Introduction
Early-stage prostate cancer can be cured by radical
surgery or radiation therapy. However, ~10–20% of men with prostate
cancer have metastatic disease, and many others develop metastases
despite primary treatment (1,2).
Although the majority of patients with advanced metastatic disease
initially respond to conventional androgen deprivation with medical
or surgical castration, the response to hormonal treatment lasts
for a median duration of 18–24 months. Thus, most patients
eventually become resistant to therapy and develop hormone
refractory prostate cancer, also known as castration-resistant
prostate cancer (CRPC) (2).
Treatment of CRPC is currently a challenge. Options
for improved survival in patients with CRPC are limited, but
docetaxel (DXT) chemotherapy is the clearly established treatment
(3). While DTX chemotherapy has
shown success with overall survival improved by 2.5 months compared
with that of mitoxantrone-based therapy, only ~48% of patients
respond, and drug resistance rapidly develops to treatment with a
combination of DXT and prednisone (4,5). Two
important mechanisms of tumor resistance, which may be exploited
therapeutically, are the overexpression of Bcl-2 and the loss of
p53 function, both of which contribute to the resistance of CRPC to
DXT (5–10).
It has been demonstrated that interferon-α-2b
(IFN-α2b) decreases the expression of Bcl-2 and increases the
expression of p53 (11,12). Similarly, IFN-α2b has been shown to
promote the effects of DTX chemotherapy in vitro(13). A low dosage of IFN-α2b showed
antitumor activity in prostate cancer patients in a recent study
with a follow-up period of >10 years (14). In addition, IFN-α2b alone or in
combination with chemotherapy drugs has been proven safe and
effective in various metastatic malignant tumors (15,16).
However, though a high dosage of IFN-α2b alone demonstrates
antitumor activity, its toxicity is intolerable for use in CRPC
(17). Conversely, toxicity levels
with a moderate dosage of IFN-α2b alone are acceptable, whereas the
efficacy is limited (18).
These facts led us to hypothesize that IFN-α2b may
be able to expand the effects of DXT chemotherapy. In this pilot
study, we conducted a prospective analysis to evaluate the efficacy
and toxicity of this regimen in patients with CRPC.
Patients and methods
Patients
From January 2007 to September 2009, 40 patients
from the Department of Urology, First Hospital of Shihezi
University (Shihezi, China) with CRPC were enrolled in this study.
To be eligible for this study, patients had to have an Eastern
Cooperative Oncology Group (ECOG) performance status of 0 or 1
(http://ecog.dfci.harvard.edu/general/perf_stat.html),
and evidence of progressive metastatic disease despite androgen
deprivation therapy. Patients were required to have a serum
testosterone concentration of <50 ng/dl and prior treatment with
maximum androgen blockade with evidence of treatment failure.
Patients were required to have a measurable soft tissue lesion.
Patients had to have adequate function of bone marrow, liver,
heart, kidney and lung, defined as white blood cell count
≥4,000/mm3, granulocytes ≥2,000/mm3, platelet
count ≥100,000/mm3, bilirubin ≤1.5 mg/dl, alanine
aminotransferase and aspartate aminotransferase ≤2-fold the
institutional upper limit of normal, creatinine ≤2.0 mg/dl or a
calculated creatinine clearance ≥50 ml/min, left ventricular
ejection fraction ≥50% as demonstrated by echocardiography, and
forced expiratory volume in one second/forced vital capacity ≥70%.
Patients were required to have no history of myocardial infarction
within 6 months of study entry, and no history of deep venous
thrombosis.
The study was performed after approval by the Human
Investigations Committee of the Medical College of Shihezi
University (Shihezi, China). Informed consent was obtained from
each patient.
