Introduction
Hepatocellular carcinoma is one of the most common
malignancies in humans that severely threatens people’s health.
Surgical therapy is the most effective method for patients who
suffer from non-advanced hepatic carcinoma (1). However, the majority of patients with
hepatocellular carcinoma have poor prognosis and succumb within
several months of diagnosis. Traditional chemotherapy is often used
in patients with unresectable hepatocellular carcinoma. However,
common problems include the severe toxicity to normal tissue and
the high resistance to the majority of chemotherapeutic drugs.
Therefore, a drug with low toxicity that is relatively selective
for cancer cells and has a synergistic effect with chemotherapeutic
drugs is extremely important. It is the key to increasing the
survival rate of liver cancer patients, particularly for advanced
patients.
The Aurora kinase family consists of
serine/threonine kinases (2). They
are critical in regulating the majority of mitotic processes and
are frequently highly expressed in human cancers. Increased
cellular levels of these kinases may be related to genetic
instability and are evident in various cancer types, including
breast, ovarian, colon and pancreatic cancer. In mammalian cells,
according to their location, Aurora kinases are divided into three
types: Aurora A, Aurora B and Aurora C.
A number of studies have demonstrated that Aurora A
and Aurora B are overexpressed in lung cancer (3), colorectal cancer (4), prostate cancer (5), renal carcinoma (6), hepatocellular carcinoma (7), ovarian cancer (8) and bladder cancer (9). Enhancing their expression causes cell
mitotic errors, cell malignant transformation and genome
instability. By contrast, suppressing their expression inhibits
cell proliferation and promotes cell apoptosis (10). Therefore, the Aurora kinase family
members have become potentially valuable antitumor therapeutic
targets.
A number of Aurora kinase inhibitors have been
discovered (11,12), including VX680, ENMD-2076, ZM447439
and MLN8237. VX-680 has been shown to disrupt mitosis and induce
apoptosis in a wide variety of tumor cell lines (13). VX-680 was also the foremost Aurora
kinase inhibitor to be studied in clinical trials (14). The clinical studies of Aurora kinase
inhibitors have already reached phase II trials; however, their
potential application in the treatment of hepatocellular carcinoma
(HCC) remains to be investigated.
In the present study, we aimed to determine whether
VX680 is able to effectively reduce the toxicity of cisplatin
chemotherapy and effectively inhibit the growth of hepatoma cells.
Accordingly, we first used VX680, cisplatin and a combination of
the two to explore their effects on HepG2 cells. Then, we
investigated the effect and mechanism of VX680 on the growth
inhibition of HepG2 cells, and the synergistic effect with
cisplatin.
Materials and methods
Cell and reagents
The HepG2 cell line was kindly provided by the
Medical College of Three Gorges University (Yichang, China). The
cells were cultured in RPMI-1640 (HyClone, Logan, UT, USA)
supplemented with 10% fetal bovine serum and 100 U/ml
penicillin/streptomycin at 37°C in a humidified atmosphere
containing 5% CO2. After cell growth reached 70–80%
confluency in the bottom of the culture bottle, logarithmic phase
cells were used for the experiment. VX680 was purchased from
Selleck Chemicals (Houston, TX, USA), and was dissolved in dimethyl
sulfoxide (Sigma-Aldrich, St. Louis, MO, USA), stored at −80°C and
diluted in fresh medium immediately before use. Cisplatin was
purchased from Qilu Pharmaceutical Co., Ltd. (Shandong, China).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay for cell growth inhibition
Logarithmic phase cells were cultured in 96-well
plates and treated with varying doses of VX680 (3.125–50 μmol/l)
and cisplatin (0.125–2 μg/ml) for 24–72 h at 37°C in a humidified
atmosphere containing 5% CO2. Following incubation with
20 μl MTT (5 mg/ml) for 4 h, 150 μl DMSO was added to each well.
Subsequently, the 96-well plates were agitated for 15 min at
micro-oscillator oscillation. The optical density (OD) value at 490
nm was measured by automatic enzyme-linked immunosorbent assay
readers. The inhibition rate was calculated using the following
equation: (1 − average OD value of experimental group/average OD
value of control group) ×100. Whether the two drugs had synergistic
or antagonistic effects was determined according to the following
formula (15): Q = E(A + B)/[(EA +
EB) − (EA × EB)], where a Q-value of 0.85–1.15 indicates the sum of
the effects and a Q-value >1.15 indicates a synergistic effect.
