Introduction
Liver cancer is one of the five most common types of
cancer and a main cause for the mortality of patients with cancer
(1). Liver cancer involves a
rapidly growing tumor with a poor prognosis (2). However, the effects of the main
therapeutic methods, including liver transplantation and surgical
resection, remain limited (3).
Therefore, the investigation of tumor-related genes may provide
benefits for new treatment options.
Astrocyte elevated gene-1 (AEG-1), also named MTDH
or LYRIC, was first cloned as an HIV- and TNF-α-inducible gene in
primary human fetal astrocytes (9).
Studies have suggested that AEG-1 expression was remarkably higher
in glioma, breast, prostate, esophagus and liver cancers compared
with that in the respective normal tissues (4–8).
Previous studies have shown that AEG-1 synergizes with oncogenic
Ha-ras to enhance the soft agar colony formation of non-tumorigenic
immortalized melanocytes (10).
Knockdown of AEG-1 induces apoptosis of prostate cancer cells
through the downregulation of Akt activity and the upregulation of
forkhead box 3a activity (6). In
addition, AEG-1 may promote the progression of hepatocellular
carcinoma by activating Wnt/β-catenin signaling (7). The data suggest that AEG-1 is able to
induce growth promotion in liver cancer, but the molecular
mechanism remains to be elucidated.
Gene silencing by RNA interference (RNAi) has become
a powerful tool for functional genetic analyses due to the ability
to create specific loss-of-function phenotypes. In the present
study, RNAi was used to obtain the liver cancer HepG2 cell line
with stably silenced expression of AEG-1. The effects of AEG-1 on
proliferation and apoptosis were examined in the HepG2 cells. IL-6
is a significant inflammatory cytokine and play a key role in
growth promotion in several human cancers, including biliary tract
epithelial cancers, prostate cancer and multiple myeloma (11–12).
IL-6 levels in patients with liver cancer are markedly higher than
those in normal individuals (13).
Furthermore, the high serum level of IL-6 has been shown to be
closely associated with the progression of liver cancer (14). Data have suggested that IL-6 may
activate several signaling pathways, including phosphoinositide
3-kinase, JAK/STAT and p38 mitogen-activated protein kinase by
autocrine or paracrine pathways (15). Stat3, a key downstream target of
IL-6 signaling, promotes the expression of several genes that are
associated with cell survival, leading to tumor cell proliferation,
apoptosis inhibition and an increased metastatic potential. The
present study aimed to investigate the effects of AEG-1 on the
proliferation and apoptosis of HepG2 cells, and explored the
molecular mechanism of tumor growth in liver cancer.
Materials and methods
Cell culture and AEG-1-knockdown
cells
Human liver cancer Hep3B, HepG2, SMMC-7721,
MHCC-97H, HCC-LM3 and SK-HEP-1 cell lines were cultured in
Dulbecco’s modified Eagle Medium (DMEM) with 10% fetal calf serum
(Invitrogen Gibco, Carlsbad, CA, USA) and incubated at 37°C with 5%
CO2. Two shRNA oligonucleotide duplexes against the
AEG-1 sequence (NM_178812) were synthesized by GeneChem (Shanghai,
China) according to the study by Yoo et al(7). The AEG-1 shRNAs were inserted into the
psilencer2.0 vector. The successful plasmid construction was
verified by DNA sequencing. The psilencer2.0-shAEG-1-1 and −2 and
the psilencer2.0 control vector, respectively, were transfected
into semi-confluent HepG2 cells using Lipofectamine 2000 reagent
(Invitrogen Gibco). After 24 h, the transfected cells were
trypsinized and replated into six-well plates (1:10), then selected
for 14 days with 600 μg/ml G418 to produce stable AEG-1-knockdown
cell lines. A single colony of stable cells was selected for
further culture and the concentration of G418 was subsequently
reduced by half and maintained in cultivation.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay and flow cytometry analysis
A total of 5×103 cells per well were
plated into a 96-well plate. The cell growth was determined using
the MTT assay with a wavelength of 550 nm. For the apoptosis
analyses, the cells were harvested and stained using a
phycoerythrin-Annexin V apoptosis detection kit (BD Pharmingen, San
Diego, CA, USA) according to the manufacturer’s instructions. For
the cell cycle analyses, the cells were harvested, washed twice
with phosphate-buffered saline and fixed with precooled 75%
ethanol. Prior to the flow cytometry analyses, the ethanol was
removed and 500 μl freshly made dying solution, containing 0.05
mg/ml propidium iodide and 0.025 mg/ml RNase, was added. Subsequent
to being stained for 30 min, the cells were subjected to flow
cytometry analysis. A total of three replicates were performed.
