Introduction
Organ transplant recipients are at increased risk of
developing malignancies, with an overall cancer incidence estimated
at 1,375 cases/100,000 person-years (1). The incidence of lung cancer has been
shown to be approximately two-fold higher during the first three
years after kidney transplantation than in the general population,
with the majority of patients being diagnosed at an advanced stage
(2). The treatment of lung cancer
in transplant recipients is complicated, as the complex clinical
situation and immunosuppressive drug administration often require
conflicting therapeutic approaches. The present study reports the
first case of stage IV de novo lung cancer developing four
months after renal transplantation. The patient developed
drug-induced interstitial pneumonitis while receiving
immunosuppressive drugs and oral-targeted therapy concomitantly.
Written informed consent was obtained from the patient.
Case report
The patient was a 66-year-old male who presented
with a history of chronic glomerulonephritis of >30 years and
had been receiving dialysis for five years. In April 2010, the
patient underwent allogenic renal transplantation followed by
immunosuppressive therapy with cyclosporin A (CsA; 50 mg twice
daily), mycophenolate mofetil (MMF; 500 mg twice daily) and
prednisolone (5 mg twice daily). The serum creatinine level
remained between 90 and 110 μmol/l and there were no episodes of
acute rejection. The patient had stopped smoking two years prior to
transplantation, but had a 40-year history of smoking 20
cigarettes/day. There was no family history of lung cancer. The
patient did not complain of coughing, expectoration, hemoptysis or
chest pain prior to kidney transplantation, and a chest X-ray
revealed no signs of abnormality.
A follow-up chest X-ray in August 2010 showed
evidence of a suspicious nodule in the right upper lobe of the
lung. A chest computed tomography (CT) scan revealed a 6-mm
diameter nodule, with a surrounding cavity and fibrous lesions
(Fig. 1). Bronchoscopic biopsy,
abdominal B ultrasound, cranial MRI and bone scans excluded distant
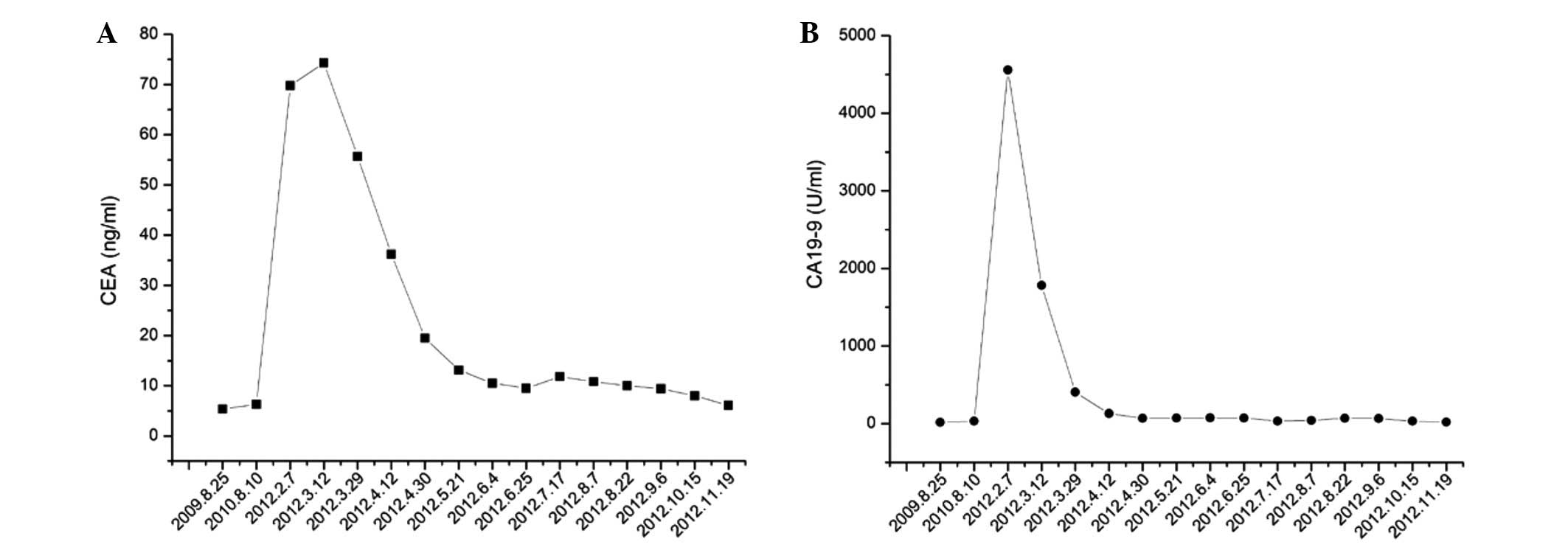
metastasis. The carcinoembryonic antigen (CEA) levels were 5.7
ng/ml (normal range, 0–5 ng/ml) and the carbohydrate/cancer antigen
19–9 (CA19–9) level was 15.7 U/ml (normal range, 0–37 U/ml)
(Fig. 2). All other serum tumor
markers were within the normal range. A follow-up chest CT in
December 2010 showed that the nodule had become enlarged, and
identified multiple ipsilateral subpleural nodules, all of which
were <5 mm in diameter. Based on these findings, the
immunosuppression protocol was switched to rapamycin (0.5 mg once
daily), MMF (500 mg twice daily) and prednisolone (5 mg once
daily).
