Introduction
Gastric carcinoma is the fourth most common cancer,
with 1 million new cases per year, and the second leading cause of
cancer-related mortality worldwide (1). A diagnosis often occurs in the
advanced stages and thus the prognosis is usually poor. Although
new cytotoxic therapies are being tested (2–4), the
median overall survival (OS) is ~10 months. Encouraging results
from targeted therapies in other cancers have led to an interest in
such therapies and the identification of biomarkers in gastric
cancer. To date, it has been shown that targeted therapies are
useful in gastric cancer in the form of treatment using the
anti-HER2 antibody, trastuzumab, which is used to treat patients
with HER2 overexpression. HER2 is a member of the epidermal growth
factor receptor (EGFR) family (5).
EGFR is a member of the tyrosine kinase receptor family, which
consists of four structurally similar, but functionally varied
receptors, including erbB1 (HER1/EGFR), erbB2 (HER2/neu), erbB3
(HER3) and erbB4 (HER4). All these transmembrane receptors contain
intrinsic kinase activities and are activated by modified tyrosine
residues. It is believed that the aberrant activation of the
signaling pathway contributes to tumorigenic events, including
increased cellular proliferation, prevention of apoptosis, tumor
cell invasion and metastasis (6).
In numerous studies, it has been shown that the EGFR status is an
independent prognostic factor in various tumor types (7). The present study aimed to evaluate the
prognostic significance of EGFR expression in patients with
advanced gastric cancer.
Patients and methods
Patients
A retrospective cohort study was conducted in
Istanbul Bilim University, (Istanbul, Turkey) involving 40 patients
with gastric adenocarcinoma who were diagnosed using a histological
stomach tissue sample examination by the Department of Pathology.
The patients were followed by the Medical Oncology Clinic of
Istanbul Bilim University between 2008 and 2011. A total of eight
patients were excluded from the study, one due to a second primary
tumor, four due to the presence of stage II disease and three due
to their pathology blocks being unavailable. All patients that were
included in the study already had metastatic disease or developed
it during the follow-up period. At the time of the present
analysis, 18 of the patients (60%) had succumbed to their diseases.
This study was approved by the ethics committee of Istanbul Bilim
University. Informed consent was obtained from the patients.
Pathology and immunohistochemistry
(IHC)
The formalin-fixed paraffin-embedded tumor samples
were evaluated for EGFR protein expression using IHC. Sections
(2-μm thick) were cut from the paraffin embedded tissue blocks,
fixed with 10% formaldehyde and fixed on marked slides. The slides
were maintained in an incubator at 56°C overnight and
deparaffinized by being soaked twice in Xylene for 15 min and in
96% alcohol three times for 5 min. The slides were finally
rehydrated with distilled water. Distilled water was added to
Decloaking Chamber Plus (Biocare Medical, Concord, CA, USA); a
system with a cooling fan and high pressure for a more rapid and
homogenous antigen retrieval. The slides were placed in a 10%
citrate buffer (pH, 6.0) solution. The slides were then placed in
the chamber by washing in an antigen recovery solution for 30 sec
at 125°C and 10 sec at 90°C and were allowed to cool to room
temperature for 10–20 min. The edges of the slides were dried and
the tissue boundaries were drawn using a Pap pen (Abcam, Cambridge,
MA, USA) subsequent to being washed in Tris-buffered saline plus
Tween 20 (TBST). Blockage of the endogenous peroxidase activity was
performed by dripping 3% hydrogen peroxide
(H2O2) on each tissue sample and then placing
them in a moist environment for 10 min. The samples were then
soaked in phosphate-buffered saline (PBS) for 5 min, subsequent to
being washed in distilled water. EGFR antibodies (1:60, Clone:
EGFR.25, Produnt code: NCL-EGFR-384; Leica-Novocastra, Solms,
Germany) were added to the slides, which were shaken in order to
remove excess liquid from the slides, placed into an incubation
vessel and stored for 2 h. The slides were then placed in staining
jars that contained PBS. The slides were incubated with the
secondary antibody (multispecies ultra streptavidine detection
system, HRP, Zymed, MA, USA) and streptavidine-biotin complex
(Zymed) was administered on the slides, which were incubated again
for 20 min. Streptavidin was dripped onto the slides following
PBS4, used to maintain a constant pH, and allowed to dry for 20
min. A solution was prepared using 1 ml distilled water, one drop
AEC buffer, two drops AEC chromogen and one drop concentrated
H2O2, which was then added to the slides. The
opposite coloring was performed using Mayer hematoxylin in a 2-min
application. The slides were kept in a water-based closing medium
(aquesmount, Scytec, Logan, UT, USA) following the completion of
the empurpling process in tap water.