By contrast, a Q-value <0.85 indicates the antagonistic effect
of the combined drugs. EA represents the inhibition rate for drug
A, EB represents the inhibition rate for drug B and E(A + B)
represents the inhibition rate for the combined therapy.
Apoptosis detected by flow cytometry
Cells (1×106/ml) were cultured in
six-well plates for 24 h and then treated with VX680 (3.125
μmol/l), cisplatin (0.5 μg/ml) or VX680 (3.125 μmol/l) and
cisplatin (0.5 μg/ml) for 72 h. Cells with no drugs added were used
as the control. Apoptosis was detected according to the Annexin
V-FITC Apoptosis Detection kit (BD Transduction Laboratories,
Lexington, KY, USA). Cells (1×105/ml) were centrifuged
at 1,200 × g for 5 min, then the supernatant was removed. Later,
the cells were treated with 195 μl Annexin V-FITC conjugation
liquid. After adding a further 5 μl Annexin V-FITC, the cells were
incubated at room temperature for 15 min. The above steps were
repeated two times. After staining with Annexin V-FITC away from
light, 10 μl propidium iodide was added and cells were analyzed
using a BD Accuri C6 flow cytometer (BD Biosciences, Ann Arbor, MI,
USA). Data were processed and analyzed using the Accuri CFlow Plus
software, version 1.0.227.4 (BD Biosciences).
Western blot analysis
HepG2 cells (5×106/ml) were cultured with
VX680 (3.125 μmol/l), cisplatin (0.5 μg/ml) and VX680 (3.125
μmol/l) plus cisplatin (0.5 μg/ml) for 72 h. Following this, the
cells were washed with cold phosphate-buffered saline and lysed
with radio-immunoprecipitation assay buffer (Beyotime, Shanghai,
China). The protein concentration was measured by a bicinchoninic
acid protein assay kit (Pierce, Rockford, IL, USA). Fifty
micrograms of total protein were denatured by boiling for 5 min,
then separated using 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred onto a nitrocellulose membrane
(Millipore Corp., Boston, MA, USA). The blots, with 5% non-fat milk
powder and 1 ml/l Tween-20/Tris-buffered salt solution (TTBS), were
blocked for 2 h, followed by incubation with the primary antibodies
(mouse monoclonal; 1:500 dilution) for Aurora A (Abcam, Cambridge,
MA, USA), Bcl-2, wt p53 (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) and β-actin (Wuhan Boster Biological Technology,
Ltd., Wuhan, China) for 2 h at room temperature. After extensive
washing with TTBS, the blots were incubated with a monoclonal
secondary mouse IgG antibody (1:5,000; Wuhan Boster Biological
Technology, Ltd.) for 1 h and washed with TTBS. Protein bands were
analyzed by SmartView gel imaging system (Shanghai Furi Technology
Co., Ltd., Shanghai, China).
Statistical analysis
Data were analyzed by SPSS version 13.0 software
(SPSS Inc., Chicago, IL, USA) and were expressed as the mean ± SD.