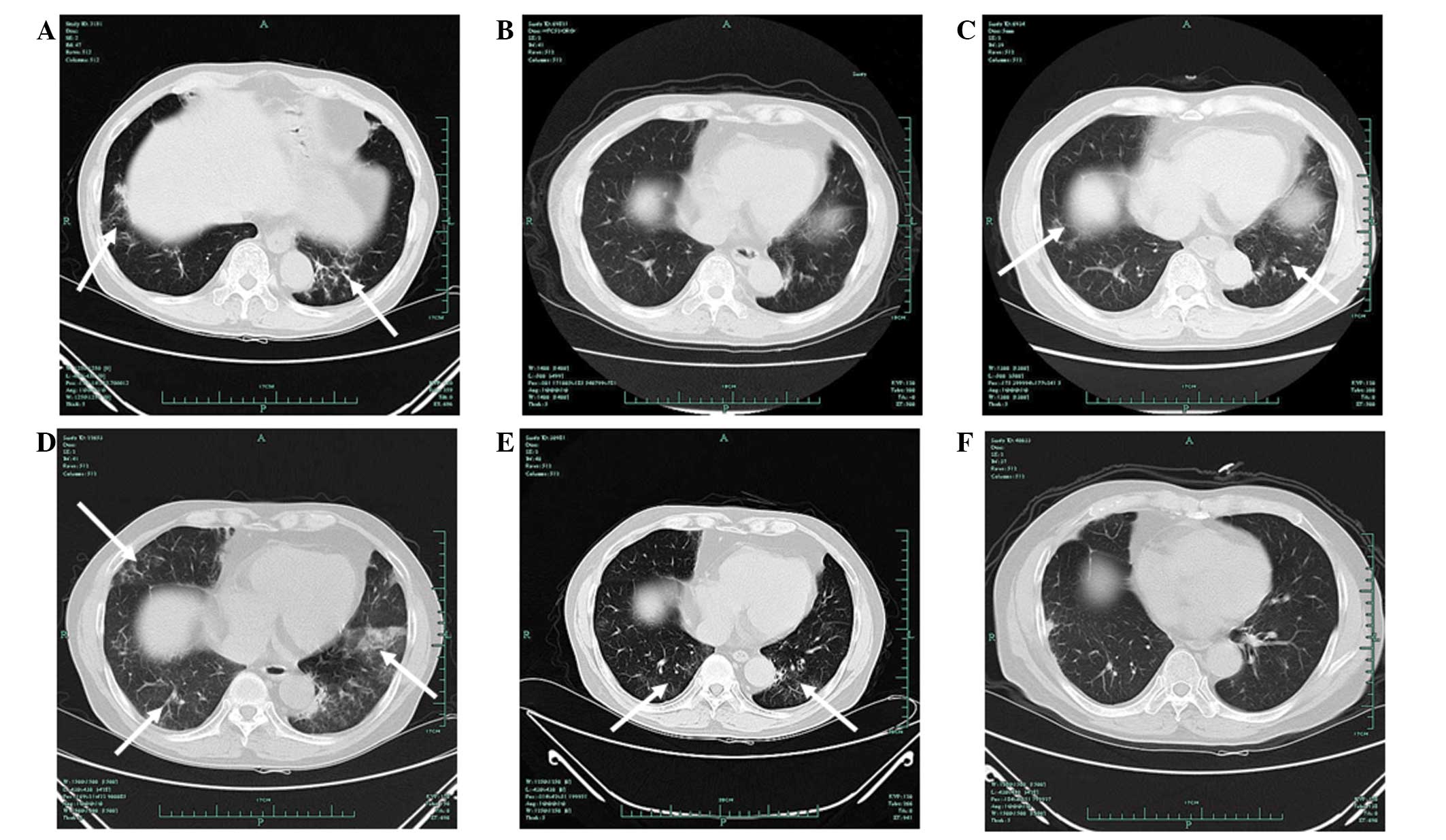
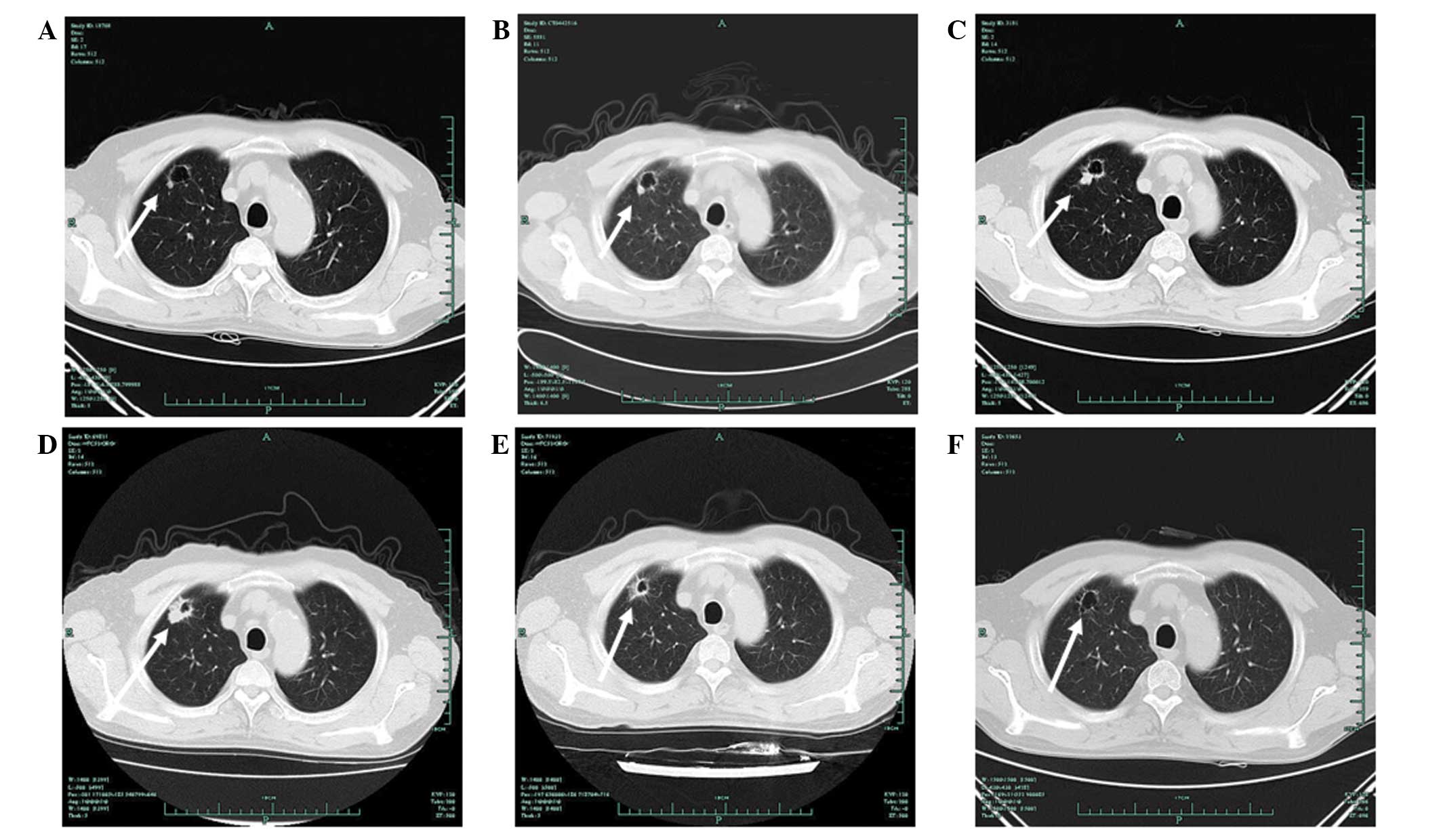 | Figure 1Chest computed tomography (CT)
findings showing evidence of a tumor response. Chest CT images
showed a tumor response prior to and after the patient received
icotinib. (A) The first appearance of a nodule on August 12, 2010,
at four months post-kidney transplant. (B) On February 9, 2011, the
nodule remained stable as previously. (C) On October 13, 2011, CT
imaging showed a marginal increase in the size of the nodule. (D)
On February 15, 2012 (prior to treatment with icotinib), the nodule
was pathologically diagnosed as adenocarcinoma. (E) Imaging results
on March 12, 2012, following one week of treatment with icotinib.
(F) Imaging results on August 7, 2012, following five months of
treatment with icotinib. The patient met the Response Evaluation
Criteria in Solid Tumors (RECIST) for a partial response. The
arrows indicate the tumor site. |
Regular CT follow-up and serum tumor marker tests
performed every three months indicated that the nodule and serum
tumor markers remained stable until the end of 2011. In February
2012, the CEA level had increased to 69.8 ng/ml and the CA19–9
level had increased to 4,559 U/ml. Chest CT imaging on February 15,
2012, revealed further significant enlargement of the nodule, with
ipsilateral multiple subpleural nodules. An abdominal contrast CT
was performed to exclude primary tumors of the digestive tract.
A CT-guided tumor biopsy enabled the nodule to be
pathologically diagnosed as adenocarcinoma, stage IV, T1aN0M1a
(3). Molecular testing undertaken
using the polymerase chain reaction-amplification refractory
mutation system (PCR-ARMS) indicated that the patient harbored a
deletion in exon 19 and an L858R point mutation in exon 21, but
there was no evidence of a T790M mutation in exon 20.
