Introduction
Tumor antigens expressed on the surface of malignant
tumor cells serve as target proteins for the host immune system,
which remains a major area of cancer research. Cancer vaccines of
various types have highlighted possible approaches for treatments
that enhance the patient’s own immunity. Dendritic cells (DCs) are
particularly suitable for cancer vaccines, as they are potent
antigen-presenting cells. DC-based antitumor vaccines have emerged
as promising cancer immunotherapies with confirmed clinical
efficacy (1,2).
Major therapies to achieve the goals for the
cellular immunotherapy of cancer are divided into the following two
groups: i) vaccination of the tumor-bearing host with tumor
antigens to elicit T cell-mediated immunity to eradicate the
established tumor; and ii) treatment of the malignancy via the
adoptive transfer of in vitro cultured tumor-reactive T
cells (3–5). However, the majority of malignant
tumor cells are poorly immunogenic and are capable of evading the
host immune surveillance. Previous results have demonstrated that
tumor cells downregulate the expression of signals that are
essential for the activation of host T cells. The mechanisms
include defective expression of major histocompatibility complex
(MHC) molecules, absence of co-stimulatory or adhesion molecules
and alteration of antigen-processing or transport, which result in
an inability to present tumor-associated antigens (6). Strategies of augmenting the host
immune responses to the tumor included the introduction of genes
encoding MHC molecules (7),
co-stimulatory molecules (8) and
cytokines (9) into tumor cells or
DCs to improve immunogenicity and antigen-presenting
capabilities.
DCs are uniquely capable of inducing primary immune
responses (10). Accumulating data
have shown that DCs induce marked antitumor immune responses in
vitro and in vivo by their exceptional capabilities to
capture antigens, process and present antigenic peptide fragments,
migrate to lymphoid organs and strengthen the primary immune
responses of CD8+ and CD4+ T cells (11).
As few human tumor antigens have been identified,
immunization with DCs pulsed by purified tumor-associated peptides
or proteins has been regarded as a limited strategy for clinical
application. Due to a lack of tumor antigens, several strategies of
using tumor cell-charged DCs as vaccines for cancer immunotherapy
have been developed (5,12,13).
It has been hypothesized that the fusion vaccine may have
capabilities of antigen expression and presentation, and elicit
effective antitumor immunity (14).
In the present study, the fusion cellular vaccine of DCs and
syngeneic tumor cells was investigated to identify whether it
effectively elicits host antitumor immunity.
Materials and methods
Mice
Female C3H/HeJ mice were purchased from the Model
Animal Research Center of Nanjing University (Nanjing, China) and
fed under specific pathogen-free conditions in the Central Animal
Facility, Nanjing University. In a typical experiment, bone marrow
was isolated from the femur of 8- to 10-week-old mice, weighing
20–22 g. All animal experiments were performed in accordance with
protocols approved by the Animal Care and Use Committee of the
Medical School, Nanjing University.
DCs culture and tumor cell line
DCs were generated according to methods described in
our previous study (15). Briefly,
monocytes were isolated from the bone marrow of mice. Marrow
monocytes were flushed out from the femur and tibia, cultured with
RPMI 1640 supplemented with 10% FBS (both Gibco-BRL, Carlsbad, CA,
USA), 50 mM 2-Mercaptoethanol, 100 mM sodium pyruvate, 100 U/ml
penicillin, 100 mg/ml streptomycin, 10 ng/ml recombinant GM-CSF and
1 ng/ml murine recombinant IL-4 (all eBioscience, Inc., San Diego,
CA, USA). On days 2 and 4, 50% of the medium was removed and fresh
media was added. The released non-adherent immature DCs (imDCs)
were collected on day 6. SCC7, a mouse head and neck carcinoma cell
line, was cultured in DMEM media supplemented with 10% FBS, 2 mM
L-glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin.
DC/tumor fusion protocol
Bone marrow-derived DCs were fused with tumor cells
at a DC to tumor ratio of 3:1 using 50% polyethylene glycol (PEG;
molecular weight, 1450)/dimethyl sulfoxide (both Sigma-Aldrich, St.
Louis, MO, USA) solution. Briefly, the DC/tumor cell suspension was
washed twice with RPMI-1640 and prewarmed to 37°C. PEG (50%; 1 ml)
was added over 1 min and the suspension was stirred gently for 1–2
min. Prewarmed RPMI-1640 (1 ml) was then added over 1 min and the
suspension was stirred. An additional 3 ml-RPMI-1640 was added over
3 min, followed by 10 ml RPMI-1640, which was added slowly.