Colony formation assay
The HepG2-shAEG-1 and HepG2-vector cells were
trypsinized and replated into six-well plates at a density of
5×102 cells per well. After two weeks, the cells were
washed twice with PBS and fixed with methanol/acetic acid (3:1;
v:v) for 15 min. The fixing solution was removed and the cells were
stained with 0.2% crystal violet for 10 min. The number of colonies
was counted under a microscope (CKX41; Olympus, Tokyo, Japan).
Quantitative polymerase chain reaction
(qPCR)
Total RNA was isolated and extracted from the cells
using TRIzol reagent according to the manufacturer’s instructions
(Invitrogen). Total RNA (0.5 μg) was used for cDNA synthesis with
PrimeScript RT reagent kit (Takara, Dalian, China). qPCR, based on
SYBR premix EX TAQ (Takara), was performed to quantify the mRNA
levels on an ABI StepOne Real-Time PCR system (Applied Biosystems,
Inc., Carlsbad, CA, USA). The value of 2−ΔΔCt was used
to determine the fold changes between the samples. The sequences of
the primers that were used in this study were as follows: Forward,
5′-CCTTGGGTCCA GTTGCCTTCT-3′ and reverse, 5′-CCAGTGCCTCTTTGCTG
CTTTC-3′ for IL-6; forward, 5′-CATGTGTGTGGAGAGCG TCCA-3′ and
reverse, 5′-GCCGGTTCAGGTACTCAGTCA-3′ for Bcl-2; forward,
5′-TCGGAGAGCACCTGTTGGA-3′ and reverse, 5′-CCATGTTCATCCTGGCGCTC-3′
for crystalin, αB (Cryab); and forward, 5′-GTTGCGTTACACCCTTTC
TTG-3′ and reverse, 5′-GACTGCTGTCACCTTCACCGT-3′ for β-actin.
Western blot analysis
For the western blot analysis, the cells were lysed
in RIPA buffer (Sigma, St Louis, MO, USA). The protein
concentration was determined using a BCA Protein Assay kit (Pierce,
Rockford, IL, USA). Total protein (50 μg) was separated on a 10%
SDS-PAGE gel and transferred to polyvinylidene difluoride membranes
(Millipore, Bedford, MA, USA). The membranes were subsequently
immunoblotted with primary antibody. Anti-AEG-1 antibody was
purchased from Abcam (Cambridge, MA, USA) and anti-glyceraldehyde
3-phosphate dehydrogenase (GAPDH) was obtained from Epitomics, Inc.
(Burlingame, CA, USA). Anti-p-Stat3 and -Stat3 antibodies were
purchased from Cell Signaling Technology (Boston, MA, USA). A
secondary goat anti-rabbit antibody solution (Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA) was finally used for
detection with an ECL kit (Pierce, Rockford, IL, USA), according to
the manufacturer’s instructions.
Enzyme-linked immunosorbent assay (ELISA)
analysis
Semi-confluent HepG2-shAEG-1 and HepG2-vector cells
were cultured in serum-free DMEM. The supernatants were collected
after 48 h, centrifuged at 210 × g for 5 min at 4°C to remove the
cellular debris, and stored at −80°C until the analysis was
performed by ELISA. Immunoreactive IL-6 was quantified using the
ELISA kit (R&D Systems, Emeryville, CA, USA), according to
basic laboratory instructions. Each data point represents readings
from a minimum of four independent assays that were performed in
triplicate.
Animal tumor model and xenograft
Eight male BALB/c-nu/nu mice (weight, ~20 g; age,
four weeks old) were purchased from the animal center of Tongji
Medical College, Huazhong University of Science and Technology
(Wuhan, China). A total of five million HepG2-shAEG-1 and
HepG2-vector cells in 0.1 ml PBS each were injected subcutaneously
into the right and left flank of each nude mouse. The length (L)
and width (W) of the tumors were measured externally using a
vernier caliper every week. The tumor volume was determined
according to the equation: V=(LxW2)/2. The growth curve
was drawn according to the change in tumor volume over time. The
mice were sacrificed following after weeks, and the tumors were
excised and analyzed by hematoxylin and eosin staining. All
experiments were performed according to the guidelines of the local
animal use and care committee.