On March 1, 2012, the patient was scheduled to
receive molecular-targeted therapy with oral icotinib (125 mg three
times a day) for six days. However, the drug was discontinued after
five days due to personal reasons. A chest CT on March 12, 2012,
indicated a substantial remission of the lung nodule, with no
change in the subpleural nodules. The patient restarted treatment
with icotinib (125 mg three times a day) on March 12, 2012. On
March 29, 2012, the CEA level had dropped to 55.7 ng/ml and the
CA19-9 level had dropped to 406.6 U/ml. A follow-up chest CT scan
subsequent to more than one month of icotinib treatment showed
evidence of a further decrease in the size of the pulmonary nodule
(Fig. 1), and a partial response
(PR) was evaluated according to the Response Evaluation Criteria in
Solid Tumors (3). MMF was
discontinued, and rapamycin (0.75 mg once daily) and prednisolone
(5 mg once daily) were used as the ongoing immunosuppressive
protocol. By April 30, 2012, the CEA level had decreased to 19.5
ng/ml and the CA19-9 level to 69.4 U/ml (Fig. 2).
The patient received icotinib for a further five
months with a maintained RECIST PR for all disease parameters.
Icotinib was extremely well tolerated, with only grade 2 skin
toxicity appearing one week after the onset of treatment.
A chest CT on August 7, 2012, showed bilateral
patchy and diffuse interstitial infiltrates, which were signs of
interstitial pneumonitis (Fig. 3).
The patient did not complain of any discomfort, and infectious
causes and other pulmonary diseases were excluded. A review of the
previous chest CT images identified an unnoticed, transient
interstitial pneumonitis on October 13, 2011 (Fig. 3). Lung auscultation identified
wheezing, and pulmonary function tests indicated a modest decrease
in diffusing capacity.
 | Figure 3Computed tomography (CT) evidence of
interstitial pneumonitis. (A) On October 13, 2011, the CT image
showed for the first time scattered, patchy shadows in the two
lower lobes of the lung. (B) On February 15, 2012, no shadows were
detected. (C) CT findings on May 12, 2012, three months after the start of
icotinib. (D) CT findings on August 7, 2012, five months after the
start of icotinib.
(E) CT findings on October 15, 2012, two months after the patient
discontinued rapamycin and icotinib. (F) CT findings on November
10, 2012, one month
after the patient underwent a segmentectomy. The arrows
indicate the site of the interstitial lung disease. |
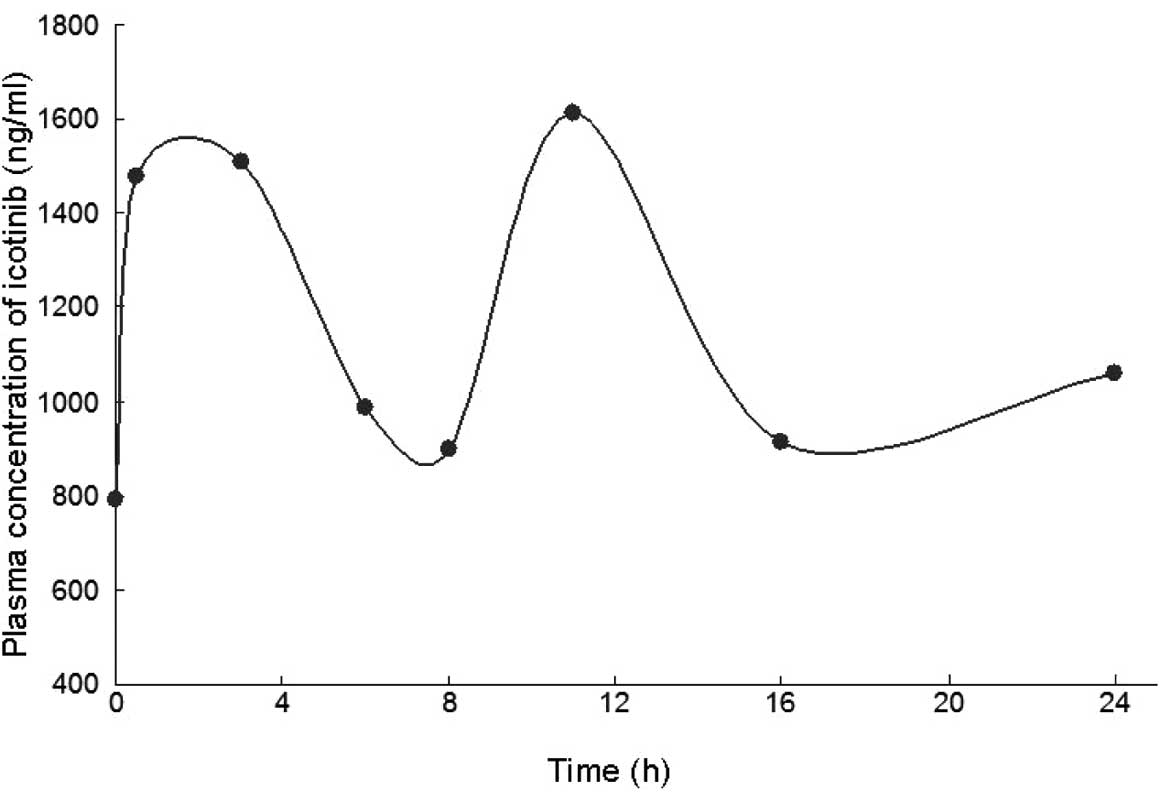
The plasma concentrations of icotinib were
determined by Beta Pharma Co., Ltd. (Hangzhou, China) using a
high-performance liquid chromatography method. The results
(Fig. 4) showed concentrations
similar to those reported in the published phase I trial of
icotinib (4), indicating that the
interstitial pneumonitis was not due to the increase in icotinib
plasma concentration. The pharmacokinetic data from the patient was
also similar to that estimated in the phase I population (Table I).