Following incubation for 5 min at 37°C, the resultant cell mixture
was pelleted and grown overnight in CM with GM-CSF. The fused cells
were irradiated (50 Gy), prior to injecting into mice to render
them non-proliferative.
Confocal microscopy
In total, ~2×104 DC/tumor fused cells
were spread on the coverslip, and DC and tumor cells were stained
with Cy5.5 and CFSE (Molecular Probes, Eugene, OR, USA),
respectively. Cells were washed, fixed and analyzed using a Laser
Scanning Confocal microscope (Fluoview, Fv10i; Olympus Corp.,
Tokyo, Japan).
FACS analysis of DC/tumor cells
The post-fusion ratio of DC, SCC7 and DC/SCC7 fused
cells was determined by FACS analysis. Briefly, 3×106
DCs and 1×106 SCC7 cells were incubated with Cy5.5 (10
μg/ml) and CFSE (5 μM) for 8 h, respectively and then washed with
FACS buffer. The fused cells were collected and fixed with 1.0%
paraformaldehyde. FACS analysis was performed on a FACS flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Tumor lysate-pulsed DC preparation
DCs were pulsed with freeze-thawed tumor lysates at
a DC to tumor ratio of 1:3. Briefly, SCC7 tumor cells
(1×107 cells in 500 μl RPMI) were lysed by repetitive
rapid freezing in liquid nitrogen and thawing in a 37°C bath, which
was repeated three times. Cellular debris were centrifuged (300 × g
for 3 min) and only the lysate supernatants were used to pulse DCs.
Pulsed DCs were harvested and washed twice following incubation for
12 h and then injected into mice.
Activation and proliferation of T cells
by mixed lymphocyte reaction (MLR)
DC/tumor fusion cells and DCs pulsed with tumor
lysate were collected and washed. Purified CD4+ T cells
were obtained by magnetic bead (Miltenyi Biotech, Bergisch
Gladbach, Germany) method from the lymph nodes (axillar, inguinal
and mesenteric) of normal C3H/He mice. Syngeneic T cells
(1×105/well) were incubated with fused cells or DCs
pulsed with tumor lysate at the ratio of 10:1 in flat-bottomed
96-well plates. Following incubation for 3 days, the proliferation
of T cells was determined by Cell Counting Kit-8 (Dojindo,
Kunamoto, Japan) incorporation assay. By contrast, the same level
of T cells were incubated with ConA (final concentration, 5
μg/ml).
Animal experiments
For therapeutic experiments, mice received s.c.
injection of 2×106 SCC7 cells in the right flank on day
0. On day 7, mice were randomized into cohorts of eight mice, each
exhibiting average tumor sizes of 20–30 mm2. On day 7,
tumor-bearing mice were treated with s.c. injections in the two
foot pads of 1×106 DC/tumor cells or DCs pulsed with
tumor lysate in a total volume of 40 μl PBS. On day 14, the two
sides of inguinal lymph nodes were injected with the same number of
cells as described previously. Tumor sizes were then assessed every
3 days, recorded in mm2 and determined as the product of
orthogonal measurements collected using vernier calipers. The
volume was calculated using the following formula: (short
diameter)2 × (long diameter) × 0.52. Data are presented
as the mean tumor area ± standard deviation (SD).
Enzyme-linked immunosorbent assay
(ELISA)
Following the sacrifice of animals, serum was
separated. ELISA was performed using a mouse IFN-γ ELISA kit
(Dakewe Biotech Co., Ltd., Shenzhen, China), according to the
manufacturer’s instructions. Briefly, serums were added into the
96-well plates coated with IFN-γ monoclonal antibody in duplicate
and incubated for 1 h at 37°C. Next, the plate was washed with
washing buffer three times. The HRP-conjugated secondary antibody
was then added into each well and the plate was incubated for 1 h
at 37°C. After washing the plate three times, TMB substrate
solution was added into each well and incubated for 15 min at room
temperature in the dark. Subsequently, the stop solution was used
to terminate the reaction. The absorbance was then determined at
450 nm by using a Synergy HT microplate reader (Bio-Tek
Instruments, Inc., Winooski, VT, USA).