All the slides that were stained using hematoxylin
and eosin (HE) and IHC were examined by two pathologists who were
experienced in gastrointestinal cancers. In all the cases, the
antibodies were evaluated separately in the invasive tumor and
surrounding gastric tissue slides. Cytoplasmic and membranous
staining was obtained for EGFR. Placental tissue was used as a
control for EGFR. A scoring system was used, as the staining
intensities were variable. The result that was obtained
semi-quantitatively by multiplying the percentage of the
positively-stained cells and the staining intensity was recorded as
the immunoreactive score (IRS). The staining intensities were
graded as follows: 0, negative; 1, weakly positive; 2,
medium-intensity positive; and 3, strongly positive. The percentage
of the positively-stained cells was graded as: 0, <5%; 1, 5–25%;
2, 26–50%; 3, 51–75%; and 4, >75%. On the basis of the values
that were obtained, IRS values of 0–3 were recorded as 0, 4–6 as
1+, 7–9 as 2+ and 10–12 as 3+. IRS results of 0 or 1+ were
evaluated as positive and scores of 2 or 3+ were considered to be
negative.
Statistical analysis
OS was calculated as the time between the beginning
of chemotherapy and mortality or the last assessment.
Progression-free survival (PFS) was calculated as the time from the
beginning of the treatment to progression of the disease.
Disease-free survival was calculated for patients without
metastases initially and was determined as the elapsed time from
the first date of the gastric cancer diagnosis until the date that
the disease became metastatic. For the statistical analysis, PASW
Statistics for Windows Version 18 (SPSS, Inc., Chicago, IL, USA)
software was used. Kaplan-Meier survival curves were drawn and a
log-rank test was performed to obtain and compare the survival
durations. A χ2 analysis was used for comparing the
rates. P<0.05 was considered to indicate a statistically
significant difference. If there were two comparisons with an
expected value of <5, Fisher’s exact test was used.
Results
The patients were aged between 34 and 85 years, with
a median age of 58.5±15.3 years. Of the total patients, 67% (n=20)
were male and 33% (n=10) were female. The median follow-up period
from the occurrence of metastasis was 12.1±9.2 months (range,
2–25.3 months; 95% confidence interval, 10–17 months). The median
survival duration from metastasis was 12.7±7.3 months (range,
2–25.3 months; 95% confidence interval, 9.5–16.8 months).
When the stages at the time of the application were
examined, it was noted that half of the patients (n=15) were
initially metastatic (16.7%; n=5) with stage IIIC diseases, 13.2%
(n=4) exhibited stage IIIB disease and 6.7% (n=2) of patients were
observed to have stage IIA, IIB and IIIA diseases. There were no
patients with stage I disease in the present study (Table I).
 | Table IDistribution of the stage status at
diagnosis. |
Table I
Distribution of the stage status at
diagnosis.
| Stage | n (%) |
|---|
| IIA | 2 (6.7) |
| IIB | 2 (6.7) |
| IIIA | 2 (6.7) |
| IIIB | 4 (13.2) |
| IIIC | 5 (16.7) |
| IV | 15 (50.0) |
Tumor localization
The tumor was localized to the esophageal-gastric
junction in 10% (n=3) of the patients, at the cardia in 13.3%
(n=4), at the antrum in 23.3% (n=7), at the corpus in 23.3% (n=7)
and at the corpus and antrum in 20% (n=6). However, localization
was not determined in 10% (n=3).