Medical protocol
Throughout the treatment period, patients in group A
received DXT (75 mg/m2) by intravenous administration
over 60 min on day 1, IFN-α2b (3 mIU/m2) was
subcutaneously injected on days 16, 18 and 20; and oral
prednisolone (5 mg) was administered twice daily on days 1 to 21,
on each 21 day cycle. Patients in group B were treated as
abovementioned, but were not administered IFN-α2b. For the first
cycle of treatment, all patients were hospitalized for 8–10 days to
check renal and liver functions and to observe adverse events. For
the second course of treatment, all patients were treated on an
outpatient basis. The treatment was continued until the disease
progressed or unacceptable adverse events occurred. Patients were
hospitalized if grade 3 to 4 neutropenia was observed, and
granulocyte-colony stimulating factor (G-CSF) was used. The dose of
DXT was reduced by 10 mg/m2 in subsequent treatment
cycles if granulocytes remained at ≤2,000/mm3 after the
G-CSF treatment in patients with grade 3 to 4 neutropenia.
Treatment evaluation
The endpoints of this study comprised
prostate-specific antigen (PSA) response, tumor response, the time
to PSA progression, and toxicity. PSA response was defined as a
reduction of at least 50% in the baseline levels for 4 weeks. PSA
progression was defined as a rise in PSA levels exceeding 25% of
the baseline level. The tumor response was documented using
radiographs according to response evaluation criteria in solid
tumors (RECIST) (19) after four
cycles of therapy (performed by a single radiologist). Tumor
complete response was defined as the disappearance of all evidence
of a tumor and all symptoms of cancer for at least 12 weeks.
Partial response was defined as ≥50% decrease in the sum of the
products of diameters of all measured lesions persisting for at
least 12 weeks. Tumor progression was defined as a rise in the
diameter exceeding 25% of the baseline level. Progression-free
survival (PFS) was defined from the date of the first chemotherapy
treatment to the first occurrence of PSA and tumor progression.
Overall survival (OS) was defined from the date of the first
chemotherapy treatment to the date of death from any cause. The
Cancer Institute Common Toxicity Criteria, version 4.0 was used to
evaluate patients for toxicity (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm).
Statistical analysis
Descriptive statistics were used to characterize
patients at study entry. Patient age and the number of cycles of
treatment undergone were compared using the Mann-Whitney U test.
Other patient demographics, response rates and toxicity rates were
compared using Fisher’s exact test. The Kaplan-Meier method was
used to characterize the PFS in terms of PSA response. P<0.05
was considered to indicate a statistically significant difference
(two-sided).
Results
Patient characteristics
Patient characteristics are summarized in Table I. Baseline clinicopathological
characteristics were generally well-balanced between the two groups
and no significant difference between the two groups was
observed.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Index | Group A, n=20 | Group B, n=20 | P-value |
|---|
| Age (years) |
| Median (range) | 68 (58–77) | 66 (62–76) | 0.642 |
| ECOG performance
score, n (%) |
| 0 | 11 (55) | 7 | 0.341 |
| 1 | 9 (45) | 13 | 0.200 |
| Prior treatment, n
(%) |
| Prostatectomy +
hormonal therapy | 5 (25) | 7 (35) | 0.501 |
| Radiotherapy +
hormonal therapy | 13 (65) | 11 (55) | 0.748 |
| Hormonal
therapy | 2 (10) | 2 (10) | 1.000 |
| Site of metastasis, n
(%) |
| Bone | 18 (90) | 19 (95) | 0.501 |
| Lymph node | 10 (50) | 8 (40) | 0.748 |
| Liver | 3 (10) | 2 (10) | 0.695 |
| Lung | 6 (30) | 8 (40) | 1.000 |
| PSA |
| Median (range) | 49.05
(10.84–1328.53) | 63.27
(12.20–1324.48) | 0.317 |
| Biopsy Gleason score,
n (%) |
| ≤6 | 8 (40) | 10 (50) | 0.751 |
| 7 | 6 (30) | 3 (15) | 0.451 |
| 8–10 | 6 (30) | 7 (35) | 1.000 |
| Time from start of
ADT to CRPC (months) |
| Median (range) | 17.50
(7.45–67.16) | 15.00
(6.68–50.43) | 0.131 |
Efficacy
The treatment and efficacy are listed in Table II. One of the 20 patients in group
B discontinued treatment by choice and was admitted to a hospice
during the second week of the study. The most frequent reason for
stopping treatment was disease progression. The PSA response rates
were 65% in group A and 47.4% in group B among evaluated patients,
and there was no statistically significant difference between the
two groups (P=0.341).