A single-factor analysis of variance was used to compare the
differences between groups. For all analyses, P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of VX680 and cisplatin on the
proliferation of HepG2 cells
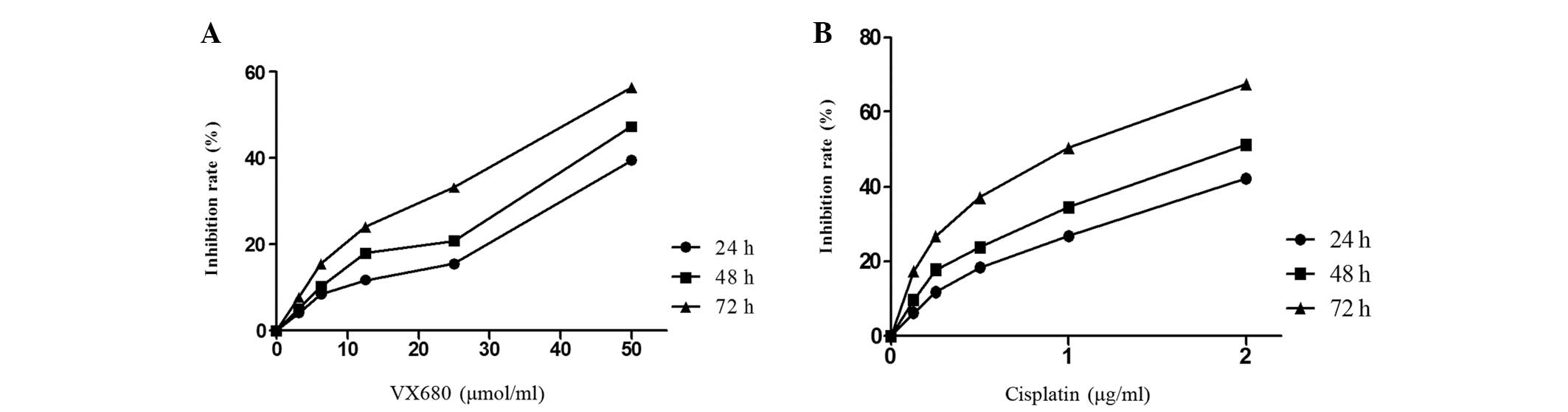
Following culture with VX680 or cisplatin, HepG2
cellular proliferation was monitored by MTT assay daily for 24, 48
and 72 h. Cell proliferation was significantly suppressed by VX680
and cisplatin in a time- and dose-independent manner (Fig. 1).
In order to determine whether VX680 synergistically
enhances the effect of cisplatin, HepG2 cells were cultured with
3.125 μmol/l VX680 (10% cytotoxicity) and cisplatin (0.125–2 μg/ml)
for 72 h. The synergistic effect for cisplatin is presented in
Table I (Q>1.15). The inhibition
of the combined group was significantly greater than the single
group. The Q value (Q>1.15) implied the two drugs can produce a
synergistic effect.
 | Table IInhibitory effect of VX680 combined
with cisplatin on HepG2 cells. |
Table I
Inhibitory effect of VX680 combined
