Introduction
Breast carcinoma is the most frequently occurring
malignancy in females, representing 22% of all forms of cancer
(1). Patients who develop these
types of tumor benefit from surgical therapy in association with
systemic or local adjuvant therapy, which increases the long-term
survival rate. Administration of systemic adjuvant therapy may lead
to a number of side effects with major impacts on patient quality
of life; therefore, optimal patient selection is necessary
(2). The traditional classification
of breast carcinoma is based strictly on evident morphological
criteria found on tissue preparations, using hematoxylin and eosin
staining (3,4). Based on gene expression in the tumor
cells of breast carcinomas, Perou et al(5) successfully identified several tumor
subtypes with specific properties with regard to epidemiology,
natural evolution and response to systemic and local adjuvant
therapy. Immunohistochemistry (IHC) was used as a surrogate method
for molecular subtyping, based on estrogen receptors (ERs),
progesterone receptors (PRs), EGFR, HER2 and cytokeratin-5
expression (6–8). Additional studies have shown the
necessity of refining criteria for IHC characterization of these
tumor subtypes. Currently, in addition to these markers, Ki-67
expression is taken into consideration, enabling quantification of
the tumor proliferation index (6).
A significant feature of malignant tumors is their
uncontrolled ability to proliferate. Proliferation may be evaluated
in various ways, including assessment of the mitotic score by
counting mitosis on stained preparations (a mandatory step in
determining the histological grade), incorporation of labeled
nucleotides into DNA and flow cytometry of the fraction of cells in
S phase (9). The most common method
used is IHC, which allows for the identification of antigen Ki-67
at the nuclear level using a highly specific antibody. Results are
presented as the Ki-67 proliferation index (IK), which represents
the percentage of Ki-67-positive tumor cells (9). As shown by Urruticoechea et al
in 2005 (10), 17 of the 18 studies
that included >200 patients showed a statistically significant
association between Ki-67 expression and prognosis, providing
compelling evidence for a biological correlation. However, the
cutoffs to distinguish ‘Ki-67-high’ from ‘Ki-67-low’ varied between
1 and 28.6%, severely limiting its clinical utility. In addition,
the numerous steps of the evaluation introduce variability into the
results of these assays. Moreover, according to the St. Gallen
International Expert Consensus on the Primary Therapy of Early
Breast Cancer (2009)(11), breast
carcinomas may be stratified into three groups (high, moderate and
low) depending on the IK, guiding to a specific therapeutic
approach (hormone and/or chemotherapy). In addition, an IK value of
14% represents a threshold for determining the A and B luminal
tumors more clearly (6). However,
as shown by Recommendations from the International Ki67 in Breast
Cancer Working Group, a significant factor that affects the value
of the IK is the interpretation and implementation approach. This
method is based on visual or automated methods to count labeled
tumor nuclei for Ki-67 antigen, and is prone to errors (9,12).
The current study proposes an original method for
the quantification of Ki-67-positive tumor nuclei, enabling the
determination of the exact value of the IK, which is required for
tumor stratification based on the proliferation rate (9). It is a method that may be used for
research and diagnostic purposes, which prevents the counting
errors that occur with other methods of quantification (12,13).
Material and methods
Morphological assessment
A total of 81 consecutive cases of diagnosed
invasive ductal carcinomas (IDCs) were examined. IDCs were obtained
from the Department of Pathology, Emergency County Hospital
(Timisoara, Romania). Specimens were processed using the standard
procedure for breast tumors according to World Health Organization
(WHO) recommendations (3). Primary
processing of tissues (fixation and paraffin embedding) was
performed using standard histological techniques. The ethics
committee of Ethics committee of ‘Victor Babes’ University of
Medicine and Pharmacy approved the protocol of the study and
informed written consent was obtained from all subjects according
to the World Medical Association Declaration of Helsinki.
From paraffin blocks, 5-μm-thick sections were
stained with hematoxylin and eosin and evaluated by two independent
pathologists. Quantified conventional parameters included tumor
size, histological type and grade, and lymph node status. Based on
the assessed parameters, pTNM grading and clinical stage were
determined and Nottingham Prognostic Index (NPI) was calculated.
Significant tissue fragments were selected from each case and
evaluated by IHC. Examination of the slides, morphologically and
immunohistochemically, was performed using a Nikon i80 microscope
with an acquisition and image processing system (Nikon Instruments
Inc., Tokyo, Japan). Tumor size (T) and primary tumor stage were
pathologically assessed by three-dimensional measuring; the largest
tumor size was used for assessing T stage of pTNM classification.
For tumors with an invasive and in situ component, only the
invasive component was taken into consideration to calculate tumor
size (14,15). The average tumor size was 2.8 cm
(min, 1; max, 8; median, 2.1). Lymph node status (N) was determined
by the evaluation of ≥10 lymph nodes for each case in accordance
with WHO criteria for pTNM classification (3). All cases examined ≥15 lymph nodes and
no cases showed metastasis (M0). Tumor grading was
quantified using the histological grading method, which is the most
relevant assessment method for IDCs of no specific type (4,16–18).
The histological grading system was based on the Scarff
Bloom-Richardson (SBR) score, modified by Elston and Ellis
(19), which takes into account the
degree of differentiation with formation of tubular structures,
nuclear pleomorphism and the number of cells in mitosis (15,16).
Of the three parameters for SBR score, nuclear pleomorphism is the
most subjective, presenting the highest interobserver differences
(20). Tubular differentiation was
quantified following the examination of all tumor areas,
determining the percentage of glandular tubular structures
(structures with well-defined lumen) from the total area of the
tumor examined. Nuclear pleomorphism was assessed on the least
differentiated area (16).
