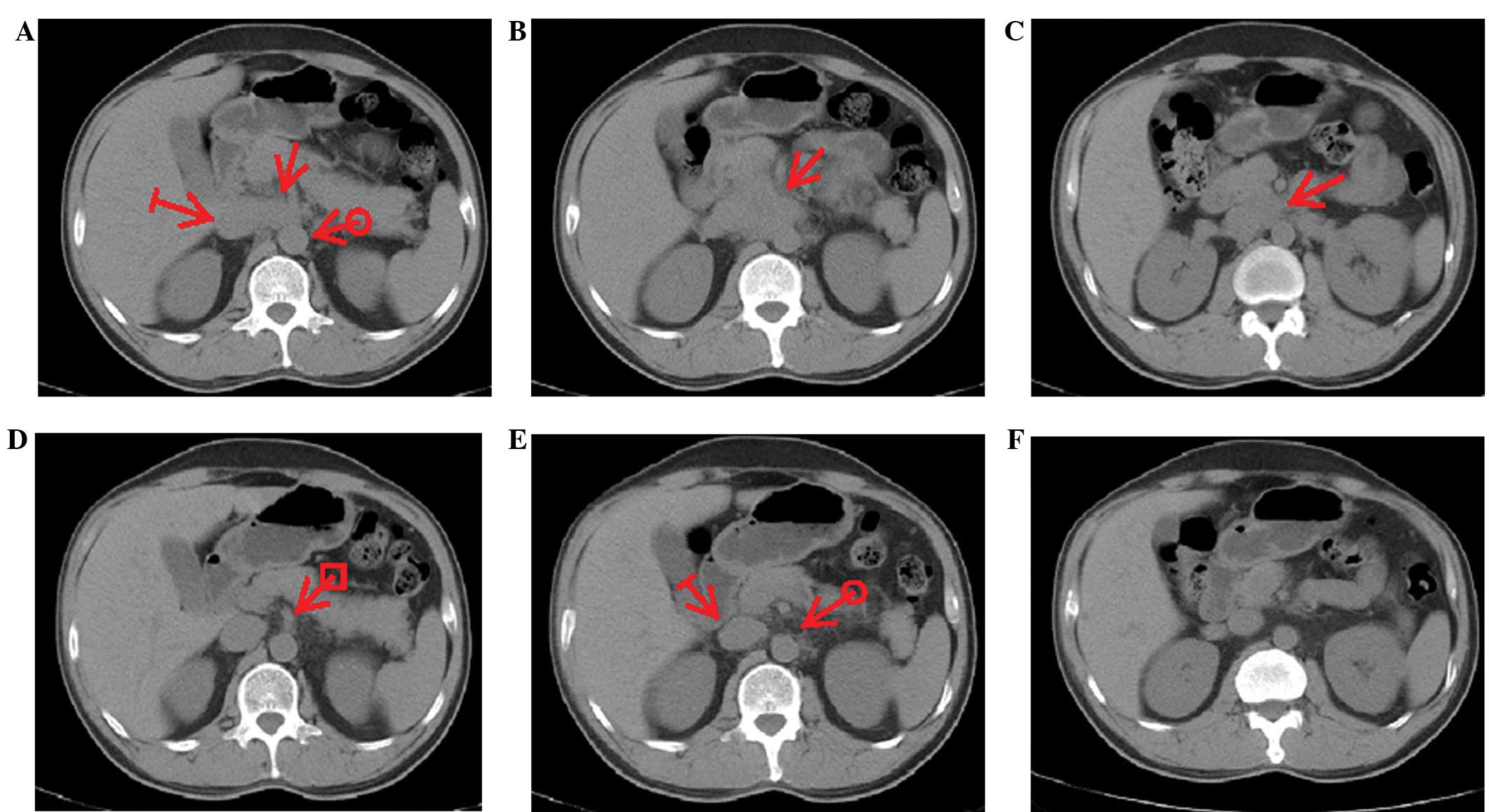
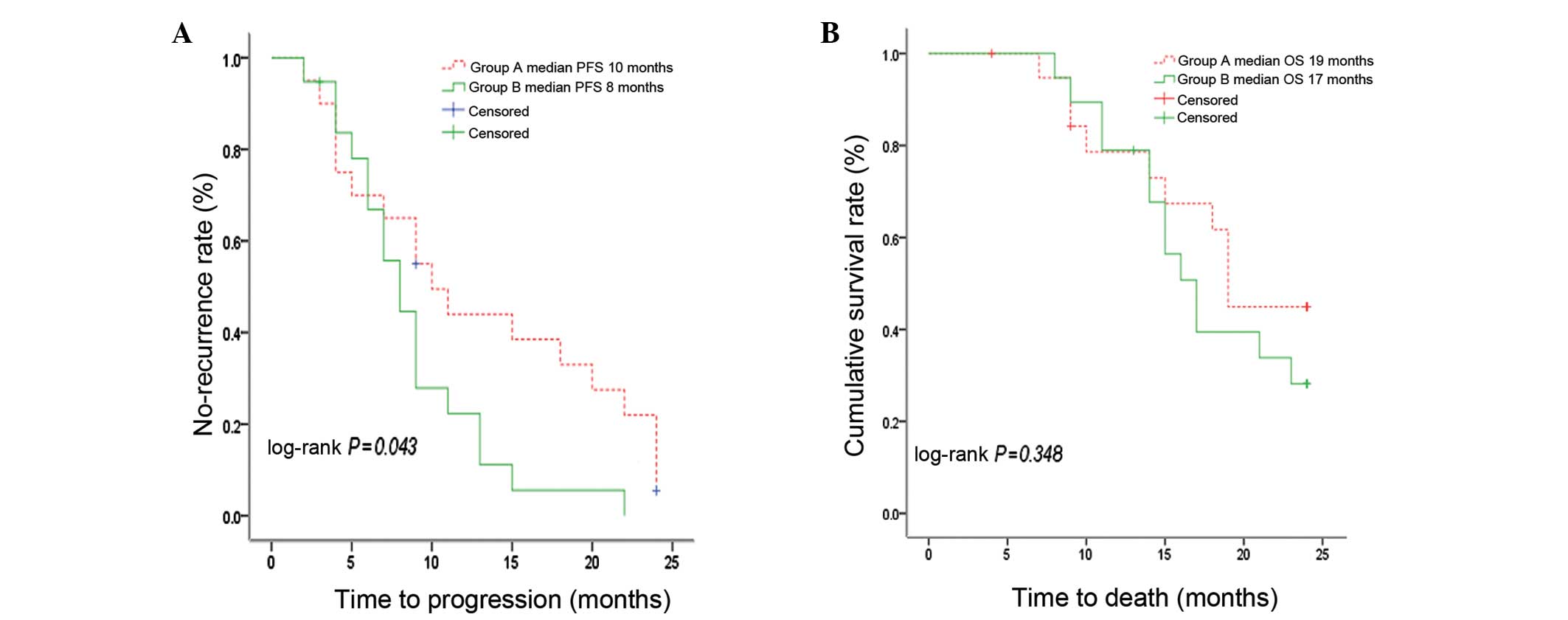
|
1
|
Feldman BJ and Feldman D: The development
of androgen-independent prostate cancer. Nat Rev Cancer. 1:34–45.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pienta KJ and Bradley D: Mechanisms
underlying the development of androgen-independent prostate cancer.
Clin Cancer Res. 12:1665–1671. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tannock IF, de Wit R, Berry WR, et al:
Docetaxel plus prednisone or mitoxantrone plus prednisone for
advanced prostate cancer. N Engl J Med. 351:1502–1512. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berthold DR, Pond GR, Soban F, de Wit R,
Eisenberger M and Tannock IF: Docetaxel plus prednisone or
mitoxantrone plus prednisone for advanced prostate cancer: updated
survival in the TAX 327 study. J Clin Oncol. 26:242–245. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Makarovskiy AN, Siryaporn E, Hixson DC and
Akerley W: Survival of docetaxel-resistant prostate cancer cells in
vitro depends on phenotype alterations and continuity of drug
exposure. Cell Mol Life Sci. 59:1198–1211. 2002. View Article : Google Scholar
|
|
6
|
McDonnell TJ, Troncoso P, Brisbay SM, et
al: Expression of the protooncogene bcl-2 in the prostate and its
association with emergence of androgen-independent prostate cancer.
Cancer Res. 52:6940–6944. 1992.PubMed/NCBI
|
|
7
|
Banerjee PP, Banerjee S and Brown TR:
Bcl-2 protein expression correlates with cell survival and androgen
independence in rat prostatic lobes. Endocrinology. 143:1825–1832.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ventura A, Kirsch DG, McLaughlin ME, et
al: Restoration of p53 function leads to tumour regression in vivo.
Nature. 445:661–665. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leprince D, Crepieux P, Laudet V, Flourens
A and Stehelin D: A new mechanism of oncogenic activation: E26
retroviral v-ets oncogene has inverted the C-terminal end of the
transcription factor c-ets-1. Virology. 194:855–857. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu C, Zhu Y, Lou W, et al: Functional p53
determines docetaxel sensitivity in prostate cancer cells.