Statistical analysis
The SPSS 16.0 software package (SPSS, Inc., Chicago,
IL, USA) was used for the statistical analysis and measurement data
are presented as the mean ± standard deviation. The statistical
significance of the differences was determined using Student’s
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Establishing AEG-1-knockdown liver cancer
cell lines
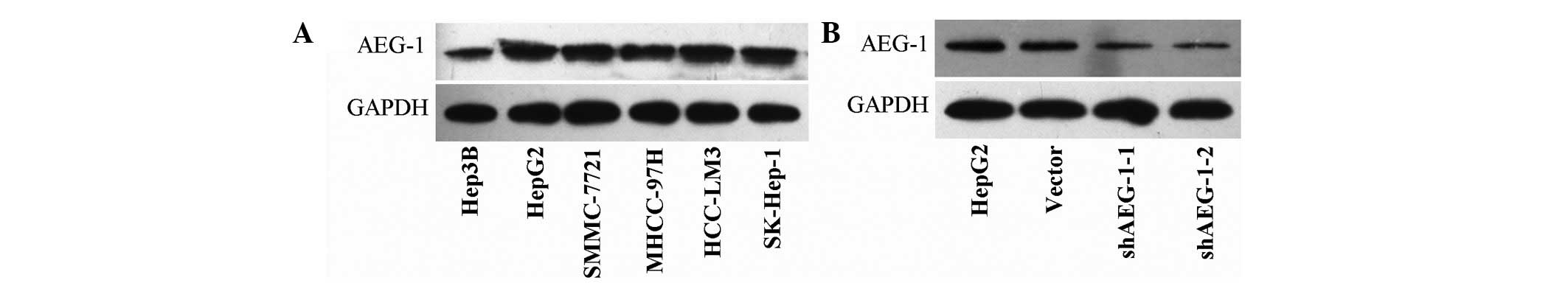
The protein expression of AEG-1 was assayed by
western blotting in a panel of liver cancer Hep3B, HepG2,
SMMC-7721, MHCC-97H, HCC-LM3, SK-Hep-1 cell lines. It was observed
that the AEG-1 protein was overexpressed in all these liver cancer
cell lines (Fig. 1A). The HepG2
cells exhibited high expression levels of AEG-1 and were selected
for AEG-1 gene silencing. The psilencer2.0 (vector),
psilencer2.0-shAEG-1-1 and 2 plasmids were stably transfected into
the HepG2 cell line. Western blotting was used to detect the effect
of AEG-1 silencing. Compared with the non-transfected HepG2 cells,
the expression level of AEG-1 protein was remarkably inhibited in
the HepG2-shAEG-1-1 and −2 cells. There was no significant
difference between the HepG2-vector cells and the untreated HepG2
cells (Fig. 1B). The
HepG2-shAEG-1-2 cells were chosen to complete the following
experiments.
Knockdown of AEG-1 inhibits cell growth
and promotes apoptosis in hepatoma HepG2 cells
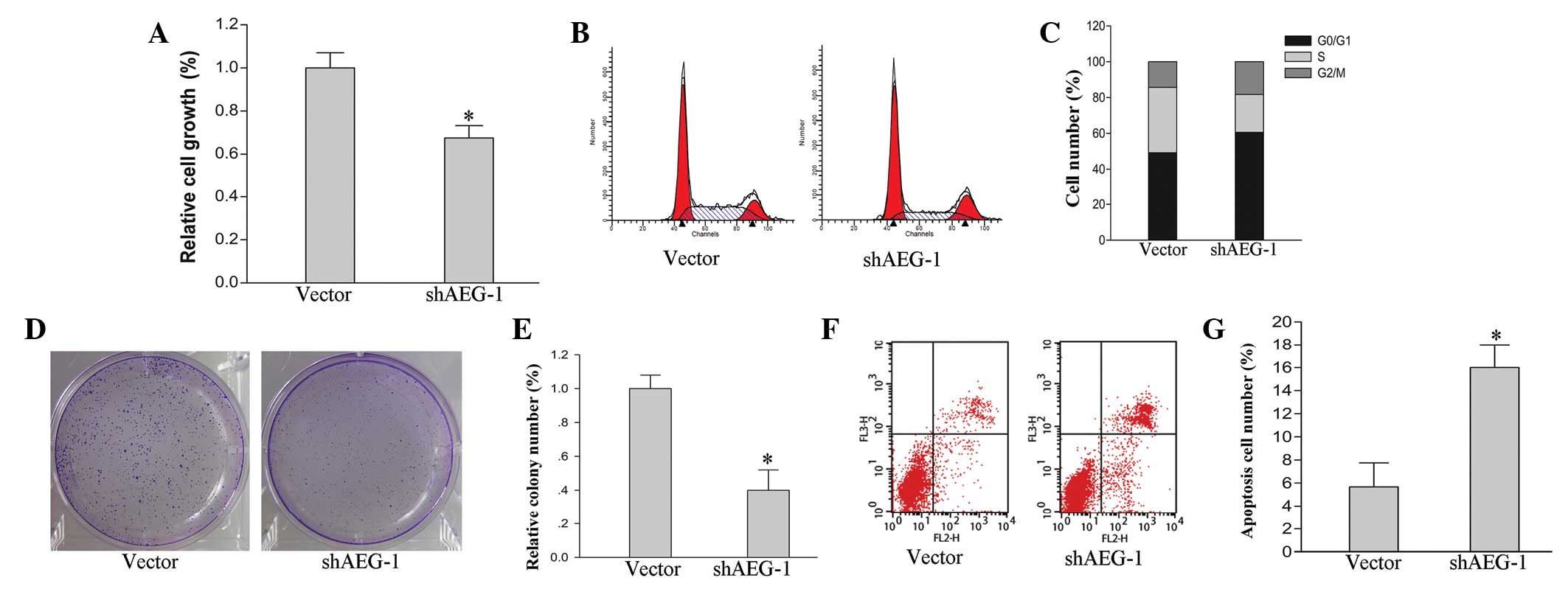
To investigate the effects of AEG-1 on the growth of
hepatoma HepG2 cells, cell cycle, cell proliferation and colony
formation assays were performed. The cell cycle was significantly
arrested in the HepG2-shAEG-1 cells. Compared with the vector
control, the ratio of the cells in the G0/G1
phase was increased by 12% and the ratio of cells in the S phase
was decreased by 16% in the HepG2-shAEG-1 cells (Fig. 2B and C). The cell proliferation
ability was also inhibited by AEG-1 silencing. Knockdown of AEG-1
suppressed cell proliferation in the HepG2 cells by 26% (Fig. 2A; P<0.05). Furthermore, the
ability of colony formation following AEG-1 silencing in the HepG2
cells was analyzed. The relative colony number was markedly
decreased in the HepG2-shAEG-1 cells compared with the vector
control (Fig. 2D and E; P<0.05).