Assessment of pleomorphism involves the size of tumor cells
relative to normal cells of breast glandular epithelium, presence
of nucleoli and their size and chromatin appearance (fine or coarse
granular) (17,21). Mitosis counting was achieved on 10
microscope fields, under a ×40 objective (0.59 mm diameter),
predominantly found at the periphery of the tumor (avoiding
necrotic areas) (21). NPI is based
on three parameters, using the following formula: NPI = [tumor size
(cm) × 0.2] + lymph node status (score 1, 2 or 3) + histological
grade (1, 2 or 3). Tumor size was used in the pTNM staging to
assess the primary tumor. Lymph node staging has three levels of
assessment, which are as follows: 1, no positive nodes; 2, ≤3
positive nodes (with metastasis); and 3, ≥4 positive nodes or
positive apical ganglion. Histological grade was obtained from the
SBR score. Numerical values were calculated according to the NPI
and its value identifies three prognostic groups, which are as
follows: i) good prognostic group (GPG), <3.4; ii) moderate
prognostic group (MPG), 3.4–5.4; and iii) poor prognostic group
(PPG), >5.4 (16,22). The clinicopathological
characteristics of patients with IDC of the breast are shown in
Table III.
 | Table IIIIK distribution according to clinical
and pathological criteria. |
Table III
IK distribution according to clinical
and pathological criteria.
| Criteria | Total, n | HighIK,%
(n) |
ModerateIK, % (n) | LowIK, %
(n) | IK |
|---|
| IDC | 81 | 37.1 (30) | 25.8 (21) | 37.1 (30) | 30.2 |
| Pre-menopause, ≤50
years | 30 | 40.0 (12) | 30.0 (9) | 30.0 (9) | 33.2 |
| Post-menopause,
>50 years | 51 | 35.3 (18) | 23.5 (12) | 41.2 (21) | 28.4 |
| Histological
grade |
| G1 | 9 | 0.0 (0) | 0.0 (0) | 100.0 (9) | 12.6 |
| G2 | 45 | 20.0 (9) | 33.3 (15) | 46.7 (21) | 24.8 |
| G3 | 27 | 77.8 (21) | 22.2 (6) | 0.0 (0) | 44.9 |
| Lymph node
metastasis |
| Yes | 51 | 47.1 (24) | 17.6 (9) | 35.3 (18) | 31.3 |
| No | 30 | 20.0 (6) | 40.0 (12) | 40.0 (12) | 28.3 |
| IDC |
| Luminal | 63 | 19.1 (12) | 33.3 (21) | 47.6 (30) | 21.7 |
| Non-luminal | 18 | 100.0 (18) | 0.0 (0) | 0.0 (0) | 59.6 |
| Stage |
| I | 12 | 0.0 (0) | 25.0 (3) | 75.0 (9) | 13.7 |
| II | 33 | 45.4 (15) | 27.3 (9) | 27.3 (9) | 35.3 |
| III | 36 | 41.7 (15) | 25.0 (9) | 33.3 (12) | 31.1 |
| NPI |
| GPG | 24 | 12.5 (3) | 25.0 (6) | 62.5 (15) | 19.1 |
| MPG | 30 | 40.0 (12) | 30.0 (9) | 30.0 (9) | 33.2 |
| PPG | 27 | 55.6 (15) | 22.2 (6) | 22.2 (6) | 36.7 |
IHC data
For IHC evaluation, the most representative paraffin
blocks were selected from each case, including primary tumor and
non-tumor glandular structures. From each selected block, four
sections (3-μm-thick) were used for IHC assessment of ER, PR, HER2
and Ki-67, and staining was performed by the second day following
sectioning. Sections were dewaxed and dehydrated, prior to internal
peroxidase inhibition with 3% hydrogen peroxide for 5 min.
Subsequently, antigen retrieval was performed for 30 min by
microwave heating in target retrieval solution (pH 6;
DakoCytomation, Glostrup, Denmark), followed by incubation with
specific primary antibodies (30 min) using various working systems.
3,3′-Diaminobenzidine hydrochloride was applied for 10 min for
visualization, followed by the staining of nuclei with hematoxylin
for 3 min. Details of antibodies and the IHC technique are shown in
Table I.
 | Table IAntibodies and working systems
used. |
Table I
Antibodies and working systems
used.
| Marker | Clone | Source | Dilution | HIER, min (pH) | WS |
|---|
| ER | 1D5 |
DakoCytomationa | RTU | MW, 30 (6) | LSAB-HRP |
| PR | PgR636 |
DakoCytomationa | RTU | MW, 30 (6) | LSAB-HRP |
| HER2 | Polyclonal |
DakoCytomationa | RTU | MW, 30 (6) | EnVision-HER |
| Ki-67 | Monoclonal,
MIB-1 |
DakoCytomationa | RTU | MW, 30 (6) | LSAB-HRP |
Quantification of IHC reactions
ER- and PR-positive cells were counted using a
semi-automated method and all cases with values of >1% were
considered positive. Tumors with ER and PR expression levels of
≤50% were considered to have low levels of receptors
(lowER and lowPR), whereas tumors with ER and
PR expression levels of >50% were considered to have high levels
of receptor expression (highER and highPR).
To assess HER2, the Dako HercepTest scoring system was used. The
current scoring system accepted by the American Society of Clinical
Oncology and College of American Pathologists uses a threshold of
≥30% tumor cells with an intense, continuous and membrane positive
reaction for HER2 (23). The
reaction to HER2 was scored as follows: 0 (negative), absent or
present in <10% of tumor cells; 1+ (negative), membranous, weak
and discontinuous in >10% of tumor cells; 2+ (questionable),
membranous, low/moderate and continuous in >10% of tumor cells
or membranous, intense and continuous in ≤30% of tumor cells; and
3+ (positive), membranous, intense and continuous in >30% of
tumor cells. Cases were stratified as luminal breast carcinoma
(ER+ and/or PR+/ ± HER2+) and
non-luminal breast carcinoma (represented by all ER- and
PR-negative cases) (5,7,8,24).