 | Table IComparison of the pharmacokinetics
data from the present patient and data obtained from a phase I
trial of icotinib. |
Table I
Comparison of the pharmacokinetics
data from the present patient and data obtained from a phase I
trial of icotinib.
| Phase I trial
(TID) | Patient (TID) |
|---|
|
|
|
|---|
| Parameter, h | 125 mg | 250 mg | 375 mg | 125 mg |
|---|
| Tmax | 2.250 | 2.500 | 2.890 | 3.000 |
| Cmax | 2.100 | 3.910 | 4.400 | 1.613 |
| Cmin | 1.020 | 2.090 | 2.310 | 0.957 |
|
AUClast | 34.600 | 40.600 | 42.600 | 27.897 |
Based on these findings, the patient was diagnosed
with icotinib-associated interstitial pneumonitis. Icotinib and
rapamycin were discontinued, and methylprednisolone tablets (8 mg
twice daily) were administered to treat interstitial pneumonitis. A
CT scan on August 22, 2012, showed a radiological improvement in
the interstitial pneumonitis. The immunosuppressive drug treatment
was changed to tacrolimus (FK506; 1 mg twice daily).
On October 17, 2012, the patient underwent a
video-assisted segmentectomy to remove the apicoposterior segment
of the right upper lobe. Surgical biopsy confirmed the pathological
diagnosis of adenocarcinoma; the immunohistochemistry results
showed that the specimen was CK7+, P63− and
thyroid transcription factor-1+. Molecular testing by
PCR-ARMS confirmed that the patient harbored a deletion in exon 19,
but not in exon 21 and 20.
At the time that this report was written, the
patient was being followed up by quarterly chest CT scans and serum
tumor marker testing. The most recent concentration of CEA on
November 19, 2012, was 6.1 ng/ml, and the CA19-9 levels were 19.1
U/ml. The concentration of tacrolimus was maintained within the
optimal therapeutic range of 3.1–4.4 ng/ml. The patient’s renal
function also remained good up to this time.
Discussion
Immunosuppressive agents inhibit immune
responsiveness and in the long-term have the potential to
accelerate tumor growth and metastasis. Immunosuppressive agents,
such as azathioprine, have been shown to exert a direct oncogenic
effect by causing chromosomal breakdown (5). CsA and MMF have been associated with
post-transplant malignancy, but the cancer incidence with MMF is
lower than that with CsA (6).
Rapamycin is an inhibitor of the mammalian target of
rapamycin (mTOR). Rapamycin acts as an immunosuppressant, but also
possesses antiproliferative activity, which may be useful in
post-transplant patients at increased risk of malignancy (7). Early withdrawal of CsA and a switch to
mTOR inhibitors, such as rapamycin and everolimus, have been shown
to reduce the risk of cancer in renal transplant patients (8,9). Data
from clinical trials and large registries indicate that the
incidence of de novo malignancies is less frequent among
patients receiving mTOR inhibitors than among those receiving other
forms of immunosuppressive therapy (10).
The modulation, switch or discontinuation of
immunosuppressive drugs in post-transplant patients has to be made
on a case-by-case basis. Upon the identification of a de
novo lung nodule in the present study patient, CsA was
discontinued and the dose of MMF was reduced and then discontinued.
Subsequent to being diagnosed with lung cancer, the patient was
switched to rapamycin and prednisolone. The immunosuppressive
effect of rapamycin, in combination with its antitumor effects,
make this drug an attractive treatment for post-transplant
malignancies.
On October 13, 2011, the patient developed
transient, asymptomatic and unnoticed interstitial pneumonitis,
which was considered to be the consequence of the rapamycin
treatment. It has previously been reported that as many as one in
six patients taking mTOR inhibitors develop reversible interstitial
pneumonitis (11). Sirolimus
pulmonary toxicity has also been reported in renal transplant
patients (12–14). In approximately half of the cases
this develops within six months of starting treatment. The exact
pathogenic mechanism of sirolimus-induced pulmonary toxicity is not
known, but it has been reported to be dose-dependent and
male-dominant (13).
The ongoing interstitial pneumonitis detected in the
present patient after five months of icotinib treatment was mainly
due to the administration of this drug. Radiological improvement
subsequent to the cessation of icotinib treatment indicated a
causal correlation.
The incidence of drug-induced lung disease by
molecular-targeted therapy varies among different drugs. The
incidence of interstitial lung disease (ILD) in patients receiving
erlotinib is reported to be between 1 and 3.8% (14–17),
while the incidence with gefitinib is between 1 and 8.3% (18–22). A
study in a population with a high co-incidence of pulmonary disease
proposed that the mechanism for developing epidermal growth factor
receptor (EGFR) tyrosine kinase inhibitor (TKI)-induced ILD was
most likely related to a decrease in alveolar regeneration
(23). This process was shown to be
normally regulated by EGFR. The treatment of drug-induced
interstitial pneumonitis includes discontinuation of the suspect
drug, administration of high-dose corticosteroids and mechanical
ventilation. Resuming administration of the previous drug following
the resolution of symptoms may lead to recurrence of ILD (24).