The knockdown of AEG-1-induced apoptosis by flow cytometry was
examined in the HepG2 cells. Compared with the HepG2 cells that
were transfected with empty vector plasmid, the apoptosis ratio
increased significantly in the HepG2-shAEG-1 cells (Fig. 2F and G; P<0.05).
Knockdown of AEG-1 suppresses IL-6
secretion and inhibits Stat3 activation
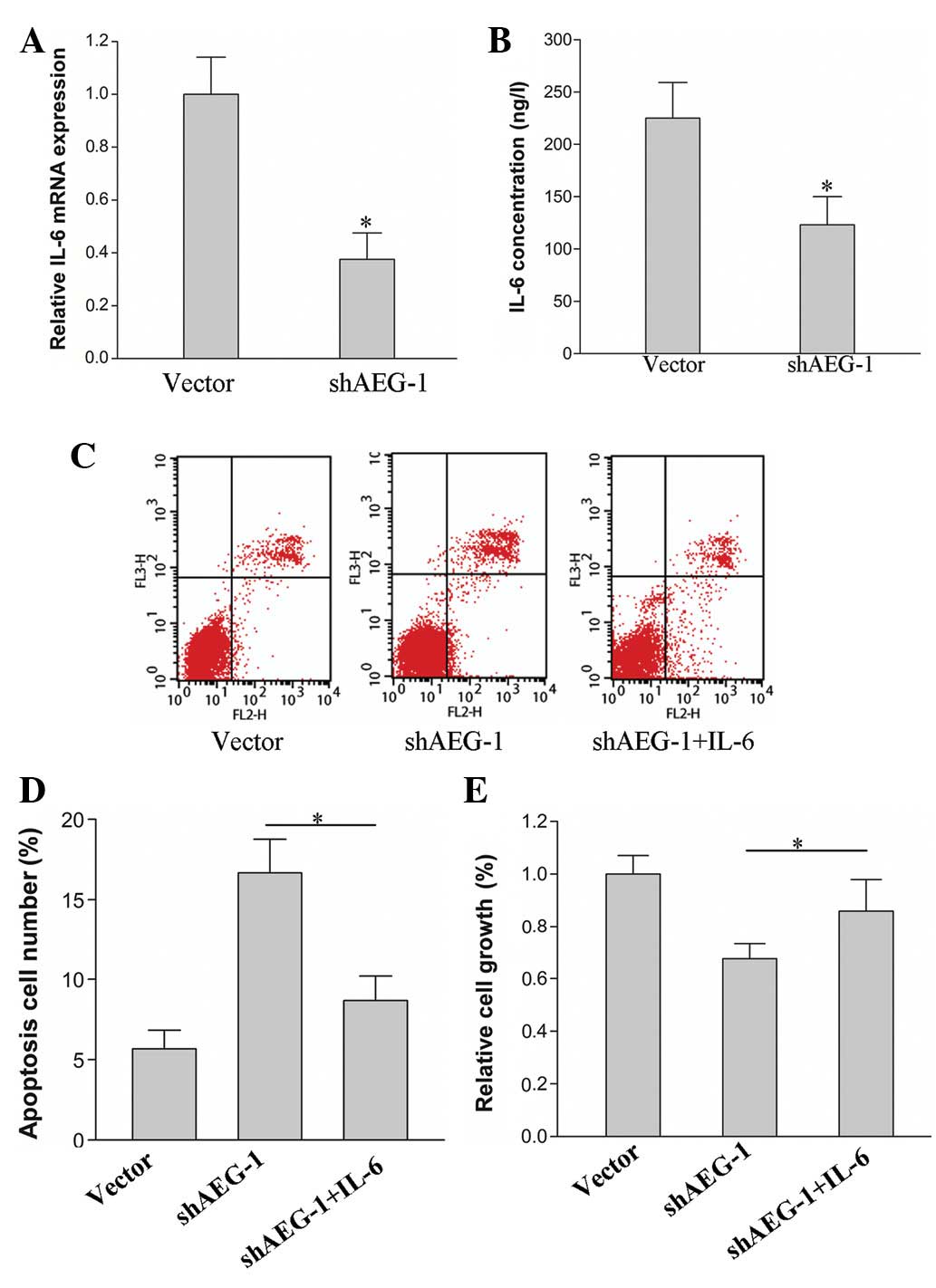
To determine whether IL-6 participates in the
knockdown of AEG-1-induced growth inhibition and apoptosis, the
expression of IL-6 in HepG2 cells was examined with AEG-1 silenced
by shRNA. The mRNA level of IL-6 was decreased in the HepG2-shAEG-1
cells compared with that in the HepG2-vector cells (Fig. 3A; P<0.05). The concentration of
IL-6 in the culture supernatants of the HepG2-shAEG-1 and
HepG2-vector cells was identified to be similar to the expression
levels of IL-6 mRNA. The secretion of IL-6 in the HepG2-shAEG-1
cells was reduced compared with that of the HepG2-vector cells
(Fig. 3B; P<0.05). In the