Ki-67 assessment
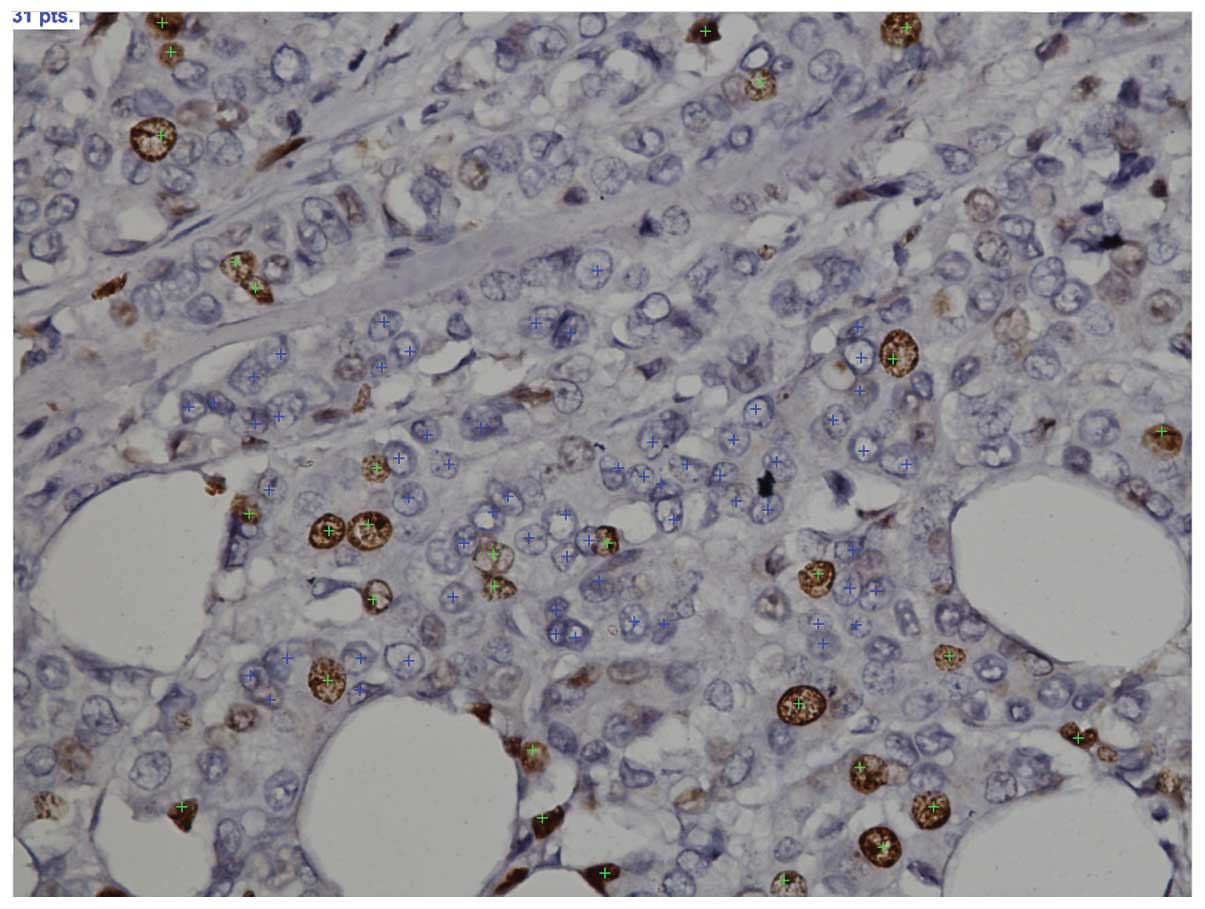
Ki-67 assessment was realized by Nikon Eclipse i80
microscope with an image acquisition and processing system,
resolution of 2,560×1,920 pixels and color depth of 24 bits
(Fig. 1). Each slide was initially
examined with a ×10 objective and areas with the highest density of
Ki-67-positive nuclei were selected, commonly located close to the
periphery of the tumor invasion front. IK was calculated using
digital images captured with a ×40 objective, taking into account
the current recommendations with regard to the requirement of a
minimum number of 1,000 nuclei to be counted for calculating the IK
(9).
The tissue fragments labeled with MIB-1 antibody
were initially examined with a ×10 objective to identify the areas
with the highest density of positive tumor nuclei, commonly located
at the periphery of the tumor fragment. Following this, the
fragments were examined with a ×40 objective and the necessary
number of digital images was captured for each case. IK was
calculated from the first digital image to determine the number of
tumor nuclei present in the image. From the values obtained, it was
clear that a number of digital images were required to be captured
for each case to achieve the minimum of 1,000 tumor nuclei to be
counted.
Since the number of tumor nuclei counted on a single
digital image was an average of 173 (median, 151) and considering
the recommendation of counting a minimum of 1,000 nuclei to
calculate the IK, an average of seven digital images were captured
for each slide (Table II). If
following seven captured images the nuclei number was <1,000,
additional images were captured to attain the recommended number. A
×40 objective was used for the assessment of the IK and the digital
image size captured with the camera had a length of 310.3 μm and a
width of 232.72 μm, which corresponds to an area of 0.072213
mm2.
 | Table IINumber of evaluated tumor nuclei and
nuclear density. |
Table II
Number of evaluated tumor nuclei and
nuclear density.
| Mean | Median | Min/max |
|---|
| Total tumor nuclei,
na | 173 | 151 | 83/585 |
| Ki-67-positive
nuclei, na | 45 | 38 | 9/134 |
| Nuclear density,
n/mm2 | 2451 | 2091 | 1149/8101 |
A special feature in the morphometry software
(NIS-Elements D 2.30, Laboratory Imaging s.r.o., Prague), called
Counts, allowed for the accurate counting of nuclei in the digital
images captured. The computer mouse is positioned on one nucleus
belonging to an immunomarked tumor cell (brown) and a marker (green
star) is placed on the nucleus (Fig.