 | Table IITreatment and efficacy. |
Table II
Treatment and efficacy.
| Index | Group A | Group B | P-value |
|---|
| No. of cycles
(months) |
| Median (range) | 8 (3–12) | 9 (2–12) | 0.573 |
| Dose reduction
(%) | 15 (3/20) | 10.5 (2/19) | 1.000 |
| PSA response
(%) | 65 (13/20) | 47.4 (9/19) | 0.341 |
| Objective tumor
response | 55 (11/20) | 21.1 (4/19) | 0.048 |
| PFS (months) |
| Median
(range) | 10 (2–24) | 8 (2–22) | 0.043 |
| Overall survival
(months) |
| Median
(range) | 19 (7–19) | 17 (8–23) | 0.348 |
In group A, among all the 20 patients with
measurable lesions, 55% (11/20) achieved a tumor response according
to the RECIST criteria. The patients with tumor response included
seven for lymph node lesions, two for liver lesions and two for
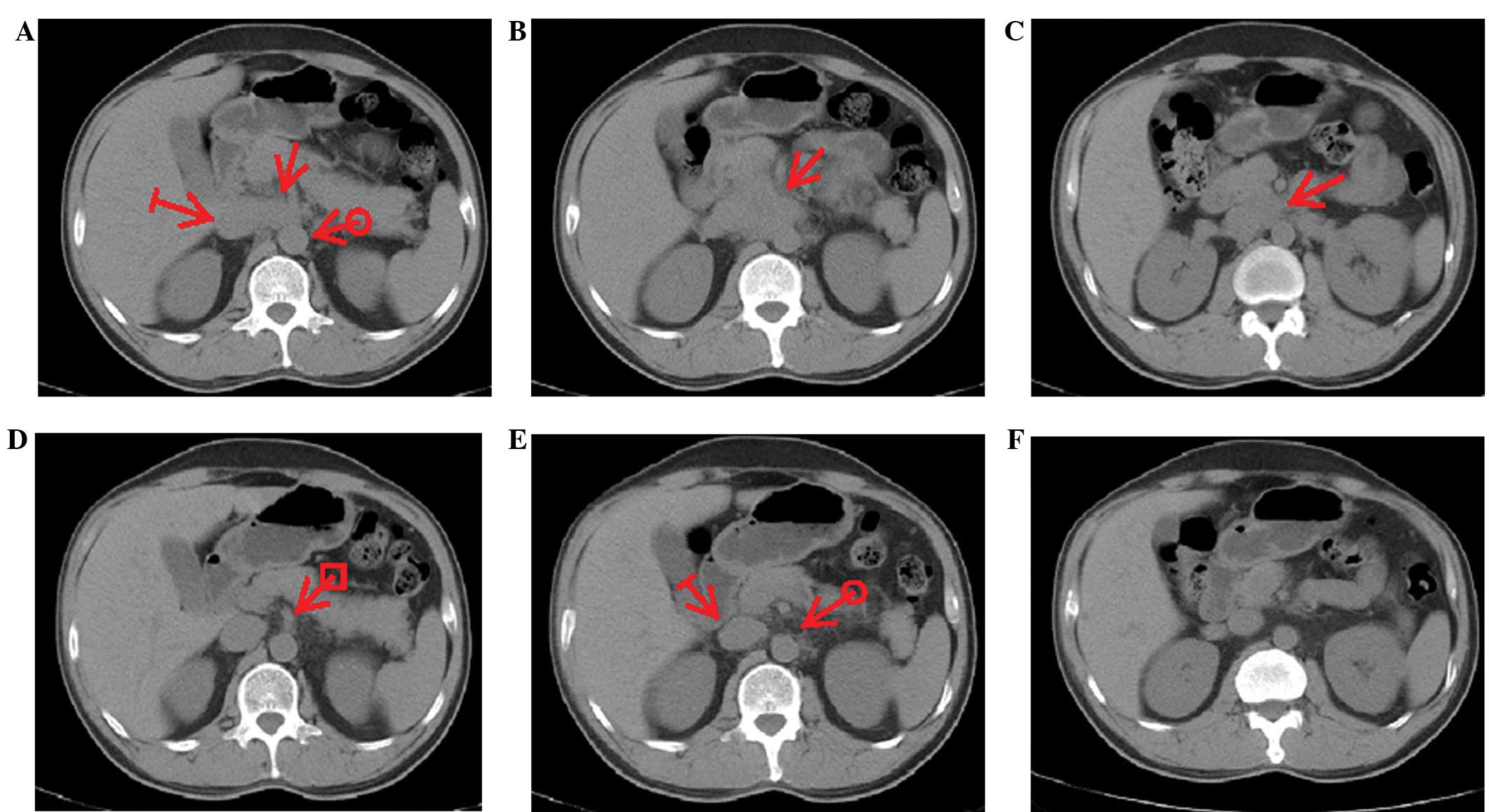
lung lesions. Notably, one patient displayed a complete response in
group A (Fig. 1). This patient was
a 58-year-old male with biopsy-proven retroperitoneal lymph node
involvement (Fig. 1A–C). Six months
after DTX plus IFN-α2b treatment, the PSA level had decreased from
755 ng/ml to an undetectable level and the retroperitoneal lymph
node mass had disappeared (Fig.
1D–F). In group B, among all the 19 cases, 21.1% (4/19) of
patients achieved a partial response. These responses were observed
lymphnode and liver lesions (two patients) and lung lesions (two
patients). However, no patients achieved a complete response in
group B. The objective tumor response rates were 55% in group A and
21.1% in group B among evaluated patients, demonstrating a
statistically significant difference between the two groups
(P=0.048).
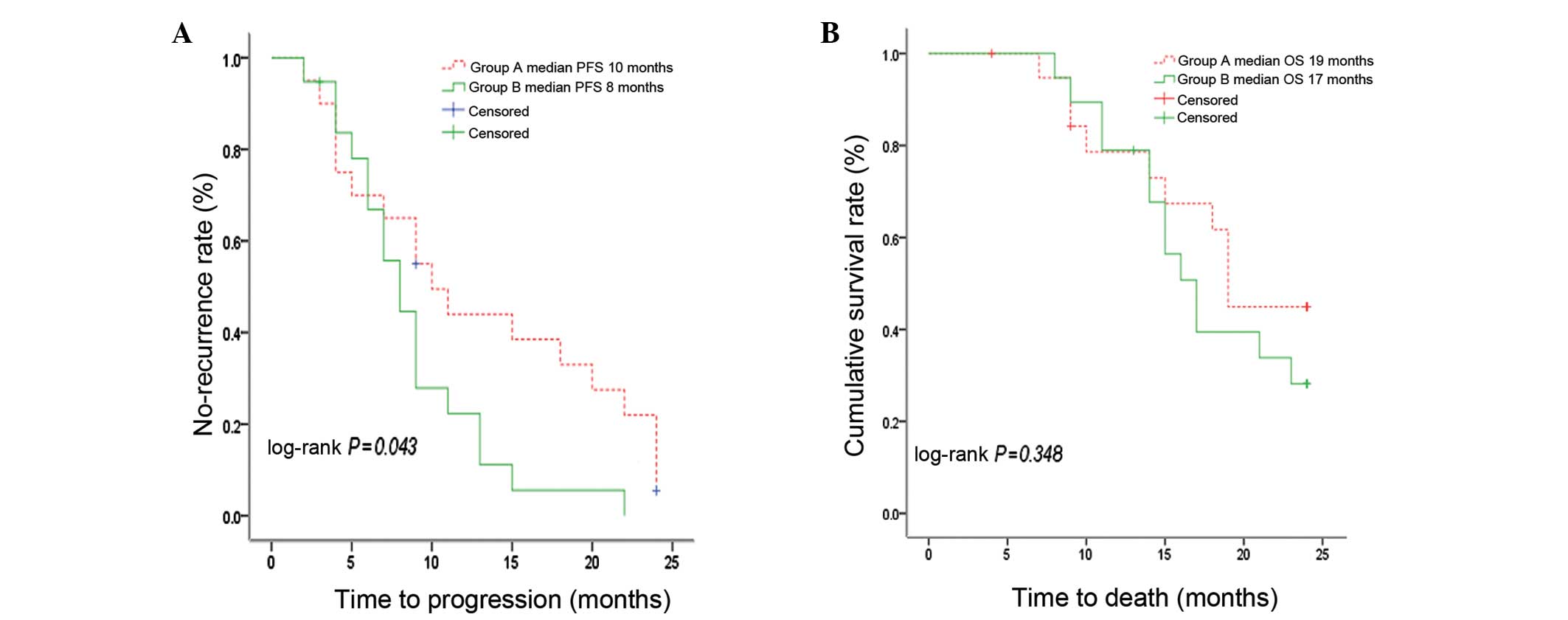
Fig. 2A shows PFS by
treatment group. The median PFS was 10 months (range 2–24; 95% CI,
5.98–14.02) for group A and 8 months (range 2–22; 95% CI,
5.94–10.06) for group B, with a 44±11 and 22±10% PFS rate at 1 year
in the two groups, respectively. There was a statistically
significant difference in PFS in groups A and B (P=0.043).