subsequent experiments, the proliferation and apoptosis of the
HepG2-shAEG-1 cells was analyzed following the treatment with IL-6
(50 ng/l). The results revealed that the proliferation ratio
increased in the HepG2-shAEG-1 cells that were treated with IL-6
compared with that in the untreated cells (Fig. 3E; P<0.05). The apoptosis ratio
decreased in the IL-6-treated cells (Fig. 3C and D; P<0.05).
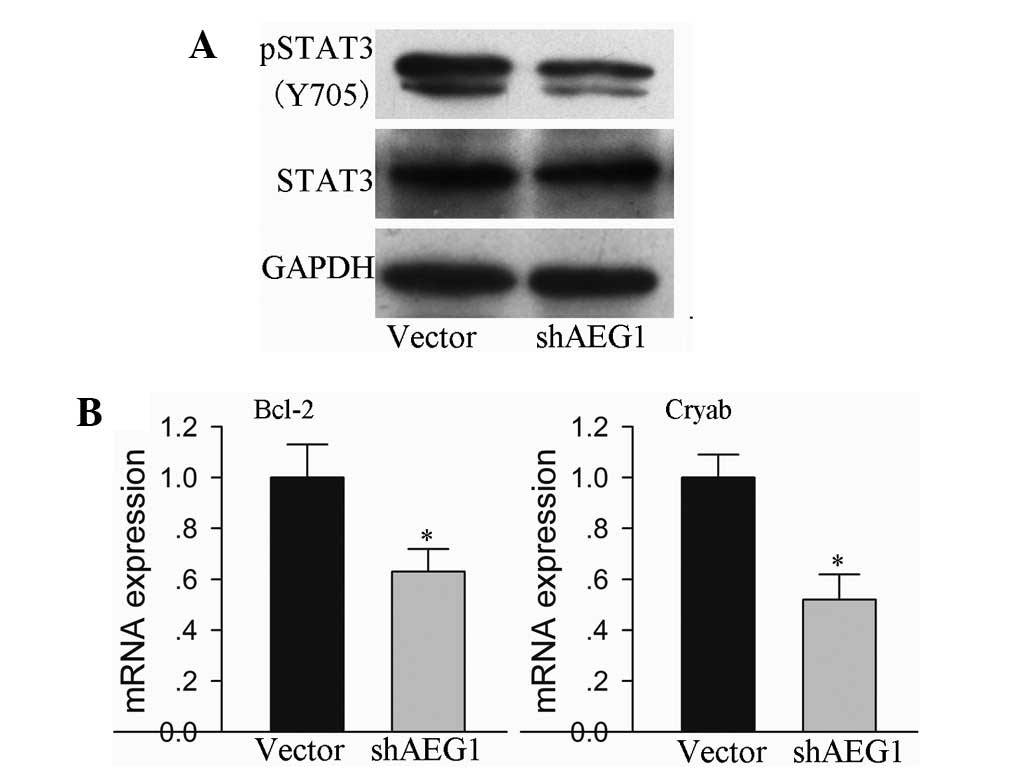
Stat3 is a significant transcription factor that is
activated by IL-6 and associated cytokines. The present study
demonstrated that AEG-1 inhibition was able to reduce the secretion
of IL-6 in hepatoma HepG2 cells. Therefore, AEG-1 inhibition was
investigated in order to determine whether it was able to
downregulate Stat3 phosphorylation. Stat3 activation was observed
to be suppressed in the HepG2 cells with shAEG-1 plasmid
transfection, compared with that in the empty vector-transfected
cells, which was confirmed by reduced tyrosine (Y705)
phosphorylation of Stat3 (Fig. 4A).