1). Automatically, the number of nuclei with markers is counted
and appears in a table. Following the completion of counting
stained nuclei, the same procedure was used to quantify negative
tumor nuclei and cells were automatically recorded into the same
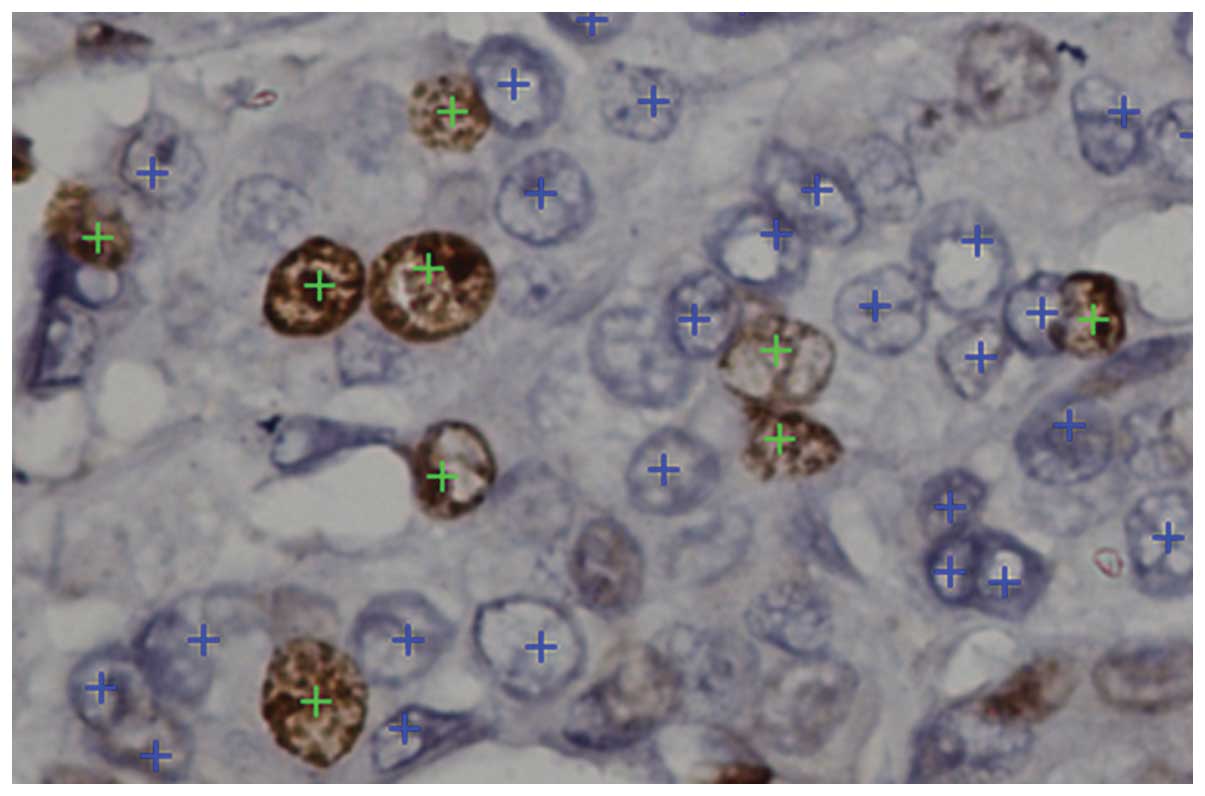
table. For high tumor nuclei densities with unclear nuclear limits,
a zoom function was used for a more exact selection (Fig. 2).
By identifying the number of stained (immunomarked)
and unstained nuclei, the percentage of stained nuclei from the
total tumor nuclei counted was calculated, obtaining the IK for
each image individually. The end result, implicating the final
value of the IK in a particular case, was calculated by taking the
arithmetic mean of all the IK values for each image individually.
Depending on the value of the IK and considering the criteria
proposed by the St. Gallen International Expert Consensus on the
Primary Therapy of Early Breast Cancer (2009)(11), luminal carcinoma
ER+/PR+/HER2− may be stratified
into exactly three subgroups, which intervene with a specific
therapeutic approach. The three subgroups were as follows: IK ≤15%,
lowIK tumors; IK 16–30%, moderateIK tumors;
and IK >30%, highIK tumors.
Results
IHC results
Positive immunoreactivity for Ki-67 antigen was
localized at the nuclear level with a granular pattern (fine or
coarse). The intensity of immunoreactivity varied slightly on this
section, without a differentiated quantification of nuclei
according to the intensity of reaction. All preparations examined
showed normal components (normal glandular lobules) associated with
the tumor and were used as internal controls to assess the IHC
reaction.
Quantification of tumor cells
The mean total number of tumor cells counted on a
digital image (area, 0.072213 mm2) was 173 cells (min,
83; max, 585; median, 151), while the number of Ki-67-positive
nuclei had an average value of 45 cells (min,9; max, 134;
median,38) (Table II).