Statistical analysis
Data are presented as the mean ± SD. Comparisons
were conducted using one-way analysis of variance, and P<0.05
was considered to indicate a statistically significant
difference.
Results
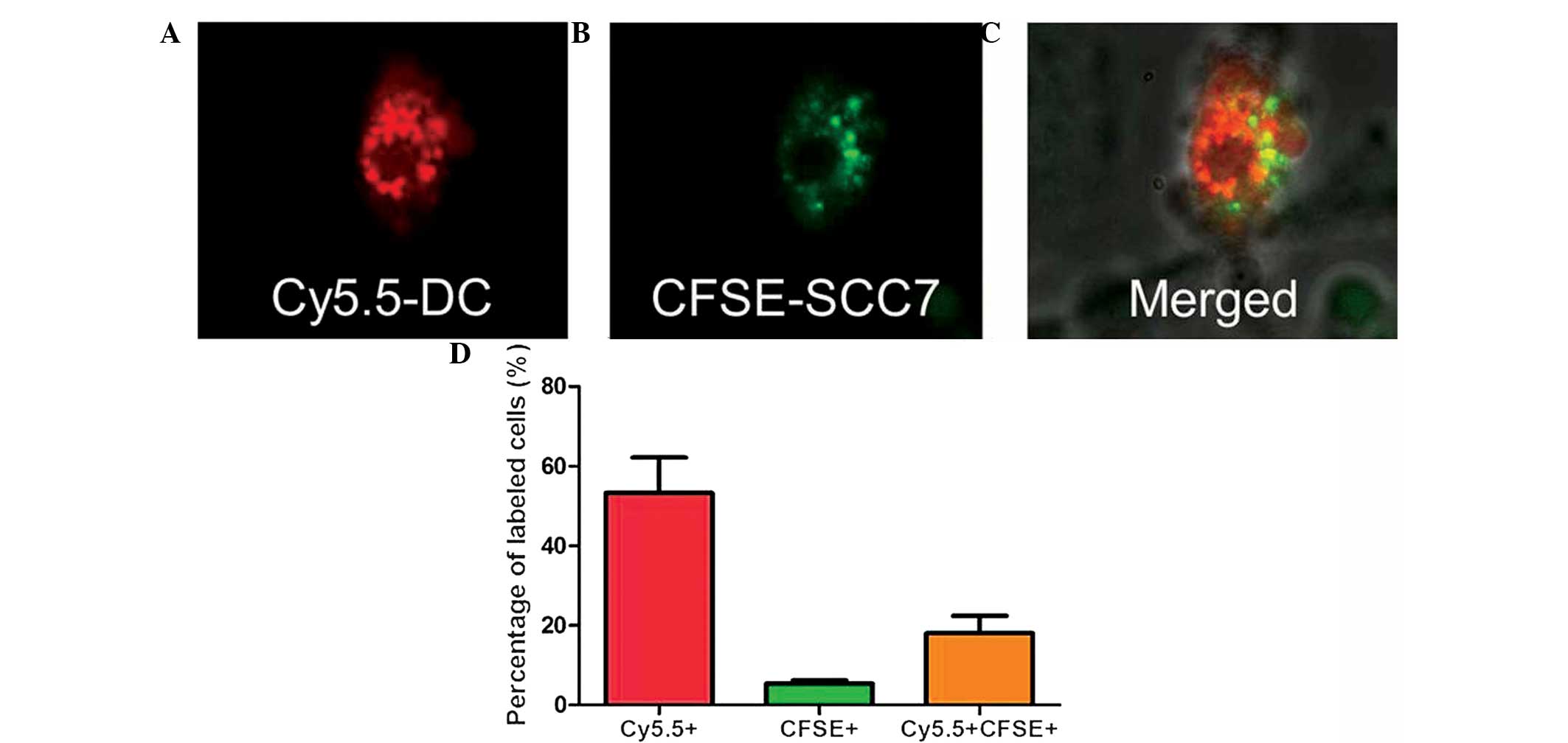
Confocal microscopy
Fused cells were first characterized by confocal
microscopy; DC surfaces were identified by Cy5.5 (red color;
Fig. 1A), while tumor cells were
identified as CFSE-positive (green cells; Fig. 1B). Overlapping these images resulted
in the detection of a subset of cells with both colors (Fig. 1C), indicating DC/tumor fused cells.