Fig. 2B shows the OS
by treatment group. Median OS was 19 months (range 7–19; 95% CI,
17.64–20.37) for group A and 17 months (range 8–23; 95% CI,
14.33–19.67) for group B, with an overall survival rate of 45±12
and 28±11% at 2 years, respectively. There was no statistically
significant difference in OS by treatment in the two groups
(P=0.348).
Toxicity
Table III shows
the hematological and non-hematological toxicities stratified by
grade and treatment group. In groups A and B, transient grade 3 to
4 neutropenia occurred in nine and six patients, grade 3 to 4
anemia occurred in three and five patients, and grade 3 to 4
general fatigue was observed in four and one patient(s),
respectively. The proportion of patients with grade 3 to 4 toxicity
displayed no statistically significant difference between the two
groups (P>0.05).
 | Table IIITreatment-related toxicity. |
Table III
Treatment-related toxicity.
| Group A, n=20 | Group B, n=19 | |
|---|
|
|
| |
|---|
| Toxicity | Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | P-value (grade
3–4) |
|---|
| Neutropenia, n
(%) | 7 (35) | 9 (45) | 8 (40) | 6 (30) | 0.514 |
| Anemia | 7 (35) | 3 (15) | 8 (40) | 5 (25) | 0.451 |
| Febrile
neutropenia | 3 (15) | 0 | 5 (25) | 0 | |
| Platelets | 2 (10) | 0 | 0 | 0 | |
| Fever | 3 (15) | 0 | 3 (15) | 0 | |
| Chills | 2 (10) | 0 | 4 (20) | 0 | |
| Allergic
reaction | 0 | 0 | 1 (5) | 0 | |
|
Nausea/vomiting | 2 (10) | 0 | 3 (15) | 0 | |
| Diarrhea | 4 (20) | 0 | 2 (10) | 0 | |
| General
fatigue | 1 (5) | 4 (20) | 2 (10) | 1 (5) | 0.342 |
| Edema | 2 (10) | 0 | 3 (15) | 0 | |
| Liver
dysfunction | 2 (10) | 0 | 4 (20) | 0 | |
Discussion
The present study demonstrated that the tumor
response rates in group A were significantly greater than those in
group B. This difference may be due to the activity of IFN-α2b
against CRPC. However, the rates of PAS and tumor response were 65
and 55%, respectively, which were higher than those observed in the