The potential synergy between the mTOR inhibitor,
rapamycin, and icotinib may have contributed to the rapid remission
of the tumor in the present case. Studies in animal models have
demonstrated in vitro synergistic effects between rapamycin
and erlotinib in non-small cell lung cancer (NSCLC) and pancreatic,
colon and breast tumors (25,26).
Rapamycin has also been shown to be effective in clinical trials
with EGFR TKIs in the treatment of glioblastoma and renal cell
carcinoma (27,28). Other mTOR inhibitors, such as
everolimus, have been administered in combination with EGFR TKIs in
NSCLC (29), providing new insights
for the treatment of post-transplant lung malignancies.
The metabolism of icotinib is undertaken mainly by
CYP3A4 and CYP2C19 (unpublished data). Thus, the combination of
icotinib with rapamycin [a known CYP3A4 substrate (30)] may have increased the potential for
unexpected side-effects. However, there are no published data on
the pharmacodynamics and interactions between the mTOR inhibitor
and icotinib. Similarly, there are no clinical trials on the safety
and efficacy of this combination in NSCLC. In the present case, we
speculate that the drug-induced interstitial pneumonitis was not
due to the interaction of the two drugs, but that it resulted from
the individual pulmonary toxicity of icotinib, since the plasma
concentrations of icotinib and rapamycin each remained within their
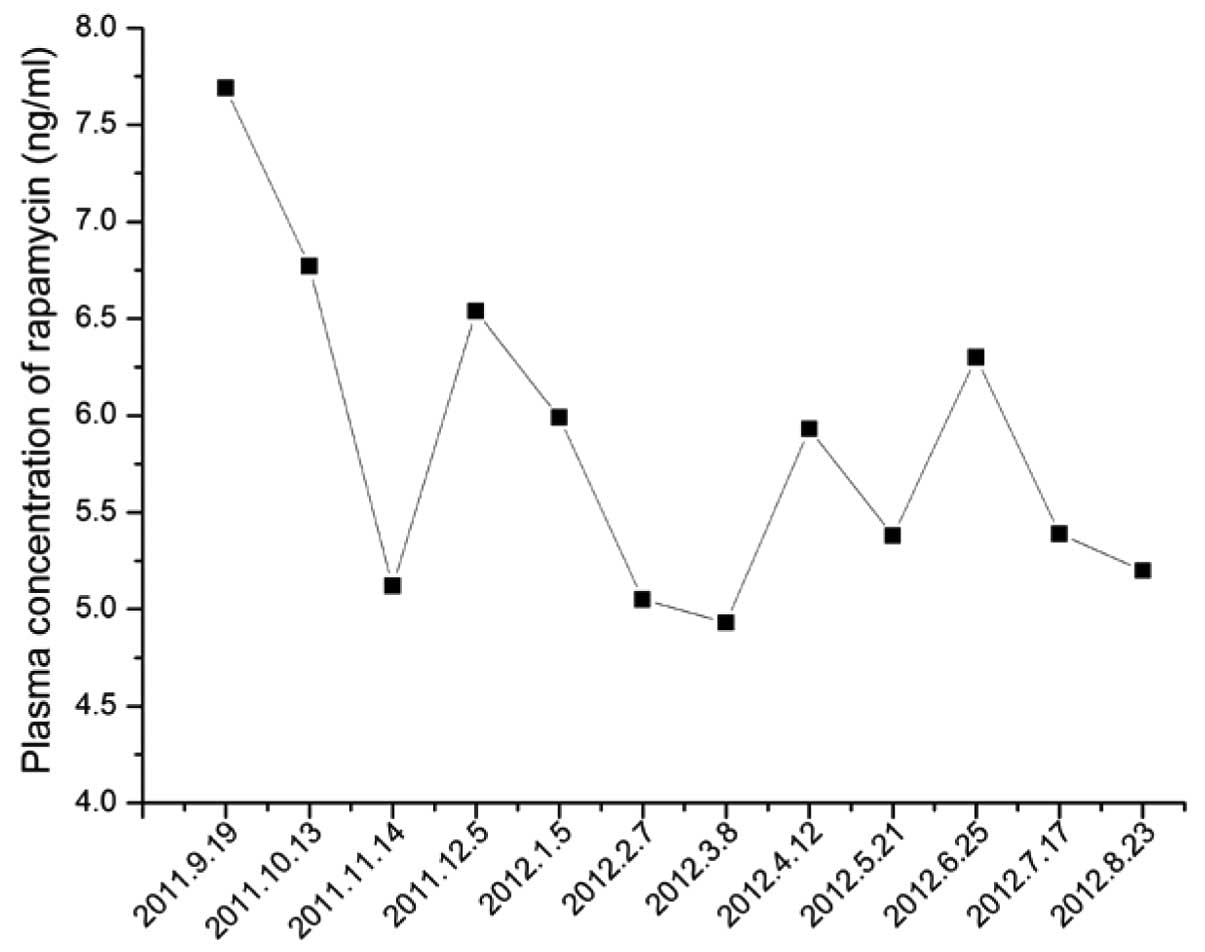
optimal ranges (Fig. 5).