Following confirmation of the fusion of Cy5.5 and CFSE dual-labeled
DC/SCC7 hybrids by confocal microscopy, cell suspensions were
prepared to be measured by FACS and the percentage of DC/SCC7
hybrids was found to be 18.03%. Almost all tumor cells were
involved in cell fusion, with ~4.99% unfused tumor cells remaining
(Fig. 1D).
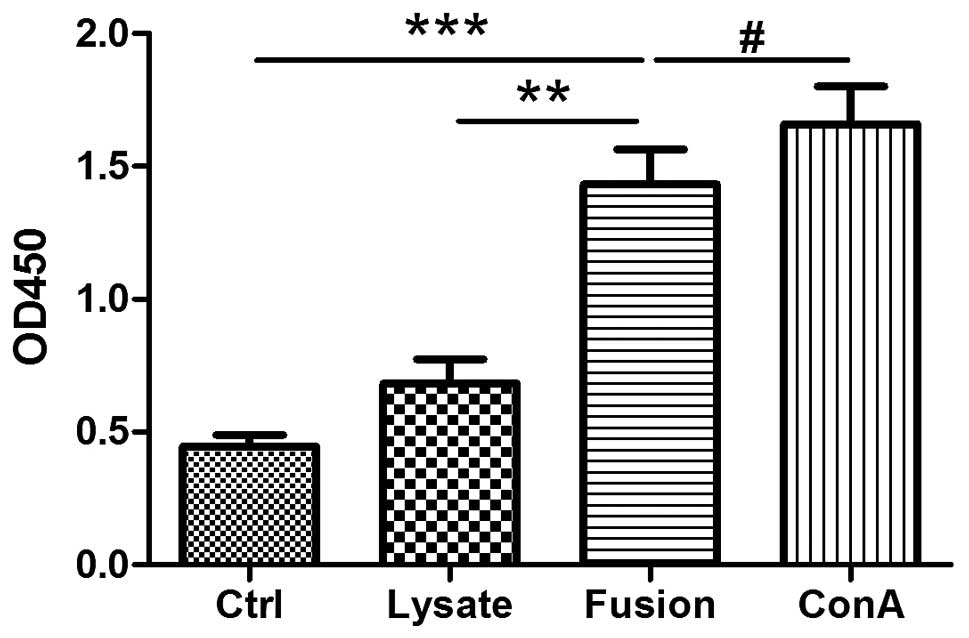
Proliferation of T cells by MLR
To confirm the capacities of antigen processing and
presenting, naive syngeneic T cells were stimulated by DC/SCC7
hybrids and DCs loaded with SCC7 whole tumor lysate, which had been
co-cultured with T cells at a ratio of 1:10 in a 3-day MLR. As
shown in Fig. 2, DC/SCC7 hybrids
and ConA evidently promoted T cell proliferation, whereas tumor
lysate-pulsed DCs induced a smaller increase in proliferation
following stimulation, compared with the controls. These results
demonstrated that DCs and SCC7 hybrids have an improved ability to
present antigens to T cells than the lysate group (Fig. 2).
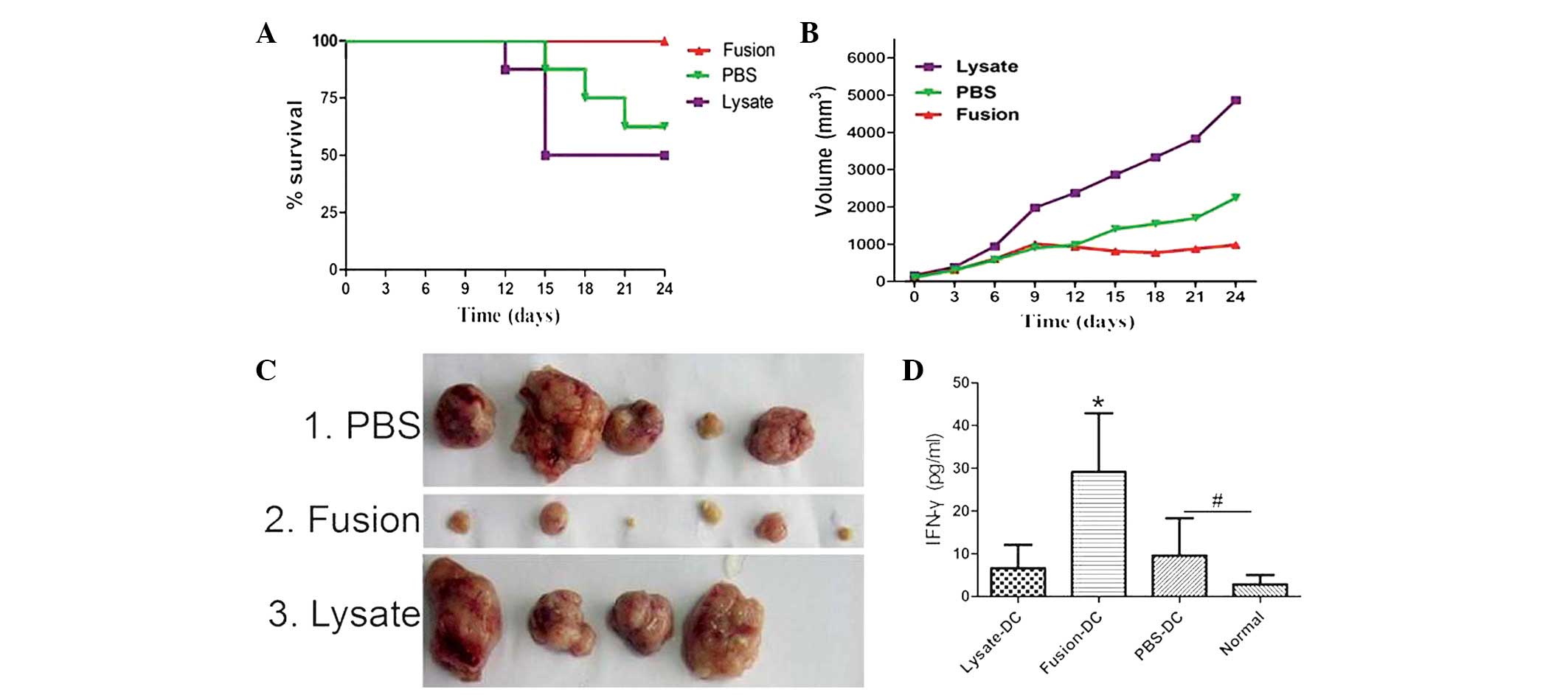
Antitumor efficacy of DC/SCC7 fused cells
in SCC7 cancer model
Mice bearing 7-day established SCC7 tumors were
vaccinated (s.c.) with DC/SCC7 hybrids and DCs pulsed with lysate
collected from parental SCC7 tumor cells. Mice treated with PBS
served as controls. Vaccination with DC/SCC7 hybrids evidently
improved survival rate (Fig. 3A)
and markedly inhibited tumor growth (Fig. 3B), compared with that of DCs pulsed
with lysate. In total, two out of eight treated mice were rendered
tumor-free and all mice survived up to sacrifice (Fig. 3C). On the 24th day, mice were
sacrificed and serum was collected and stored immediately at −20°C
until analysis. The level of IFN-γ in the serum of the fusion group
was higher compared with that of the other groups (Fig. 3D).
Discussion
The immune response of DC vaccines against solid