Digital image capture and time required
for analysis
In ~50% (n=40) of the cases studied, seven digital
images/case was sufficient for the assessment of the number of
quantified tumor nuclei to be ≥1,000. In 32% of cases (n=26), it
was necessary to evaluate more than seven images/case, with ~50% of
these cases (n=12) requiring eight images. For the remaining 18% of
the cases (n=15) investigated, 73% (n=11) required five images/case
and 27% (n=4) required only two images/case, characterized by high
nuclear density (>500 nuclei/image). The time required for tumor
nuclei counting, using the proposed method on a digital image
containing 173 nuclei, was 2 min and 30 sec and the actual time
required to count 1,000 nuclei was 15 min. An additional 5 min
(maximum) was necessary for capturing the digital images
corresponding to each case as well as ~5 min for calculating the
percentage value of the IK. Therefore, the maximum time required
for the evaluation of a case was 25 min. The high, moderate and low
levels of the IK according to clinicopathological characteristics
are shown in Table III.
IK value analysis
An IK of 68% (n=55) represents
ER+/PR+/HER2− tumors (luminal A),
of which specific tumors are likely to be selected for treatment by
chemotherapy with a parameter being highIK (Table IV). Following the proposed
assessment using a semi-automated method, it was identified that
among these tumors only approximately one in five (21.8%) exhibited
highIK.
 | Table IVIK distribution in
ER+/PR+/HER2− luminal tumors
depending on low or high levels of ER and PR. |
Table IV
IK distribution in
ER+/PR+/HER2− luminal tumors
depending on low or high levels of ER and PR.
| Levels of ER and
PR | HighIK,
% (n) |
ModerateIK, % (n) | LowIK, %
(n) | Total, n |
|---|
| Cases
(ER+/PR+/HER2−) | 21.8 (12) | 36.4 (20) | 41.8 (23) | 55 |
| ER |
| High | 15.0 (6) | 42.5 (17) | 42.5 (17) | 40 |
| Low | 40.0 (6) | 20.0 (3) | 40.0 (6) | 15 |
| PR |
| High | 18.8 (6) | 43.7 (14) | 37.5 (12) | 32 |
| Low | 26.1 (6) | 26.1 (6) | 47.8 (11) | 23 |
As shown in Table
IV, a number of ER+/PR+/HER2−
tumors (n=40) were characterized by highER, of which
only a small proportion (15%) had highIK. This tendency
was found to be the case with PR expression. The highest percentage
of highIK tumors was characteristic of those with
lowER and lowPR expression compared with
highER and highPR expression (Table V).
 | Table VIK distribution in
ER+/PR+/HER2− luminal tumors
depending on combined levels of ER and PR (high and low). |
Table V
IK distribution in
ER+/PR+/HER2− luminal tumors
depending on combined levels of ER and PR (high and low).
| Cases
(ER+/PR+/HER2−) | HighIK,
% (n) |
ModerateIK, % (n) | LowIK, %
(n) | Total |
|---|
| HighER
and highPR | 13.1 (3) | 60.8 (14) | 26.1 (6) | 23 |
| HighER
and lowPR | 17.6 (3) | 17.6 (3) | 64.8 (11) | 17 |
| LowER
and highPR | 33.3 (3) | 0.0 (0) | 66.7 (6) | 9 |
| LowER
and lowPR | 50.0 (3) | 50.0 (3) | 0.0 (0) | 6 |
Discussion
The Ki-67 (MKI67) antigen is a nuclear non-histone
protein required for cell proliferation and is encoded by the MKI67
gene, which is located on the long arm of chromosome 10. The
antigen was first identified by Gerdes et al in 1980
(25) and shortly following this
discovery, the anti-Ki-67 antibody was developed. Cellular
proliferation involves several defined phases: i) G0,
resting; ii) G1, first gap; iii) S, DNA synthesis; iv)
G2, second phase of relative inactivity; and v) M,
mitotic. Cells may be recycled by entering the G1 phase
or return to the resting G0 phase (10,26). A
detailed cell cycle analysis showed that the Ki-67 nuclear antigen
is expressed in the G1, S, G2 and M phases,
but is not expressed in non-dividing/quiescent cells that are in
G0 phase (27). The
topographic distribution of Ki-67 also varies during the cell cycle
(10,28,29).
Uncontrolled cell proliferation represents the
hallmark of malignant tumors and may be assessed by various
methods, most commonly by IHC detection of the Ki-67 antigen
(30,26). Early breast cancer has a highly
variable prognosis and the benefit of existing therapies is often
unpredictable. Several morphological parameters, including tumor
size, histological grade, vascular invasion and lymph node
metastasis, are useful in this regard, but insufficient (8). This has lead to the study of tumor
molecular characteristics and currently ER, PR and HER2 are
recognized as prognostic and predictive factors (10,12).
In addition to these, Ki-67 has been added, as its prognostic role
has been recognized by previous studies, particularly when specific
subgroups of breast carcinomas have been selected (9,31–33).
Moreover, in 2009, Cheang et al(34) proposed that a panel of antibodies
formed by ER, PR, HER2 and Ki-67 allows for the segregation of the
two types of luminal tumors (A and B) taking into account the value
of the IK. Thus, identification of the exact value of Ki-67 allows
for the differentiation of the two subtypes. The international
Ki-67 in Breast Cancer Working Group reported Ki-67 measurement by
IHC as the current assay of choice for measuring and monitoring
tumor proliferation in standard pathology specimens. However, the
group recognized the poor consistency with the precise clinical
uses of Ki-67 and the substantial heterogeneity and variable levels
of validity in methods of assessment (9,33).
For the clinician, it is important to be aware of
exactly what type of systemic adjuvant therapy is administered to
patients, as this therapy causes numerous side effects and requires
optimal patient selection (2).