with cisplatin on HepG2 cells.
| VX680 (μmol/l) | Cisplatin
(μg/ml) | Inhibition rate
(%) | Q-value |
|---|
| 3.125 | 0 | 7.87±1.08 | |
| 0 | 0.125 | 17.29±1.93 | |
| 0 | 0.25 | 26.75±1.27 | |
| 0 | 0.5 | 37.19±2.37 | |
| 0 | 1 | 50.41±4.50 | |
| 0 | 2 | 67.54±5.68 | |
| 3.125 | 0.125 | 30.61±1.95 | 1.29 |
| 3.125 | 0.25 | 42.86±1.72 | 1.32 |
| 3.125 | 0.5 | 57.37±2.35 | 1.36 |
| 3.125 | 1 | 70.07±2.12 | 1.29 |
| 3.125 | 2 | 81.41±3.10 | 1.16 |
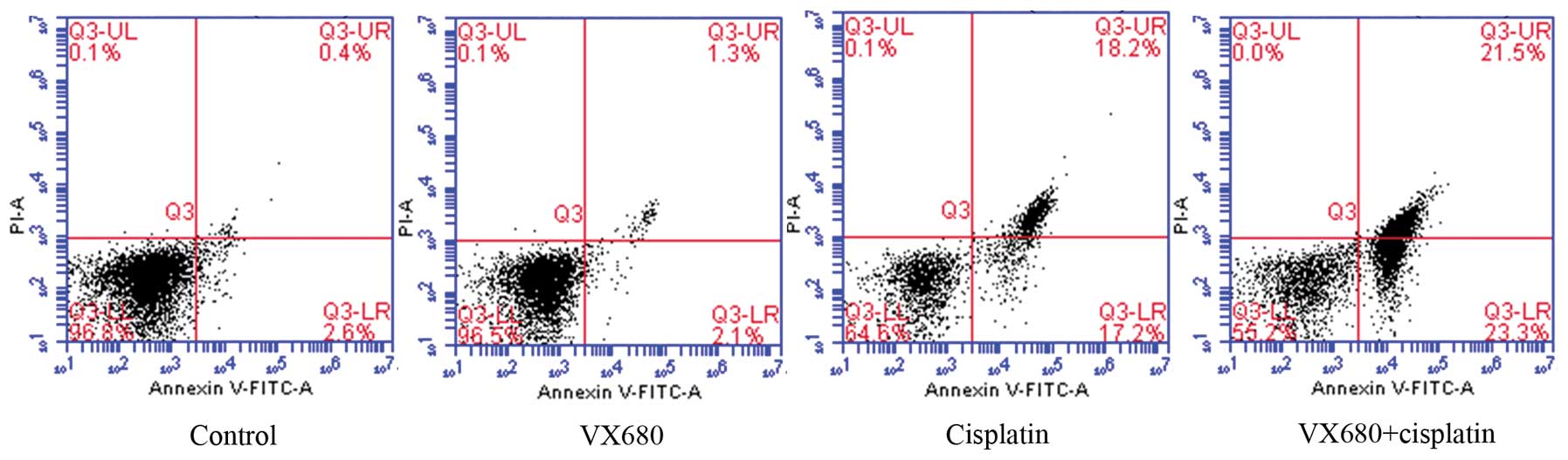
Detection of cell apoptosis
HepG2 cell apoptosis was detected using flow
cytometry. Compared with the control group, the VX680 group (3.125
μmol/l) presented no significant change in apoptosis rate. However,
the apoptosis rate in the combined group was significantly higher
than that in the cisplatin group (0.5 μg/ml) and control group
(P<0.05; Fig. 2).
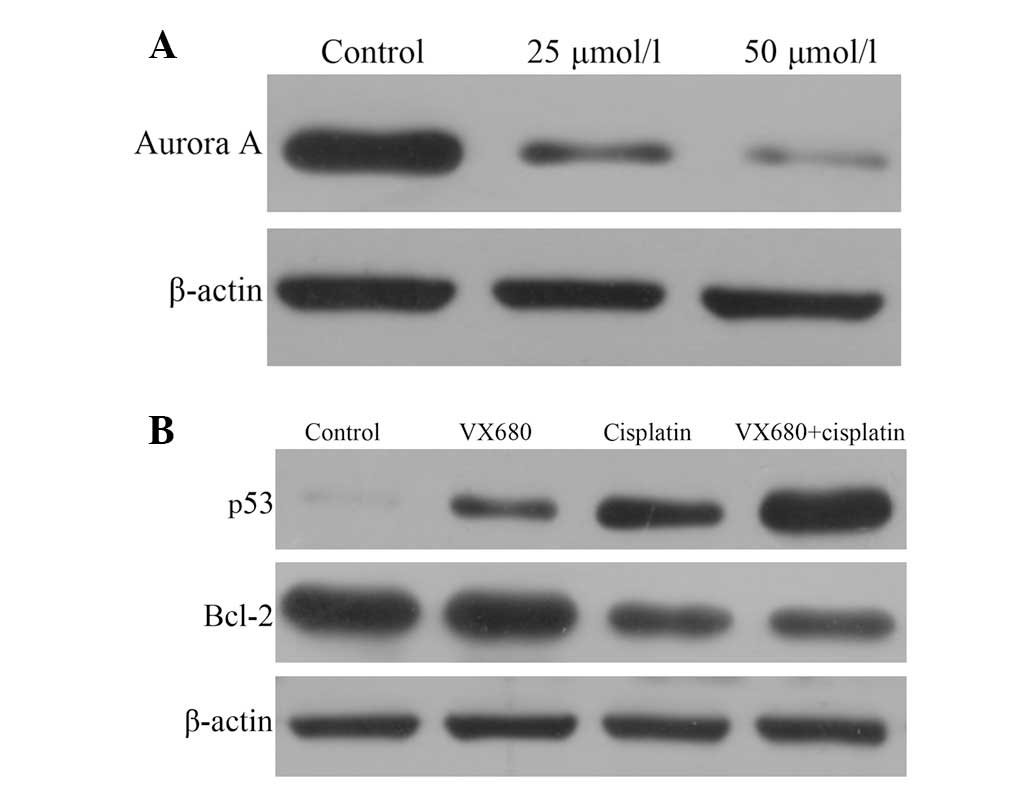
Effect of VX680 and cisplatin on Aurora
A, p53 and Bcl-2 protein expression
VX680 significantly reduced Aurora A expression in a
concentration-dependent manner (Fig.