tumors has often been shown to be weak or localized and, therefore,
there has been concern with regard to the requirement to develop a
novel approach against tumors. The advantage of the DC/tumor fusion
vaccine compared with pulsing DCs with tumor lysate may be that
endogenously synthesized antigens have improved access to the MHC
class I pathway (16). An
additional important difference between the DC/tumor fusion
strategy and the whole tumor lysates loading strategy, is that DCs,
as well as tumor cells, are independently modified while their
characters persist following fusion.
In the current study, bone marrow imDCs from 6-day
culture in GM-CSF- plus IL-4-supplemented medium were used. It is
known that imDCs have more active antigen processing capabilities
compared with those of mature DCs (mDCs). However, whether tumor
and imDC or mDC hybrids generate a more potent antitumor effect
remains to be determined. In the present study, the TNF-α cytokine
was not selected to stimulate the maturation of imDCs, which means
the hybrids also have the ability to active T cells and initiate
anti-tumor immune responses.
The results of the current study revealed that
almost all tumor cells were fused with DCs and that only a few
tumor cells, ≤5%, remained unfused. This implies that this type of
fusion technology has a satisfied fusion efficiency. Efficient
separation of the tumor/DC hybrids from parental tumor cells in the
fusion preparations remains a technical challenge, therefore, the
remaining unfused tumor cells were irradiated prior to being
injected into mice. To confirm the capacities of antigen processing
and presenting, naive syngeneic T cells were stimulated by the
fusion vaccine and DCs loaded with tumor cell lysate, which had
been tested by MLR assay. The results revealed that the hybrids
significantly induced the proliferation of T cells.
