JG: A gene hypermethylation profile of human cancer. Cancer Res.
61:3225–3229. 2001.PubMed/NCBI
|
|
19
|
Ruas M, Brookes S, McDonald NQ and Peters
G: Functional evaluation of tumour-specific variants of
p16INK4a/CDKN2A: correlation with protein structure information.
Oncogene. 18:5423–5434. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Simon B and Lubomierski N: Implication of
the INK4a/ARF locus in gastroenteropancreatic neuroendocrine
tumorigenesis. Ann NY Acad Sci. 1014:284–299. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chan AO, Kim SG, Bedeir A, Issa JP,
Hamilton SR and Rashid A: CpG island methylation in carcinoid and
pancreatic endocrine tumors. Oncogene. 22:924–934. 2003. View Article : Google Scholar : PubMed/NCBI
|