3A). Compared with the control group, cisplatin reduced Bcl-2
expression and increased the expression level of p53 protein
(P<0.05). However, VX680 only increased the expression of p53
(P<0.05) and did not reduce the expression of Bcl-2. Bcl-2 and
p53 expression levels were significantly reduced and increased,
respectively, in the combined group compared with the single drug
and control groups (P<0.05; Fig.
3B).
Discussion
Several studies have indicated that Aurora kinase is
overexpressed in the majority of hepatocellular carcinoma tissue
samples and cell lines (16,17). A
previous study used VE-465, an analog of VX-680, which
significantly reduced Aurora A expression and induced apoptosis in
HepG2 cells (18). These findings
indicated that Aurora A may serve as a molecular target against
HCC. Although the antitumor effect of Aurora kinase inhibitors has
been demonstrated, it is unclear whether they effectively enhance
the effect of cisplatin chemotherapy on HepG2 cells.
In the present study, we used VX680 to inhibit the
expression of Aurora A in HepG2 cells and analyzed the cellular
changes using an MTT assay. We found that cisplatin and VX680
inhibited the growth of HepG2 cells. Additionally, the combination
of VX680 and cisplatin had a synergistic effect (Q>1.15). This
result suggests that the suppression of Aurora A expression
enhances the sensitivity to cisplatin. Cell apoptosis detection
revealed that VX680 alone (at a low concentration) does not induce
apoptosis of tumor cells, but cisplatin alone does. When cisplatin
was combined with VX680, the apoptosis rate of HepG2 cells
increased significantly. Numerous studies have indicated that
inhibiting Aurora kinase expression may increase the
chemosensitivity of cancer cells (11,12).
The present study was consistent with these previous studies.
Moreover, western blotting results revealed that
chemosensitivity was associated with the expression of p53 and
Bcl-2 proteins. In the control group, the expression of p53 protein
was at a low level; however, when VX680 or cisplatin were added,
the p53 expression increased. The expression of p53 markedly
increased in the combined group.
The p53 gene inhibits the growth of tumor cells by
inducing cell cycle arrest or apoptosis, and also increases the
chemosensitivity of hepatocellular carcinoma (19). Furthermore, Aurora A is a key
regulatory component in the p53 pathway. Overexpression of Aurora A
leads to degradation of p53 (20).
Thus, VX680 increases the expression of p53 and increases the
chemosensitivity of HepG2 cells by increasing the expression of
Aurora A. Cell apoptosis was associated with the expression of
Bcl-2. The anti-apoptosis activity was reduced, while the
chemosensitivity to cisplatin was enhanced.
In conclusion, our results indicate that VX680
inhibits the growth of HepG2 cells and enhances the
chemosensitivity of HepG2 cells to cisplatin. Thus, the selective
inhibition of Aurora A by VX680 provides a new approach to
anticancer therapy and may serve as a single or combined agent with
existing therapies in the future.
References
|
1
|
Nathan H, Segev DL, Mayo SC, Choti MA,
Cameron AM, Wolfgang CL, Hirose K, Edil BH, Schulick RD and Pawlik
TM: National trends in surgical procedures for hepatocellular
carcinoma: 1998–2008. Cancer. 118:1838–1844. 2012.
|
|
2
|
Carmena M and Earnshaw WC: The cellular
geography of aurora kinases. Nat Rev Mol Cell Biol. 4:842–854.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang XH, Rao M, Loprieato JA, et al:
Aurora A, Aurora B and survivin are novel targets of
transcriptional regulation by histone deacetylase inhibitors in
non-small cell lung cancer. Cancer Biol Ther. 7:1388–1397. 2008.