In our previous studies, the migration of DCs
labeled with super paramagnetic iron oxide particles and enhanced
green fluorescent protein by magnetic resonance imaging and optical
imaging demonstrated that DCs migrate into the cortical area of the
draining popliteal lymph nodes by footpad injection (17). Notably, the ability of DCs to
migrate to lymph nodes was limited following intravenous infusion
(data not shown), and the antigen-specific immune responses induced
by intranodal injection were similar to those of intradermal
injection (18–20). Thus, footpad injection of the DC
vaccine was selected as the primary immunity and intranodal
infusion as the secondary dose one week later.
Previous animal studies have demonstrated that
fusion vaccines are superior to DCs loaded with tumor cell lysates
(21–23). Through cross-priming, the fusion
cells activate antigen-specific CD4+ T cells that become
multifunctional effectors, producing cytokines, particularly IFN-γ
(24–26). Moreover, the fusion cells function
as antigen-presenting cells with the ability to migrate to draining
lymph nodes, where they reside in the T cell area, interact with
CD4+ and CD8+ T cells and induce potent
antitumor immunity (24,27).
In summary, the present study demonstrated that the
novel cancer vaccine of DC/tumor hybrids promotes the proliferation
of syngeneic T cells and elicits a clear antitumor T-cell response.
The results indicate that the relatively crude preparations of DCs
and tumor cells are likely to suffice as sources of DC and tumor
fusion and, thus, indicate that DC/tumor hybrids may be of value
for the treatment of OSCC. Meanwhile, the fusion conditions for DCs
and tumor cells must be optimized for use in a clinical setting.
The current study presents an investigation into the strategies to
circumvent immune evasion of OSCC prior to the implementation of
large-scale clinical assessment.
Acknowledgements
The authors would like to thank Dr. Yayi Hou, Zichun
Hua and Guohua Xia for their technical assistance. This study was
supported by grants from the National Natural Sciences Foundation
of China (no. 81271698), the Project of Natural Science Foundation
of Jiangsu Province (no. BK2012744) and the Nanjing Medical
Development Foundation (nos. ZKX10031, ZKX12034, 201201077).
References
|
1
|
Steinman RM and Banchereau J: Taking
dendritic cells into medicine. Nature. 449:419–426. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Banchereau J and Palucka AK: Dendritic
cells as therapeutic vaccines against cancer. Nat Rev Immunol.
5:296–306. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iwahashi M, Katsuda M, Nakamori M, et al:
Vaccination with peptides derived from cancer-testis antigens in
combination with CpG-7909 elicits strong specific CD8+ T
cell response in patients with metastatic esophageal squamous cell
carcinoma. Cancer Sci. 101:2510–2517. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kono K, Mizukami Y, Daigo Y, et al:
Vaccination with multiple peptides derived from novel cancer-testis
antigens can induce specific T-cell responses and clinical
responses in advanced esophageal cancer. Cancer Sci. 100:1502–1509.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Turtle CJ and Riddell SR: Genetically
retargeting CD8+ lymphocyte subsets for cancer
immunotherapy. Curr Opin Immunol. 23:299–305. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Villarroel-Dorrego M, Speight PM and
Barrett AW: Expression of major histocompatibility complex class II
and costimulatory molecules in oral carcinomas in vitro. Med Oral
Patol Oral Cir Bucal. 10:188–195. 2005.PubMed/NCBI
|
|
7
|
Mimura K, Ando T, Poschke I, et al: T cell
recognition of HLA-A2 restricted tumor antigens is impaired by the
oncogene HER2. Int J Cancer. 128:390–401. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Slavin-Chiorini DC, Catalfamo M,
Kudo-Saito C, Hodge JW, Schlom J and Sabzevari H: Amplification of
the lytic potential of effector/memory CD8+ cells by
vector-based enhancement of ICAM-1 (CD54) in target cells:
implications for intratumoral vaccine therapy. Cancer Gene Ther.