Furthermore, the expression of cell survival-related genes that are
regulated by Stat3 were identified in the AEG-1-silenced HepG2
cells. Bcl-2 and Cryab mRNA expression levels were reduced in the
cells that were transfected with the shAEG-1 plasmid, compared with
those in the mice that were transfected with the empty vector
(Fig. 4B; P<0.05).
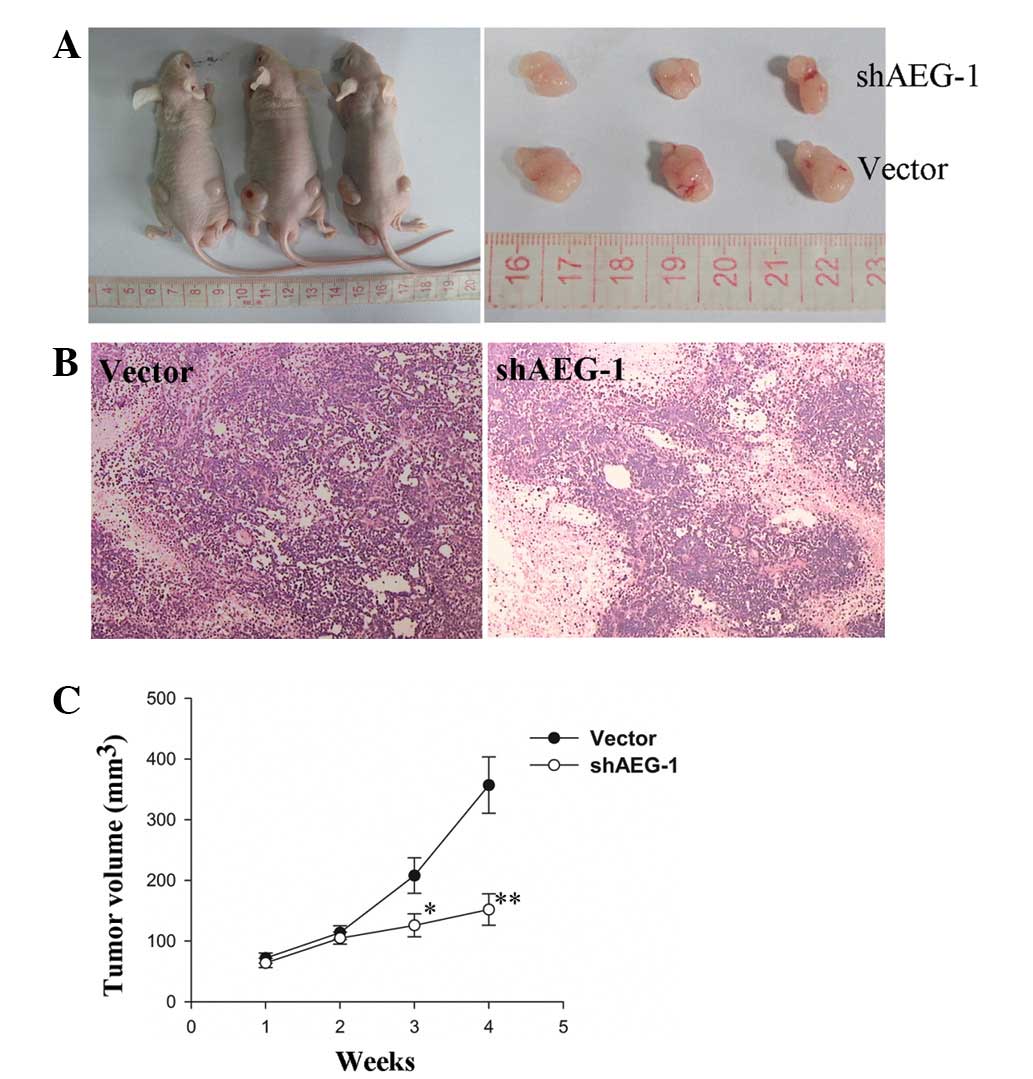
Knockdown of AEG-1 inhibits the growth of
subcutaneous tumors in nude mice
Following subcutaneous cell inoculation in BALB/C
nude mice for seven days, the rate of tumor formation of the
HepG2-shAEG-1 cells was 62.5% (5/8), whereas the tumor formation
rate of the HepG2-vector cells was 75% (6/8). Knockdown of AEG-1 in
the HepG2 cells inhibited the growth of subcutaneous tumors and the
tumor volumes were smaller than those in the mice that were
inoculated with the HepG2-vector cells (Fig. 5A). The average tumor volume and
growth rate in the HepG2-shAEG-1 cell inoculation group were
markedly reduced compared with the vector control group (Fig. 5C; P<0.05 and P<0.01).