study by DiPaola et al (23 and 15%, respectively) (20). We hypothesize that this difference
between the trial by DiPaola et al and the present study was
due to the following reasons: firstly, the DXT regimen was
different, as DiPaola et al used DXT combined with IFN-α2b
and 13-cis retinoic acid. We administered DXT combined with
prednisone and IFN-α2b. DXT with prednisone has better efficacy and
less toxicity, as has been confirmed by two large phase III
clinical trials (PSA and tumor response rates were 44.4 and 44.2%,
respectively) (3,4). Another reason is the difference in
patient characteristics, particularly the metastatic sites. The
present study had more patients with lymph node lesions than the
study by DiPaola et al. A higher response of the lymph node
lesions was also found in the study by van Haelst-Pisani et
al(17); however, in this
study, IFN-α2b was not combined with DXT and IFN-α2b toxicity is
intolerable for patients administered high-dose IFN-α2b alone (10
mIU). In particular, one patient with a metastatic retroperitoneal
lymph node (6 cm) achieved a complete tumor response in group A. In
this patient, an undetectable PSA level and complete regression of
disease persisted for more than 18 months. Complete disease
regression was also observed in the study by Emerson and Morales
(14), which used a low dosage of
IFN-α2b. The PFS in group A was longer than that in group B. This
difference may have resulted from the benefit of tumor response, as
PFS included two responses, PSA and tumor, in our present study.
These results demonstrated that IFN-α2b may expand the antitumor
activity of DXT chemotherapy. Such increased activity may result
from several mechanisms, which may not be mutually exclusive.
Firstly, IFN-α2b may be able to reduce the DXT resistance by
downregulating Bcl-2 as reported previously (11,21).
Overexpression of Bcl-2 is important in antitumor drug resistance,
including hormonal and chemotherapy resistance found in CRPC