Pathological parameters
The parameters of the tumor pathologies, including
tumor necrosis, blood vessel invasion, perineural invasion and
lymphatic invasion, are presented in Table II.
 | Table IIPathological parameters. |
Table II
Pathological parameters.
| Parameter | Yes, n (%) | No, n (%) | Undetermined, n
(%) |
|---|
| Tumor necrosis | 6 (20.0) | 6 (20.0) | 18 (60.0) |
| Blood vessel
invasion | 9 (30.0) | 8 (26.7) | 13 (43.3) |
| Perineural
invasion | 13 (43.3) | 4 (13.3) | 13 (43.3) |
| Lymphatic
invasion | 13 (43.3) | 3 (10.0) | 14 (46.6) |
Distribution of metastases
Liver metastases were present in 36.7% (n=11) of the
patients. Lung metastases were present in 13.3% (n=4). There were
bone metastases present in 16.6% (n=5) and ovarian metastases in
10% (n=3) of the cases. The percentage of patients with only local
recurrence was 6.6% (n=2), and brain metastases were also observed
in 6.6% (n=2).
Chemotherapy
While 13.3% of the patients (n=4) were administered
only supportive care (BSC), 60% (n=18) were administered one-line,
13.3% (n=4) two-line and 13.3% (n=4) three-line chemotherapy.
Frequently, docetaxel, cisplatin and fluorouracil (FU; DCF)
treatment was performed in 76.7% (n=23) as the first-line
chemotherapy. The other first-line treatments included epirubisin,
cisplatin and FU (ECF), cisplatin and capecitabine (CX), docetaxel,
cisplatin and capecitabine (DCX), docetaxel and cisplatin (DC) and
tegafur uracil (UFT). While 30% (n=9) of the patients were
administered second-line therapy, the treatments had been performed
using FU, irınotekan and leucovorin (FOLFIRI) in 33.3% (n=3) and
with capecitabine in 22.2% (n=2). Other second-line therapies were