View Article : Google Scholar
|
|
4
|
Lam AK, Ong K and Ho YH: Aurora kinase
expression in colorectal adenocarcinoma: correlations with
clinicopathological features, p16 expression, and telomerase
activity. Hum Pathol. 39:599–604. 2008. View Article : Google Scholar
|
|
5
|
Lee EC, Frolov A, Li R, Ayala G and
Greenberg NM: Targeting Aurora kinases for the treatment of
prostate cancer. Cancer Res. 66:4996–5002. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Terakawa T, Miyake H, Kumano M and
Fujisawa M: Growth inhibition and enhanced chemosensitivity induced
by down-regulation of Aurora-A in human renal cell carcinoma Caki-2
cells using short hairpin RNA. Oncol Lett. 2:713–717. 2011.
|
|
7
|
Tanaka S, Arii S, Yasen M, Mogushi K, Su
NT, Zhao C, Imoto I, Eishi Y, Inazawa J, Miki Y and Tanaka H:
Aurora kinase B is a predictive factor for the aggressive
recurrence of hepatocellular carcinoma after curative hepatectomy.
Br J Surg. 95:611–619. 2008. View
Article : Google Scholar
|
|
8
|
Kuang Y, Cai J, Li D, Han Q, Cao J and
Wang Z: Repression of Dicer is associated with invasive phenotype
and chemoresistance in ovarian cancer. Oncol Lett. 5:1149–1154.
2013.PubMed/NCBI
|
|
9
|
Park HS, Park WS, Bondaruk J, et al:
Quantitation of Aurora kinase A gene copy number in urine sediments
and bladder cancer detection. J Natl Cancer Inst. 100:1401–1411.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang XX, Liu R, Jin SQ, Fan FY and Zhan
QM: Overexpression of Aurora-A kinase promotes tumor cell
proliferation and inhibits apoptosis in esophageal squamous cell
carcinoma cell line. Cell Res. 16:356–366. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qi W, Cooke LS, Liu X, Rimsza L, Roe DJ,
Manziolli A, Persky DO, Miller TP and Mahadevan D: Aurora inhibitor
MLN8237 in combination with docetaxel enhances apoptosis and
anti-tumor activity in mantle cell lymphoma. Biochem Pharmacol.
81:881–890. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shimomura T, Hasako S, Nakatsuru Y, et al:
MK-5108, a highly selective Aurora-A kinase inhibitor, shows
antitumor activity alone and in combination with docetaxel. Mol
Cancer Ther. 9:157–166. 2010. View Article : Google Scholar
|
|
13
|
Harrington EA, Bebbington D, Moore J, et
al: VX-680, a potent and selective small-molecule inhibitor of the
Aurora kinases, suppresses tumor growth in vivo. Nat Med.
10:262–267. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fiskus W, Wang Y, Joshi R, et al:
Cotreatment with vorinostat enhances activity of MK-0457 (VX-680)
against acute and chronic myelogenous leukemia cells. Clin Cancer
Res. 14:6106–6115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin ZJ: Addition in drug combination.
Zhongguo Yao Li Xue Bao. 1:70–76. 1980.(In Chinese).
|
|
16
|
Jeng YM, Peng SY, Lin CY and Hsu HC:
Overexpression and amplification of Aurora-A in hepatocellular
carcinoma. Clin Cancer Res. 10:2065–2071. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin ZZ, Jeng YM, Hu FC, Pan HW, Tsao HW,
Lai PL, Lee PH, Cheng AL and Hsu HC: Significance of Aurora B
overexpression in hepatocellular carcinoma. Aurora B Overexpression
in HCC. BMC Cancer. 10:4612010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin ZZ, Hsu HC, Hsu CH, et al: The Aurora
kinase inhibitor VE-465 has anticancer effects in pre-clinical
studies of human hepatocellular carcinoma. J Hepatol. 50:518–527.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu Y, Zhang Y, Hu J, et al: MARVELD1
inhibited cell proliferation and enhance chemosensitivity via
increasing expression of p53 and p16 in hepatocellular carcinoma.
Cancer Sci. 103:716–722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Katayama H, Sasai K, Kawai H, Yuan ZM,
Bondaruk J, Suzuki F, Fujii S, Arlinghaus RB, Czerniak BA and Sen
S: Phosphorylation by aurora kinase A induces Mdm2-mediated
destabilization and inhibition of p53. Nat Genet. 36:55–62. 2004.
View Article : Google Scholar : PubMed/NCBI
|