Post-transplant lung cancer is often associated with
non-smokers and an adenocarcinoma histology (31). The present patient was a former
smoker and the histology of the tumor was of an adenocarcinoma.
Prior to the pathological diagnosis, the tumor had gradually
developed from stage I to stage IV disease. Molecular testing
confirmed that the patient harbored a deletion in exon 19 and an
L858R point mutation in exon 21, which indicated that EGFR TKIs may
provide some benefit. Icotinib (4,32) is
an oral EGFR TKI that has been approved by the Chinese State Food
and Drug Administration (FDA) for the treatment of advanced NSCLC.
In total, >5,000 Chinese patients have received this drug in the
last year. However, the present study is the first report of the
administration of icotinib in a post-transplant patient.
The patient achieved a PR subsequent to receiving
icotinib for six days. Research has shown that patients with
double-activating mutations in exon 19 and 21 account for ~3.4% of
unselected NSCLC patients of Chinese origin. These patients tend to
respond well to TKIs, as the sensitivity of double-mutated EGFR
TKIs is higher than that observed among patients with single
mutations (33). However, the
presence of double mutations is not only associated with higher
clinical response rates, but may also contribute to the high
incidence of pulmonary toxicity (34).
Tumors that develop following kidney transplantation
are generally more malignant, more poorly differentiated and carry
a worse prognosis compared with corresponding tumors in other
populations. Tumor screening and early diagnosis are therefore
essential prior to and following transplantation. Measures to
reduce the risk of post-transplant malignancies, including CT
screening for lung cancer and smoking cessation, should be
recommended for transplant recipients.
Rapamycin and other mTOR inhibitors are associated
with unique side-effects (7), the
majority of which are dose-related. This means that the monitoring
of drug levels should be routinely undertaken in patients receiving
icotinib as a targeted therapy.
To the best of our knowledge, this is the first
report of a concomitant administration of icotinib and rapamycin.
This combination of EGFR-TKIs and mTOR inhibitors may provide an
attractive regimen for the subset of patients that develops
advanced NSCLC following kidney transplantation. However,
consideration of the unique side-effects associated with this
combined regimen strategy requires further evaluation in randomized
double-blind trials. An improved understanding of drug-induced ILD
is also required, including more reliable data on the incidence of
events associated with different treatments and identification of
the risk factors for this type of ILD. Clinicians should remain
aware of the possibility of drug-induced pulmonary toxicity when
using mTOR inhibitors in combination with icotinib.
References
|
1
|
Engels EA, Pfeiffer RM, Fraumeni JF Jr, et
al: Spectrum of cancer risk among US solid organ transplant
recipients. JAMA. 306:1891–1901. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kasiske BL, Snyder JJ, Gilbertson DT and
Wang C: Cancer after kidney transplantation in the United States.
Am J Transplant. 4:905–913. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Detterbeck FC, Boffa DJ and Tanoue LT: The
new lung cancer staging system. Chest. 136:260–271. 2009.
View Article : Google Scholar
|
|
4
|
Zhao Q, Shentu J, Xu N, et al: Phase I
study of icotinib hydrochloride (BPI-2009H), an oral EGFR tyrosine
kinase inhibitor, in patients with advanced NSCLC and other solid
tumors. Lung Cancer. 73:195–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taylor L, Hughes RA and McPherson K: The
risk of cancer from azathioprine as a treatment for multiple
sclerosis. Eur J Neurol. 11:1412004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kauffman HM, Cherikh WS, McBride MA, Cheng
Y and Hanto DW: Post-transplant de novo malignancies in renal
transplant recipients: the past and present. Transpl Int.
19:607–620. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Webster AC, Lee VW, Chapman JR and Craig
JC: Target of rapamycin inhibitors (sirolimus and everolimus) for
primary immunosuppression of kidney transplant recipients: a
systematic review and meta-analysis of randomized trials.
Transplantation. 81:1234–1248. 2006. View Article : Google Scholar
|
|
8
|
Campistol JM, Eris J, Oberbauer R, et al:
Sirolimus therapy after early cyclosporine withdrawal reduces the
risk for cancer in adult renal transplantation. J Am Soc Nephrol.
17:581–589. 2006. View Article : Google Scholar
|
|
9
|
Kahan BD, Yakupoglu YK, Schoenberg L, et
al: Low incidence of malignancy among
sirolimus/cyclosporine-treated renal transplant recipients.
Transplantation. 80:749–758. 2005. View Article : Google Scholar
|
|
10
|
Kauffman HM, Cherikh WS, Cheng Y, Hanto DW
and Kahan BD: Maintenance immunosuppression with
target-of-rapamycin inhibitors is associated with a reduced
incidence of de novo malignancies. Transplantation. 80:883–889.