(22,23). Secondly, IFN-α2b may increase DXT
sensitivity by upregulating the activity of p53 (12,24).
Mutant p53 or the lack of functional p53 usually causes drug
resistance, while restoring p53 function leads to regression of
autochthonous lymphomas and sarcomas in mice without affecting
normal tissues (9). In addition,
IFN-α2b may synergize with DXT to promote apoptosis of tumor cells,
the rapid growth of which depends on sustained angiogenesis
(13).
Although the overall survival was longer in CRPC
patients treated with DXT + IFN-α2b, there was no statistically
significant difference between the two groups. One reason for this
finding may be due to the fact that the improved PSA and tumor
responses are not necessarily transformed into a survival
advantage. For example, a large-scale phase III clinical study was
recently conducted on the basis of the rationale that targeting
neovasculature and microtubules could enhance the clinical impact
of DXT alone (25). However,
despite the advantages of improved PSA response and delayed disease
progression, the anti-angiogenic agent bevacizumab in combination
with DXT did not significantly prolong survival compared with DXT
alone (25). The small sample size
and short follow-up time of the present study may be another reason
for these findings.
From the viewpoint of safety, while the incidence of
grade 3 to 4 toxicity was not statistically significant between the
two groups, neutropenia was still frequently observed in group A.
This side-effect may be due to suppression of the release of
granulocytes by IFN-α2b in the bone marrow (26). This adverse event can often be
safely and effectively controlled by administration of G-CSF. It
should be noted that five patients with grade 3 to 4 neutropenia
were poorly responsive to G-CSF; however, neutropenia could be
attenuated by reducing the DXT dose, increasing the interval
between doses and decreasing the number of chemotherapy cycles.