Therefore, it is necessary to determine: i) which patients are
recommended for hormone therapy; ii) which patients are likely to
be administered anti-HER2 therapy; and iii) which patients are
likely to receive chemotherapy. Administration of endocrine therapy
is selected for all cases showing a positive reaction for ER, as
the response to therapy is dependent on the level of receptor
expression. Anti-HER2 therapy with trastuzumab has been recommended
for all HER2-positive tumors (>30% positive tumor cells),
according to the American Society of Clinical Oncology and the
College of American Pathologists (23). Selection of cases for chemotherapy
is the most delicate and difficult step and considers the following
patient groups: i) HER2-positive cases, chemotherapy is
administered prior to or following the administration of
trastuzumab; ii) triple negative tumors, ER−,
PR− and HER2−; and iii) specific
ER+, PR+ and HER2− (luminal)
tumors, a subset of tumors receiving hormone and chemotherapy. The
precise determination of the subset of patients with luminal
carcinomas (ER+/PR+/HER2−) who are
suitable for chemotherapy (associated with hormone therapy) is the
main issue, and the assessment of IK has shown promising
preliminary data with regard to these issues (34,35).
According to the St. Gallen International Expert Consensus on the
Primary Therapy of Early Breast Cancer, depending on the IK of
ER+/PR+/HER2− tumors, therapeutic
management is different. Patients with highIK are a
tumor subgroup receiving chemotherapy and hormone therapy,
moderateIK patients may benefit from hormone therapy and
chemotherapy and lowIK patients are likely to benefit
only from hormone therapy (11,33).
In addition, an IK value of >14% is used to identify luminal B
tumors (6). These results show the
importance of accurate IK values for all these cases and that low
intra- and interobserver variability for IK values are required
(33). As shown by Urruticoechea
et al in 2005 (10), using
various antibodies, immunomarking techniques and protocols for
assessing score, without a minimum standard with regard to the
number of tumor cells to be quantified and optimal thresholds for
defining subgroups, are all causes of heterogeneity and obstacles
against the use of these methods in clinical practice (9,11,36).
Previous studies have shown that limitations for the clinical use
of Ki-67 have been due to a lack of standardization concerning the
following four groups of parameters: i) preanalytical (type of
fixative, time used for fixation and how to preserve the tissue
fragments); ii) analytical (use of antigen retrieval, clone of
antibody used and the staining of nuclei with hematoxylin); iii)
interpretation and implementation of the score (quantification of
positive nuclei and/or intensity, assessment of the tumor area and
visual/automatic quantification methods); and iv) data analysis
(cut point) (9). Tissue fragments
to be assessed using the IK were fixed in 10% neutral buffered
formalin (3.7% formaldehyde; pH 7.2) for 24 h. It is important to
avoid delay in tissue fixation, as the IK value may decrease by 6%.
Data from previous studies show that the optimal fixative solution
recommended to preserve tissue for IHC evaluation of Ki-67 is
neutral buffered formalin and possibly non-buffered formalin. The
recommended optimal fixation period is between 6 h and up to 3 days
(9), although, there have been
previous studies showing that prolonged fixation (154 days) did not
significantly reduce Ki-67 immunomarking (37). However, the current study identified
that all tumors fixed in unbuffered formalin for >70 days were
Ki-67-negative.
Once the tissues are paraffin-embedded, they may be
stored at room temperature for a number of years without affecting
Ki-67 immunomarking (38). The
paraffin blocks used in the current study were selected
retrospectively, and the maximum time that passed following the
preparation of the blocks and IHC evaluation of Ki-67 was not >2
years. Paraffin sections that are placed on slides and stored at
room temperature retain their antigenicity for 3 months (39). Sections stored on glass slides at
room temperature for 2 weeks do not change in IK value (9). In the current study, IHC reactions
were performed on the second day following the sectioning of the
paraffin blocks for Ki-67 and other markers (ER, PR and HER2). With
regard to the antibody used for evaluating the IK, the most widely
used and recommended is the mouse anti-human Ki-67 monoclonal MIB-1
antibody. Proteases and low pH (<5) must be avoided for antigen
retrieval (9,40). The best results are obtained on
tissues fixed for a minimum of 6 h and a maximum of 3 days using
neutral buffered formalin or non-buffered formalin (pH 5) (41). The working technique of the current
study was to fix the specimens for 24 h in neutral buffered
formalin at pH 7.2 using clone MIB-1 and antigen retrieval solution
at pH 6 (DakoCytomation).
Typically, Ki-67 expression appears at the nuclear
level, although, cytoplasmic expression is possible as a result of
using MIB-1 antibody, particularly in grade 3, HER2-positive and
ER-negative breast cancer with squamous metaplastic changes
(42). However, this is not a
serious issue for interpretation; for the IK, appreciation of
nuclear expression is taken into account, which is rarely masked by
cytoplasmic reactions. Scoring systems are based on the percentage
of tumor cells stained by the antibody. This requires counting
≥1,000 tumor cells with nuclear staining under a high-powered field
(magnification, ×40) with laboratory limitations. Certain
pathologists estimate the percentage of nuclei staining, whereas
others count several hundred consecutive nuclei in various areas of
tumors to determine an overall average index. However, estimating
the percentage of cells is poorly reproducible and manual counting
is tedious, with high interobserver variability (12,13,43).
Therefore, automated readers have been used for scoring large
series of samples (44). A
significant concern is that automated methods may count
non-malignant nuclei, whereas a manual count is likely to exclude
this potential error. There are also significant discrepancies of
the IK value in these cases when using automated and visual
methods, particularly in tumors with heterogeneous Ki-67 expression
(45). The proposed semi-automated
method excludes errors and technical issues that may occur by
visual and automated counting methods.
Advantages of the semi-automated method are as
follows: i) accurate identification of positive tumor nuclei,
preventing the counting of other possible positive cells, including
lymphoid, normal epithelial and hyperplastic (12); ii) precise quantification of nuclei,
even when the limits between nuclei are difficult to identify when
tumor fragments have an increased cell density where the
nucleus/cytoplasm ratio is clearly in favor of the nucleus and the
distance between nuclei is small; iii) precise quantification of
negative tumor nuclei, preventing the counting of any other
non-tumor cells, including increased nuclear density areas; iv)
storage of images in a database, including the assessments, with
easy access to data when required; and v) it may be used on virtual
slides in telepathology. It is likely that the only disadvantage of
the semi-automated method is that it is time consuming, however,
the trained pathologist is able to make this count relatively
rapidly (maximum, 25 min/case). This method requires the use of a
digital image acquisition and processing optical system, however,
pathology departments and research centers where the evaluation of
the IK is performed have these systems, and therefore, this method
may be used routinely.