2005. View Article : Google Scholar
|
|
11
|
Barber NA and Ganti AK: Pulmonary
toxicities from targeted therapies: a review. Target Oncol.
6:235–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singer SJ, Tiernan R and Sullivan EJ:
Interstitial pneumonitis associated with sirolimus therapy in
renal-transplant recipients. N Engl J Med. 343:1815–1816. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pham PT, Pham PC, Danovitch GM, et al:
Sirolimus-associated pulmonary toxicity. Transplantation.
77:1215–1220. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morelon E, Stern M, Israel-Biet D, et al:
Characteristics of sirolimus-associated interstitial pneumonitis in
renal transplant patients. Transplantation. 72:787–790. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Herbst RS, Prager D, Hermann R, et al:
TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774)
combined with carboplatin and paclitaxel chemotherapy in advanced
non-small-cell lung cancer. J Clin Oncol. 23:5892–5899. 2005.
View Article : Google Scholar
|
|
16
|
Moore MJ, Goldstein D, Hamm J, et al:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: a phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar
|
|
17
|
Gemma A: Drug-induced interstitial lung
diseases associated with molecular-targeted anticancer agents. J
Nippon Med Sch. 76:4–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hotta K, Kiura K, Takigawa N, et al:
Comparison of the incidence and pattern of interstitial lung
disease during erlotinib and gefitinib treatment in Japanese
Patients with non-small cell lung cancer: the Okayama Lung Cancer
Study Group experience. J Thorac Oncol. 5:179–184. 2010. View Article : Google Scholar
|
|
19
|
Cohen MH, Williams GA, Sridhara R, et al:
United States Food and Drug Administration Drug Approval summary:
Gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res. 10:1212–1218.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ando M, Okamoto I, Yamamoto N, et al:
Predictive factors for interstitial lung disease, antitumor
response, and survival in non-small-cell lung cancer patients
treated with gefitinib. J Clin Oncol. 24:2549–2556. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hotta K, Kiura K, Tabata M, et al:
Interstitial lung disease in Japanese patients with non-small cell
lung cancer receiving gefitinib: an analysis of risk factors and
treatment outcomes in Okayama Lung Cancer Study Group. Cancer J.
11:417–424. 2005. View Article : Google Scholar
|
|
22
|
Takano T, Ohe Y, Kusumoto M, et al: Risk
factors for interstitial lung disease and predictive factors for
tumor response in patients with advanced non-small cell lung cancer
treated with gefitinib. Lung Cancer. 45:93–104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Danson S, Blackhall F, Hulse P and Ranson
M: Interstitial lung disease in lung cancer: separating disease
progression from treatment effects. Drug Saf. 28:103–113. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suzuki M, Asahina H, Konishi J, Yamazaki K
and Nishimura M: Recurrent gefitinib-induced interstitial lung
disease. Intern Med. 47:533–536. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Buck E, Eyzaguirre A, Brown E, et al:
Rapamycin synergizes with the epidermal growth factor receptor
inhibitor erlotinib in non-small-cell lung, pancreatic, colon, and
breast tumors. Mol Cancer Ther. 5:2676–2684. 2006. View Article : Google Scholar
|
|
26
|
Costa LJ, Gemmill RM and Drabkin HA:
Upstream signaling inhibition enhances rapamycin effect on growth
of kidney cancer cells. Urology. 69:596–602. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reardon DA, Desjardins A, Vredenburgh JJ,
et al: Phase 2 trial of erlotinib plus sirolimus in adults with
recurrent glioblastoma. J Neurooncol. 96:219–230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Flaig TW, Costa LJ, Gustafson DL, et al:
Safety and efficacy of the combination of erlotinib and sirolimus
for the treatment of metastatic renal cell carcinoma after failure
of sunitinib or sorafenib. Br J Cancer. 103:796–801. 2010.
View Article : Google Scholar
|
|
29
|
Kris MG, Riely GJ, Azzoli CG, et al:
Combined inhibition of mTOR and EGFR with everolimus (RAD001) and
gefitinib in patients with non-small cell lung cancer who have
smoked cigarettes: A phase II trial. J Clin Oncol. 25(Suppl 18):
403S2007.
|
|
30
|
Weir MR, Diekmann F, Flechner SM, et al:
mTOR inhibition: the learning curve in kidney transplantation.
Transpl Int. 23:447–460. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ajithkumar TV, Parkinson CA, Butler A and
Hatcher HM: Management of solid tumours in organ-transplant
recipients. Lancet Oncol. 8:921–932. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tan F, Shen X, Wang D, et al: Icotinib
(BPI-2009H), a novel EGFR tyrosine kinase inhibitor, displays
potent efficacy in preclinical studies. Lung Cancer. 76:177–182.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang GC, Lin JY, Wang Z, et al: Epidermal
growth factor receptor double activating mutations involving both
exons 19 and 21 exist in Chinese non-small cell lung cancer
patients. Clin Oncol (R Coll Radiol). 19:499–506. 2007. View Article : Google Scholar
|
|
34
|
Paez JG, Janne PA, Lee JC, et al: EGFR
mutations in lung cancer: correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|