X, CF, CX, DCF and capecitabine and irinotecan (XELIRI). While the
proportion of patients who were administered third-line therapy was
13.3%, these treatments had been chosen as ECF or FOLFIRI.
EGFR status
For EGFR, the patients were grouped into those with
no staining, 36.6% (n=11), those with cytoplasmic staining, 46.7%
(n=14), and those with membranous staining, 16.7% (n=5). For the
purposes of the analysis, the patients with cytoplasmic and
membranous staining were accepted as EGFR+ and the
patients with no staining were considered EGFR−. In the
present study, the number of EGFR+ patients was recorded
as 19 (63.3%).
Survival analysis
No differences were observed in OS between the
stages of the disease. No significant differences were observed
between the survival duration following recurrence in the patients
with and without metastasis, between those who had undergone
surgery and those who had not or between those who were
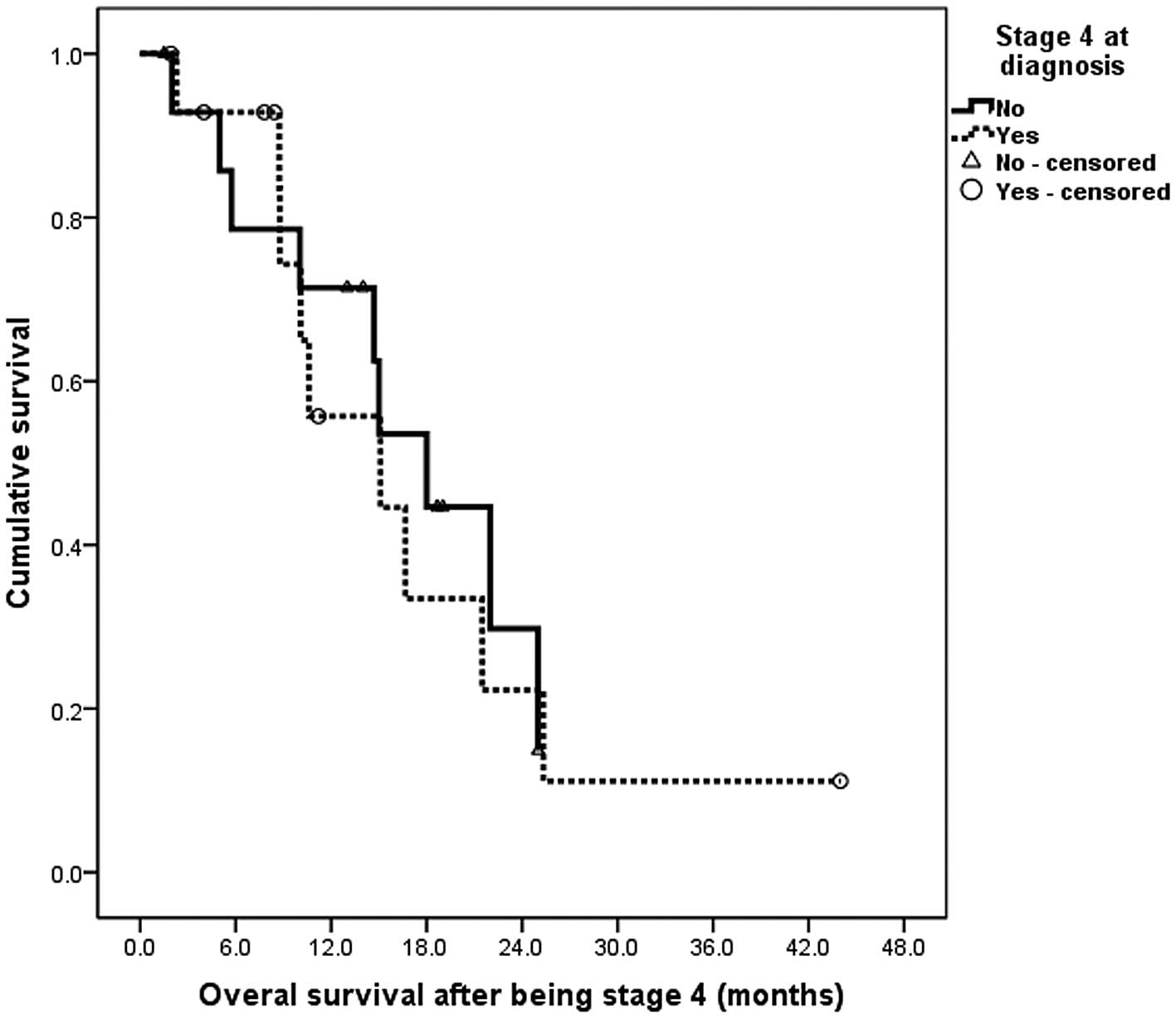
administered adjuvant therapy and those who were not. The median
survival time following metastasis in the patients without
metastasis initially was 18 months (95% confidence interval,
12.7–23.2 months). In the patients with an initial metastatic
diagnosis, the median survival time was 15.1 months (95% confidence
interval, 2.8–27.4 months) and the difference between them was not
statistically significant. (log-rank, P=0.841; Fig. 1).
By grouping the patients as stage IV and others,
their associations were assessed using the EGFR status (Table III).
 | Table IIIEGFR status association with
stage. |
Table III
EGFR status association with
stage.