Discussion
The present study shows that the knockdown of AEG-1
may inhibit cell proliferation and promote apoptosis in the liver
cancer HepG2 cell line, and the molecular mechanism may involve the
suppression of IL-6 secretion and the inhibition of Stat3
activation.
Despite the fact that AEG-1 was first identified in
2002, studies on AEG-1 have increased gradually in the last five
years and have mainly focused on the progression of tumors.
Previous studies have suggested that AEG-1 is able to promote
invasion and metastasis of hepatocellular carcinoma (7,16,17).
However, few studies have investigated the role of AEG-1 in the
growth of liver cancer cells. Proliferation and apoptosis are two
significant aspects during the tumor growth process (18). The poor prognosis of patients with
liver cancer is mainly due to rapid growth and metastasis (2). Therefore, it is necessary to identify
the association between tumor-related genes and the growth of liver
cancer.
In the present study, the stable HepG2 cells were
acquired with AEG-1 silencing. Inhibited proliferation, increased
apoptosis and cell cycle arrest were observed in the HepG2-shAEG-1
cells. The tumor microenvironment is now considered to be a
significant stimulative factor for tumors. Inflammatory cytokines,
the main component of the tumor microenvironment, are closely
associated with the development and progression of liver cancer
(19). Our previous expression
microarray analysis suggested that IL-6 and IL-1β levels were
markedly changed in AEG-1-overexpressed and -silenced HCC cells
(unpublished data). The subsequent experiments demonstrated that
IL-6 secretion was inhibited in AEG-1 knockdown cells, and
exogenous IL-6 was able to reverse AEG-1 knockdown-induced
anti-proliferation and apoptosis.
The IL-6 family of cytokines, including IL-6, ILF
and oncostatin M, are known to be able to activate the Stat3
transcription factor (a downstream target of IL-6 signaling)
through autocrine or paracrine pathways. Aberrant Stat3 activation
has been reported to exist in numerous human carcinomas (20–22).
Upregulated phosphorylation of Stat3 is closely associated with the
promotion of growth and inhibition of apoptosis in tumor cells
(23,24). IL-6 is able to bind to IL-6R/gp130
and activate the Jak/Stat3 pathway. IL-6 is a downstream regulator
of AEG-1 in the HepG2 cells. The phosphorylation of Stat3 was
further investigated and it was observed that the activation of
Stat3 was reduced in the AEG-1-silenced cells. Furthermore, the
cell survival-related genes, Bcl-2 and Cryab, which are regulated
by Stat3, were downregulated in the AEG-1-silenced cells. Thus, the
inhibition of proliferation and the promotion of apoptosis that was
induced by AEG-1 silencing in the HepG2 cells was likely to be
mediated by the inactivation of Stat3 and the downregulated
expression of Bcl-2 and Cryab. However, whether the molecular
mechanism identified in the present study is functional in other
liver cancer cells and other tumors requires further study.
In conclusion, the present study has demonstrated
that AEG-1 plays a significant role in the proliferation and
apoptosis of liver cancer HepG2 cells via downregulated IL-6
secretion and Stat3 activation. Furthermore, AEG-1 knockdown
inhibits the tumor growth in vivo. Therefore, this study
provides an improved understanding of the role of AEG-1 in the
growth of liver cancer.
Acknowledgements
This study was supported by grants from the National
Science Foundation of China (no. 81070333) and the Natural Science
Foundation of Hubei Province of China (no. 2012FFB02318).
References
|
1
|
Yang JD and Roberts LR: Hepatocellular
carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 7:448–458.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pang RW, Joh JW, Johnson PJ, Monden M,
Pawlik TM and Poon RT: Biology of hepatocellular carcinoma. Ann
Surg Oncol. 15:962–971. 2008. View Article : Google Scholar
|
|
3
|
O’Neil BH and Venook AP: Hepatocellular
carcinoma: the role of the North American GI Steering Committee
Hepatobiliary Task Force and the advent of effective drug therapy.
Oncologist. 12:1425–1432. 2007.PubMed/NCBI
|
|
4
|
Brown DM and Ruoslahti E: Metadherin, a
cell surface protein in breast tumors that mediates lung
metastasis. Cancer Cell. 5:365–374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu L, Wu J, Ying Z, Chen B, Han A, Liang
Y, Song L, Yuan J, Li J and Li M: Astrocyte elevated gene-1
upregulates matrix metalloproteinase-9 and induces human glioma
invasion. Cancer Res. 70:3750–3759. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kikuno N, Shiina H, Urakami S, Kawamoto K,
Hirata H, Tanaka Y, Place RF, Pookot D, Majid S, Igawa M and Dahiya
R: Knockdown of astrocyte-elevated gene-1 inhibits prostate cancer
progression through upregulation of FOXO3a activity. Oncogene.