We hypothesize that the semi-automated method for
counting nuclei offers the most accurate method of assessing the IK
and avoids counting errors that may occur through other automatic
or manual counting methods. This method is used to assess the rate
of proliferation in IDC (and other non-mammary tumors) in our
institutions.
The current study proposes the use of this method
for the evaluation of any nuclear marker that requires the
quantification of breast carcinomas, particularly ER, PR, AR, p53
and other markers. In addition, the semi-automated method may be
used to identify the tumor proliferation rate of all tumor entities
(other than breast), which requires the determination of the IK by
using the methodology adapted for the specific evaluation of
tumors.
Acknowledgements
The current study was supported by a grant from The
Romanian Ministry of Education and Research (no. UEFISCDI
345/2011).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
Statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Cianfrocca M and Goldstein LJ: Prognostic
and predictive factors in early-stage breast cancer. Oncologist.
9:606–616. 2004. View Article : Google Scholar
|
|
3
|
Tavassoli FA and Devilee P: TNM
classification of carcinomas of the breast. World Health
Classification of Tumours Pathology and Genetics of Tumours of the
Breast and Female Genital Organs. IARC Press; Lyon: pp. 11–12.
2003
|
|
4
|
Rosen PP: Invasive duct carcinoma:
assessment of prognosis, morphologic prognostic markers, and tumor
growth rate. Rosen’s Breast Pathology. 3rd edition. Lippincott
Williams & Wilkins; Philadelphia, PA: pp. 358–404. 2009
|
|
5
|
Perou CM, Sørlie T, Eisen MB, et al:
Molecular portraits of human breast tumours. Nature. 406:747–752.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goldhirsch A, Wood WC, Coates AS, et al:
Panel members: Strategies for subtypes - dealing with the diversity
of breast cancer: highlights of the St. Gallen International Expert
Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann
Oncology. 22:1736–1747. 2011. View Article : Google Scholar
|
|
7
|
Carey L, Perou CM, Livasy CA, et al: Race,
breast cancer subtypes and survival in the Carolina Breast Cancer
Study. JAMA. 295:2492–2502. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mullan PB and Millikan RC: Molecular
subtyping of breast cancer: opportunities for new therapeutic
approaches. Cell Mol Life Sci. 64:3219–3232. 2007.PubMed/NCBI
|
|
9
|
Dowsett M, Nielsen OT, A’Hern R, et al;
International Ki-67 in Breast Cancer Working Group. Assessment of
Ki67 in breast cancer: recommendations from the International Ki67
in Breast Cancer working group. J Natl Cancer Inst. 103:1656–1664.
2011. View Article : Google Scholar
|
|
10
|
Urruticoechea A, Smith IE and Dowsett M:
Proliferation marker Ki-67 in early breast cancer. J Clin Oncol.
23:7212–7220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goldhirsch A, Ingle JN, Gelber RD, et al:
Panel members: Thresholds for therapies: highlights of the St
Gallen International Expert Consensus on the primary therapy of
early breast cancer 2009. Ann Oncol. 20:1319–1329. 2009. View Article : Google Scholar
|
|
12
|
Yerushalmi R, Woods R, Ravdin PM, et al:
Ki67 in breast cancer: prognostic and predictive potential. Lancet
Oncol. 11:174–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Varga Z, Diebold J, Dommann-Scherrer C, et
al: How reliable is Ki-67 immunohistochemistry in grade 2 breast
carcinomas? A QA study of the Swiss Working Group of Breast- and
Gynecopathologists. PLoS One. 7:e373792012. View Article : Google Scholar
|
|
14
|
Fitzgibbons PL, Page DL, Weaver D, et al:
Prognostic factors in breast cancer. College of American
Pathologists Consensus Statement 1999. Arch Pathol Lab Med.
124:966–978. 2000.PubMed/NCBI
|
|
15
|
Edge SB, Byrd DR, Compton CC, et al:
Breast. AJCC Cancer Staging Manual. 7th edition. Springer; New
York, NY: pp. 345–376. 2010
|
|
16
|
Derek AC: Breast carcinoma. Histopathology
Reporting Guidelines for Surgical Cancer. Springer-Verlag; London:
pp. 213–235. 2006
|
|
17
|
Moinfair F: Infiltrating ductal carcinoma
(NOS type). Essentials of Diagnostic Breast Pathology. A Practical
Approach. Springer; Berlin Heidelberg, New York, NY: pp. 180–181.
2007
|
|
18
|
Rakha EA, Reis-Filho JS, Baehner F, et al:
Breast cancer prognostic classification in the molecular era: the
role of histological grade. Breast Cancer Res.
12:2072010.PubMed/NCBI
|
|
19
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I The value of histological
grade in breast cancer: experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar
|
|
20
|
Dunne B and Going JJ: Scoring nuclear
pleomorphism in breast cancer. Histopathology. 39:259–265. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Elston CW and Ellis IO: Assessment of
histological gradee. Systemic Pathology. The Breast. 13. 3rd
edition. Churchill Livingstone; Philadelphia, PA: pp. 365–384.