Thus, careful follow-up is required. However, a few reports have
shown that the toxicity of IFN-α2b is not well-tolerated in CRPC
(20,27). We hypothesize that the toxicities in
the previous studies and the present study are different due to the
following reasons. First, the IFN-α2b dosage was different. We
employed a lower dose of IFN-α2b [3 vs. 6
mIU/m2(20) or 10
mIU/m2(17)]. The second
reason is the differences in infusion time of IFN-α2b. DXT-induced
hematological toxicities most frequently occurred during the first
10 days of every cycle, particularly on days 5 and 7 (28). DXT combined with IFN-α2b may
increase the patient’s adverse events during this period. Patients
were administered in the third week of the cycle (days 16, 18 and
20) in the present study. However, IFN-α2b was used on days 1 and 2
of each week in the trial by DiPaola et al(20). In addition, the patients had a
better performance status prior to chemotherapy [ECOG ≤1 vs. ECOG
≤2 (17,19)] in the present study.
In conclusion, a low dosage of IFN-α2b may expand
the effects of DXT chemotherapy with improvements in tumor response
and PFS in patients with CRPC. The main side-effects were
neutropenia, fever and general fatigue, which may be safely
controlled by administration of G-CSF and symptomatic treatment.
Further evaluation of a large number of patients with a longer
follow-up period is required.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 30860281). The authors would
like to thank Dr Jianjun Wu (Xi’an Jiaotong University) for
comments on the study.
References
|
1
|
Feldman BJ and Feldman D: The development
of androgen-independent prostate cancer. Nat Rev Cancer. 1:34–45.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pienta KJ and Bradley D: Mechanisms
underlying the development of androgen-independent prostate cancer.
Clin Cancer Res. 12:1665–1671. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tannock IF, de Wit R, Berry WR, et al:
Docetaxel plus prednisone or mitoxantrone plus prednisone for
advanced prostate cancer. N Engl J Med. 351:1502–1512. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berthold DR, Pond GR, Soban F, de Wit R,
Eisenberger M and Tannock IF: Docetaxel plus prednisone or
mitoxantrone plus prednisone for advanced prostate cancer: updated
survival in the TAX 327 study. J Clin Oncol. 26:242–245. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Makarovskiy AN, Siryaporn E, Hixson DC and
Akerley W: Survival of docetaxel-resistant prostate cancer cells in
vitro depends on phenotype alterations and continuity of drug
exposure. Cell Mol Life Sci. 59:1198–1211. 2002. View Article : Google Scholar
|
|
6
|
McDonnell TJ, Troncoso P, Brisbay SM, et
al: Expression of the protooncogene bcl-2 in the prostate and its
association with emergence of androgen-independent prostate cancer.
Cancer Res. 52:6940–6944. 1992.PubMed/NCBI
|
|
7
|
Banerjee PP, Banerjee S and Brown TR:
Bcl-2 protein expression correlates with cell survival and androgen
independence in rat prostatic lobes. Endocrinology. 143:1825–1832.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ventura A, Kirsch DG, McLaughlin ME, et
al: Restoration of p53 function leads to tumour regression in vivo.
Nature. 445:661–665. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leprince D, Crepieux P, Laudet V, Flourens
A and Stehelin D: A new mechanism of oncogenic activation: E26
retroviral v-ets oncogene has inverted the C-terminal end of the
transcription factor c-ets-1. Virology. 194:855–857. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu C, Zhu Y, Lou W, et al: Functional p53
determines docetaxel sensitivity in prostate cancer cells.
Prostate. 73:418–427. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Panaretakis T, Pokrovskaja K, Shoshan MC
and Grander D: Interferon-alpha-induced apoptosis in U266 cells is
associated with activation of the proapoptotic Bcl-2 family members
Bak and Bax. Oncogene. 22:4543–4556. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Davol PA, Goulette FA, Frackelton AR Jr
and Darnowski JW: Modulation of p53 expression by human recombinant
interferon-alpha2a correlates with abrogation of cisplatin
resistance in a human melanoma cell line. Cancer Res. 56:2522–2526.
1996.PubMed/NCBI
|
|
13
|
Huang SF, Kim SJ, Lee AT, et al:
Inhibition of growth and metastasis of orthotopic human prostate
cancer in athymic mice by combination therapy with pegylated
interferon-alpha-2b and docetaxel. Cancer Res. 62:5720–5726.