26:7647–7655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoo BK, Emdad L, Su ZZ, Villanueva A,
Chiang DY, Mukhopadhyay ND, Mills AS, Waxman S, Fisher RA, Llovet
JM, et al: Astrocyte elevated gene-1 regulates hepatocellular
carcinoma development and progression. J Clin Invest. 119:465–477.
2009. View
Article : Google Scholar
|
|
8
|
Yu C, Chen K, Zheng H, Guo X, Jia W, Li M,
Zeng M, Li J and Song L: Overexpression of astrocyte elevated
gene-1 (AEG-1) is associated with esophageal squamous cell
carcinoma (ESCC) progression and pathogenesis. Carcinogenesis.
30:894–901. 2009. View Article : Google Scholar
|
|
9
|
Su ZZ, Kang DC, Chen Y, Pekarskaya O, Chao
W, Volsky DJ and Fisher PB: Identification and cloning of human
astrocyte genes displaying elevated expression after infection with
HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid
subtraction hybridization, RaSH. Oncogene. 21:3592–3602. 2002.
View Article : Google Scholar
|
|
10
|
Kang DC, Su ZZ, Sarkar D, Emdad L, Volsky
DJ and Fisher PB: Cloning and characterization of HIV-1-inducible
astrocyte elevated gene-1, AEG-1. Gene. 353:8–15. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meng F, Yamagiwa Y, Taffetani S, Han J and
Patel T: IL-6 activates serum and glucocorticoid kinase via
p38alpha mitogen-activated protein kinase pathway. Am J Physiol
Cell Physiol. 289:C971–C981. 2005. View Article : Google Scholar
|
|
12
|
Kobayashi S, Werneburg NW, Bronk SF,
Kaufmann SH and Gores GJ: Interleukin-6 contributes to Mcl-1
up-regulation and TRAIL resistance via an Akt-signaling pathway in
cholangiocarcinoma cells. Gastroenterology. 128:2054–2065. 2005.
View Article : Google Scholar
|
|
13
|
Tilg H, Wilmer A, Vogel W, Herold M,
Nölchen B, Judmaier G and Huber C: Serum levels of cytokines in
chronic liver diseases. Gastroenterology. 103:264–274. 1992.
|
|
14
|
Matzaraki V, Alexandraki KI, Venetsanou K,
Piperi C, Myrianthefs P, Malamos N, Giannakakis T, Karatzas S,
Diamanti-Kandarakis E and Baltopoulos G: Evaluation of serum
procalcitonin and interleukin-6 levels as markers of liver
metastasis. Clin Biochem. 40:336–342. 2007. View Article : Google Scholar
|
|
15
|
Johnson C, Han Y, Hughart N, McCarra J,
Alpini G and Meng F: Interleukin-6 and its receptor, key players in
hepatobiliary inflammation and cancer. Transl Gastrointest Cancer.
1:58–70. 2012.
|
|
16
|
Srivastava J, Siddiq A, Emdad L, et al:
Astrocyte elevated gene-1 promotes hepatocarcinogenesis: novel
insights from a mouse model. Hepatology. 56:1782–1791. 2012.
View Article : Google Scholar
|
|
17
|
Sarkar D: AEG-1/MTDH/LYRIC in liver
cancer. Adv Cancer Res. 120:193–221. 2013. View Article : Google Scholar
|
|
18
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leonardi GC, Candido S, Cervello M,
Nicolosi D, Raiti F, Travali S, Spandidos DA and Libra M: The tumor
microenvironment in hepatocellular carcinoma (review). Int J Oncol.
40:1733–1747. 2012.
|
|
20
|
Walter M, Liang S, Ghosh S, Hornsby PJ and
Li R: Interleukin 6 secreted from adipose stromal cells promotes
migration and invasion of breast cancer cells. Oncogene.
28:2745–2755. 2009. View Article : Google Scholar
|
|
21
|
Lin MT, Lin BR, Chang CC, Chu CY, Su HJ,
Chen ST, Jeng YM and Kuo ML: IL-6 induces AGS gastric cancer cell
invasion via activation of the c-Src/RhoA/ROCK signaling pathway.
Int J Cancer. 120:2600–2608. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tu B, Du L, Fan QM, Tang Z and Tang TT:
STAT3 activation by IL-6 from mesenchymal stem cells promotes the
proliferation and metastasis of osteosarcoma. Cancer Lett.
325:80–88. 2012. View Article : Google Scholar
|
|
23
|
Johnston PA and Grandis JR: STAT3
signaling: anticancer strategies and challenges. Mol Interv.
11:18–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu H and Jove R: The STATS of cancer - new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar
|