1998
|
|
22
|
Rampaul RS, Pinder SE, Elston CW and Ellis
IO; Nottingham Breast Team. Prognostic and predictive factors in
primary breast cancer and their role in patient management: The
Nottingham Breast Team. Eur J Surg Oncol. 27:229–238. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wolff AC, Hammond HE, Schwartz JN, et al;
American Society of Clinical Oncology; College of American
Pathologists. American Society of Clinical Oncology/College of
American Pathologists guideline recommendations for human epidermal
growth factor receptor 2 testing in breast cancer. J Clin Oncol.
25:118–145. 2007. View Article : Google Scholar
|
|
24
|
Rakha EA, Elsheikh SE, Aleskandarany MA,
et al: Triple-negative breast cancer: distinguishing between basal
and nonbasal subtypes. Clin Cancer Res. 15:2302–2310. 2009.
View Article : Google Scholar
|
|
25
|
Gerdes J, Schwab U, Lemke H and Stein H:
Production of a mouse monoclonal antibody reactive with a human
nuclear antigen associated with cell proliferation. Int J Cancer.
31:13–20. 1983. View Article : Google Scholar
|
|
26
|
Beresford MJ, Wilson GD and Makris A:
Measuring proliferation in breast cancer: practicalities and
applications. Breast Cancer Res. 8:2162006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gerdes J, Li L, Schlueter C, et al:
Immunobiochemical and molecular biologic characterization of the
cell proliferation-associated nuclear antigen that is defined by
monoclonal antibody Ki-67. Am J Pathol. 138:867–873. 1991.
|
|
28
|
du Manoir S, Guillaud P, Camus E, et al:
Ki-67 labeling in postmitotic cells defines different Ki-67
pathways within the 2c compartment. Cytometry. 12:455–463.
1991.PubMed/NCBI
|
|
29
|
Bruno S and Darzynkiewicz Z: Cell cycle
dependent expression and stability of the nuclear protein detected
by Ki-67 antibody in HL-60 cells. Cell Prolif. 25:31–40. 1992.
View Article : Google Scholar
|
|
30
|
Gerdes J, Lemke H, Baisch H, et al: Cell
cycle analysis of a cell proliferation-associated human nuclear
antigen defined by the monoclonal antibody Ki-67. J Immunol.
133:1710–1715. 1984.
|
|
31
|
Park D, Kåresen R, Noren T and Sauer T:
Ki-67 expression in primary breast carcinomas and their axillary
lymph node metastases: clinical implications. Virchows Arch.
451:11–18. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Aleskandarany MA, Green AR, Benhasouna AA,
et al: Prognostic value of proliferation assay in the luminal,
HER2-positive and triple-negative biologic classes of breast
cancer. Breast Cancer Res. 14:R32012. View Article : Google Scholar
|
|
33
|
Luporsi E, André F, Spyratos F, et al:
Ki-67: level of evidence and methodological considerations for its
role in the clinical management of breast cancer: analytical and
critical review. Breast Cancer Res Treat. 132:895–915. 2012.
View Article : Google Scholar
|
|
34
|
Cheang MC, Chia SK, Voduc D, et al: Ki67
index, HER2 status, and prognosis of patients with luminal B breast
cancer. J Natl Cancer Inst. 101:736–750. 2009. View Article : Google Scholar
|
|
35
|
Yamaguchi T and Mukai H: Ki-67 index
guided selection of preoperative chemotherapy for HER2-positive
breast cancer: a randomized phase II trial. Jpn J Clin Oncol.
42:1211–1214. 2012. View Article : Google Scholar
|
|
36
|
Jalava P, Kuopio T, Juntti-Patinen L, et
al: Ki67 immunohistochemistry: a valuable marker in prognostication
but with a risk of misclassification: proliferation subgroups
formed based on Ki67 immunoreactivity and standardized mitotic
index. Histopathology. 48:674–682. 2006. View Article : Google Scholar
|
|
37
|
Arber DA: Effect of prolonged formalin
fixation on the immunohistochemical reactivity of breast markers.
Appl Immunohistochem Mol Morphol. 10:183–186. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Camp RL, Charette LA and Rimm DL:
Validation of tissue microarray technology in breast carcinoma. Lab
Invest. 80:1943–1949. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
DiVito KA, Charette LA, Rimm DL and Camp
RL: Long-term preservation of antigenicity on tissue microarrays.
Lab Invest. 84:1071–1078. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Boon ME: Microwave-antigen retrieval: the
importance of pH of the retrieval solution for MIB-1 staining. Eur
J Morphol. 34:375–379. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mengel M, von Wasielewski R, Wiese B, et
al: Inter-laboratory and inter-observer reproducibility of
immunohistochemical assessment of the Ki-67 labelling index in a
large multi-centre trial. J Pathol. 198:292–299. 2002. View Article : Google Scholar
|
|
42
|
Faratian D, Munro A, Twelves C and
Bartlett JM: Membranous and cytoplasmic staining of Ki67 is
associated with HER2 and ER status in invasive breast carcinoma.
Histopathology. 54:254–257. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tawfik O, Kimler BF, Davis M, et al:
Grading invasive ductal carcinoma of the breast: advantages of
using automated proliferation index instead of mitotic count.
Virchows Arch. 450:627–636. 2007. View Article : Google Scholar
|
|
44
|
Viale G, Giobbie-Hurder A, Regan MM, et
al; Breast International Group Trial 1-98. Prognostic and
predictive value of centrally reviewed Ki-67 labeling index in
postmenopausal women with endocrine-responsive breast cancer:
results from Breast International Group Trial 1-98 comparing
adjuvant tamoxifen with letrozole. J Clin Oncol. 26:5569–5575.
2008. View Article : Google Scholar
|
|
45
|
Mohammed ZM, McMillan DC, Elsberger B, et
al: Comparison of visual and automated assessment of Ki-67
proliferative activity and their impact on outcome in primary
operable invasive ductal breast cancer. Br J Cancer. 106:383–388.
2012. View Article : Google Scholar
|