11:665–680. 2004.PubMed/NCBI
|
|
9
|
Malvicini M, Alaniz L, Bayo J, et al:
Single low-dose cyclophosphamide combined with interleukin-12 gene
therapy is superior to a metronomic schedule in inducing immunity
against colorectal carcinoma in mice. Oncoimmunology. 1:1038–1047.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
O’Neill DW, Adams S and Bhardwaj N:
Manipulating dendritic cell biology for the active immunotherapy of
cancer. Blood. 104:2235–2246. 2004.PubMed/NCBI
|
|
11
|
Xu Q, Liu G, Yuan X, et al:
Antigen-specific T-cell response from dendritic cell vaccination
using cancer stem-like cell-associated antigens. Stem Cells.
27:1734–1740. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Williams BJ, Bhatia S, Adams LK, et al:
Dendritic cell based PSMA immunotherapy for prostate cancer using a
CD40-targeted adenovirus vector. PLoS One. 7:e469812012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Noguchi T, Kato T, Wang L, et al:
Intracellular tumor-associated antigens represent effective targets
for passive immunotherapy. Cancer Res. 72:1672–1682. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee WT: Dendritic cell-tumor cell fusion
vaccines. Adv Exp Med Biol. 713:177–186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mou Y, Chen B, Zhang Y, et al: Influence
of synthetic superparamagnetic iron oxide on dendritic cells. Int J
Nanomedicine. 6:1779–1786. 2011.PubMed/NCBI
|
|
16
|
Benencia F, Courrèges MC and Coukos G:
Whole tumor antigen vaccination using dendritic cells: comparison
of RNA electroporation and pulsing with UV-irradiated tumor cells.
J Transl Med. 6:212008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mou Y, Hou Y, Chen B, et al: In vivo
migration of dendritic cells labeled with synthetic
superparamagnetic iron oxide. Int J Nanomedicine. 6:2633–2640.
2011.PubMed/NCBI
|
|
18
|
Verdijk P, Aarntzen EH, Lesterhuis WJ, et
al: Limited amounts of dendritic cells migrate into the T-cell area
of lymph nodes but have high immune activating potential in
melanoma patients. Clin Cancer Res. 15:2531–2540. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Creusot RJ, Yaghoubi SS, Chang P, et al:
Lymphoid-tissue-specific homing of bone-marrow-derived dendritic
cells. Blood. 113:6638–6647. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huck SP, Tang SC, Andrew KA, Yang J,
Harper JL and Ronchese F: Activation and route of administration
both determine the ability of bone marrow-derived dendritic cells
to accumulate in secondary lymphoid organs and prime
CD8+ T cells against tumors. Cancer Immunol Immunother.
57:63–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shimizu K, Kuriyama H, Kjaergaard J, Lee
W, Tanaka H and Shu S: Comparative analysis of antigen loading
strategies of dendritic cells for tumor immunotherapy. J
Immunother. 27:265–272. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu Z, Liu S, Mai X, Hu Z and Liu C:
Anti-tumor effects of fusion vaccine prepared by renal cell
carcinoma 786-O cell line and peripheral blood dendritic cells of
healthy volunteers in vitro and in human immune reconstituted SCID
mice. Cell Immunol. 262:112–119. 2010. View Article : Google Scholar
|
|
23
|
Deng YJ, Zhang LJ, Su XD, et al: Dendritic
cell-tumor cell fusion vaccine prevents growth of subcutaneous
transplanted esophageal carcinomas. Ai Zheng. 28:1067–1071.
2009.(In Chinese).
|
|
24
|
Koido S, Tanaka Y, Chen D, Kufe D and Gong
J: The kinetics of in vivo priming of CD4 and CD8 T cells by
dendritic/tumor fusion cells in MUC1-transgenic mice. J Immunol.
168:2111–2117. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koido S, Hara E, Torii A, et al: Induction
of antigen-specific CD4- and CD8-mediated T-cell responses by
fusions of autologous dendritic cells and metastatic colorectal
cancer cells. Int J Cancer. 117:587–595. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koido S, Ohana M, Liu C, et al: Dendritic
cells fused with human cancer cells: morphology, antigen
expression, and T cell stimulation. Clin Immunol. 113:261–269.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Phan V, Errington F, Cheong SC, et al: A
new genetic method to generate and isolate small, short-lived but
highly potent dendritic cell-tumor cell hybrid vaccines. Nat Med.
9:1215–1219. 2003. View
Article : Google Scholar : PubMed/NCBI
|