2002.
|
|
14
|
Emerson L and Morales A: Intralesional
recombinant alpha-interferon for localized prostate cancer: a pilot
study with follow-up of >10 years. BJU Int. 104:1068–1070.
2009.
|
|
15
|
Shinohara N, Abe T, Sazawa A, et al:
Interferon-α-based immunotherapy in metastatic renal cell carcinoma
patients with the primary tumor in situ. Jpn J Clin Oncol.
42:113–119. 2012.
|
|
16
|
Mocellin S, Pasquali S, Rossi CR and Nitti
D: Interferon alpha adjuvant therapy in patients with high-risk
melanoma: a systematic review and meta-analysis. J Natl Cancer
Inst. 102:493–501. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van Haelst-Pisani CM, Richardson RL, Su J,
et al: A phase II study of recombinant human alpha-interferon in
advanced hormone-refractory prostate cancer. Cancer. 70:2310–2312.
1992.PubMed/NCBI
|
|
18
|
Bulbul MA, Huben RP and Murphy GP:
Interferon-beta treatment of metastatic prostate cancer. J Surg
Oncol. 33:231–233. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Padhani AR and Ollivier L: The RECIST
(Response Evaluation Criteria in Solid Tumors) criteria:
implications for diagnostic radiologists. Br J Radiol. 74:983–986.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
DiPaola RS, Chen YH, Stein M, et al: A
randomized phase II trial of mitoxantrone, estramustine and
vinorelbine or bcl-2 modulation with 13-cis retinoic acid,
interferon and paclitaxel in patients with metastatic
castrate-resistant prostate cancer: ECOG 3899. J Transl Med.
8:202010. View Article : Google Scholar
|
|
21
|
Anatol P, Danuta P, Janusz D and Bozena P:
Expression of bcl-2 protein in chronic hepatitis C: effect of
interferon alpha 2b with ribavirin therapy. World J Gastroenterol.
11:2949–2952. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Amato RJ and Mohammad T: Interferon-alpha
plus capecitabine and thalidomide in patients with metastatic renal
cell cancer. J Exp Ther Oncol. 7:41–47. 2008.
|
|
23
|
Rosevear HM, Lightfoot AJ, Nepple KG and
O’Donnell MA: Usefulness of the Spanish Urological Club for
Oncological Treatment scoring model to predict nonmuscle invasive
bladder cancer recurrence in patients treated with intravesical
bacillus Calmette-Guérin plus interferon-α. J Urol. 185:67–71.
2011.
|
|
24
|
Panasiuk A, Prokopowicz D and Dzieciol J:
p53 protein expression in chronic hepatitis C; effect of interferon
alpha 2b therapy. Hepatogastroenterology. 52:1176–1179.
2005.PubMed/NCBI
|
|
25
|
Kelly WK, Halabi S, Carducci M, et al:
Randomized, double-blind, placebo-controlled phase III trial
comparing docetaxel and prednisone with or without bevacizumab in
men with metastatic castration-resistant prostate cancer: CALGB
90401. J Clin Oncol. 30:1534–1540. 2012. View Article : Google Scholar
|
|
26
|
Koirala J, Gandotra SD, Rao S, et al:
Granulocyte colony-stimulating factor dosing in pegylated
interferon alpha-induced neutropenia and its impact on outcome of
anti-HCV therapy. J Viral Hepat. 14:782–787. 2007. View Article : Google Scholar
|
|
27
|
Chang AY, Fisher HA, Spiers AS and Boros
L: Toxicities of human recombinant interferon-alpha 2 in patients
with advanced prostate carcinoma. J Interferon Res. 6:713–715.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
ten Tije AJ, Verweij J, Carducci MA, et
al: Prospective evaluation of the pharmacokinetics and toxicity
profile of docetaxel in the elderly. J Clin Oncol. 23:1070–1077.
2005.PubMed/NCBI
|