| Stage | |
|---|
|
| |
|---|
| Status | Others | Stage 4 | Total |
|---|
| EGFR− | 3 | 8 | 11 |
| EGFR+ | 12 | 7 | 19 |
| Total | 15 | 15 | 30 |
The EGFR+ patients tended to be diagnosed
at an earlier stage than the EGFR− patients. This was
not statistically significant (P=0.058). Also, in the
EGFR+ patients, a significant correlation was identified
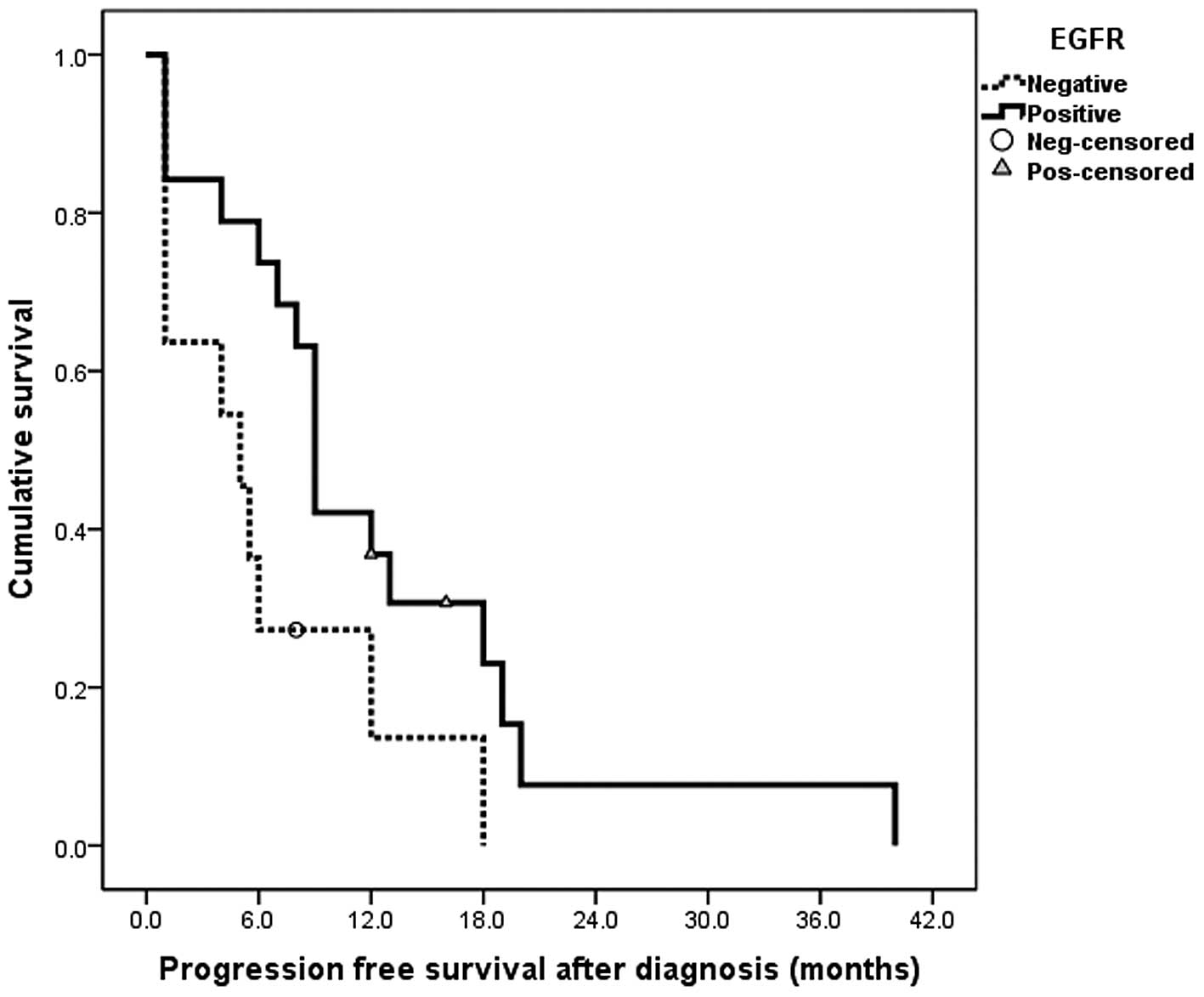
in OS (P=0.011) and PFS (P=0.039). Thus, the EGFR+
patients were diagnosed earlier and had an improved survival
compared with those who were EGFR−. The median duration
of PFS was five months (95% confidence interval, 0.2–9.9 months) in
the EGFR− patients and nine months (95% confidence
interval, 7.9–10.0 months) in the EGFR+ patients. The
difference between the two was statistically significant (log-rank,
P=0.039; Fig. 2).
The median survival time following metastasis was 18
months in the EGFR+ patients and 10.6 months in the
EGFR− patients. However, this difference was not
statistically significant (log rank, P=0.110; Fig. 3).