Prostate. 73:418–427. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Panaretakis T, Pokrovskaja K, Shoshan MC
and Grander D: Interferon-alpha-induced apoptosis in U266 cells is
associated with activation of the proapoptotic Bcl-2 family members
Bak and Bax. Oncogene. 22:4543–4556. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Davol PA, Goulette FA, Frackelton AR Jr
and Darnowski JW: Modulation of p53 expression by human recombinant
interferon-alpha2a correlates with abrogation of cisplatin
resistance in a human melanoma cell line. Cancer Res. 56:2522–2526.
1996.PubMed/NCBI
|
|
13
|
Huang SF, Kim SJ, Lee AT, et al:
Inhibition of growth and metastasis of orthotopic human prostate
cancer in athymic mice by combination therapy with pegylated
interferon-alpha-2b and docetaxel. Cancer Res. 62:5720–5726.
2002.
|
|
14
|
Emerson L and Morales A: Intralesional
recombinant alpha-interferon for localized prostate cancer: a pilot
study with follow-up of >10 years. BJU Int. 104:1068–1070.
2009.
|
|
15
|
Shinohara N, Abe T, Sazawa A, et al:
Interferon-α-based immunotherapy in metastatic renal cell carcinoma
patients with the primary tumor in situ. Jpn J Clin Oncol.
42:113–119. 2012.
|
|
16
|
Mocellin S, Pasquali S, Rossi CR and Nitti
D: Interferon alpha adjuvant therapy in patients with high-risk
melanoma: a systematic review and meta-analysis. J Natl Cancer
Inst. 102:493–501. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van Haelst-Pisani CM, Richardson RL, Su J,
et al: A phase II study of recombinant human alpha-interferon in
advanced hormone-refractory prostate cancer. Cancer. 70:2310–2312.
1992.PubMed/NCBI
|
|
18
|
Bulbul MA, Huben RP and Murphy GP:
Interferon-beta treatment of metastatic prostate cancer. J Surg
Oncol. 33:231–233. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Padhani AR and Ollivier L: The RECIST
(Response Evaluation Criteria in Solid Tumors) criteria:
implications for diagnostic radiologists. Br J Radiol. 74:983–986.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
DiPaola RS, Chen YH, Stein M, et al: A
randomized phase II trial of mitoxantrone, estramustine and
vinorelbine or bcl-2 modulation with 13-cis retinoic acid,
interferon and paclitaxel in patients with metastatic
castrate-resistant prostate cancer: ECOG 3899. J Transl Med.
8:202010. View Article : Google Scholar
|
|
21
|
Anatol P, Danuta P, Janusz D and Bozena P:
Expression of bcl-2 protein in chronic hepatitis C: effect of
interferon alpha 2b with ribavirin therapy. World J Gastroenterol.
11:2949–2952. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Amato RJ and Mohammad T: Interferon-alpha
plus capecitabine and thalidomide in patients with metastatic renal
cell cancer. J Exp Ther Oncol. 7:41–47. 2008.
|
|
23
|
Rosevear HM, Lightfoot AJ, Nepple KG and
O’Donnell MA: Usefulness of the Spanish Urological Club for
Oncological Treatment scoring model to predict nonmuscle invasive
bladder cancer recurrence in patients treated with intravesical
bacillus Calmette-Guérin plus interferon-α. J Urol. 185:67–71.
2011.
|
|
24
|
Panasiuk A, Prokopowicz D and Dzieciol J:
p53 protein expression in chronic hepatitis C; effect of interferon
alpha 2b therapy. Hepatogastroenterology. 52:1176–1179.
2005.PubMed/NCBI
|
|
25
|
Kelly WK, Halabi S, Carducci M, et al:
Randomized, double-blind, placebo-controlled phase III trial
comparing docetaxel and prednisone with or without bevacizumab in
men with metastatic castration-resistant prostate cancer: CALGB
90401. J Clin Oncol. 30:1534–1540. 2012. View Article : Google Scholar
|
|
26
|
Koirala J, Gandotra SD, Rao S, et al:
Granulocyte colony-stimulating factor dosing in pegylated
interferon alpha-induced neutropenia and its impact on outcome of
anti-HCV therapy. J Viral Hepat. 14:782–787. 2007. View Article : Google Scholar
|
|
27
|
Chang AY, Fisher HA, Spiers AS and Boros
L: Toxicities of human recombinant interferon-alpha 2 in patients
with advanced prostate carcinoma. J Interferon Res. 6:713–715.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
ten Tije AJ, Verweij J, Carducci MA, et
al: Prospective evaluation of the pharmacokinetics and toxicity
profile of docetaxel in the elderly. J Clin Oncol. 23:1070–1077.
2005.PubMed/NCBI
|