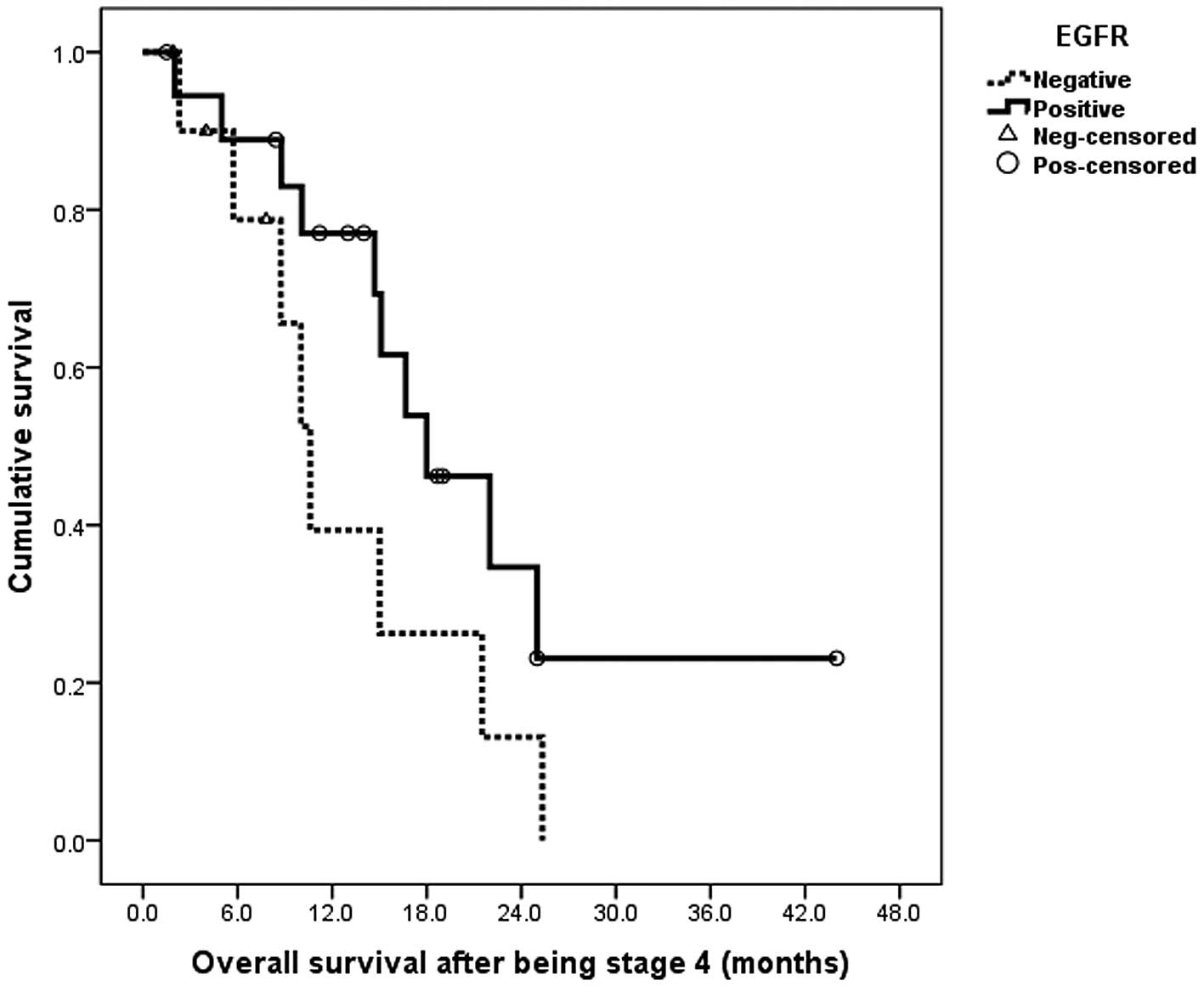
The analyses were repeated using a calculation of OS
from the moment of diagnosis by including the patients that were
without metastases at the time of diagnosis, regardless of the
treatment of the patients. Thus, the prognosis was measured based
on whether the patient was administered adjuvant treatment or not.
The median survival from the time of diagnosis was 34.7 months (95%
confidence interval, 19.5–49.9 months) in the EGFR+
patients and 10.6 months (95% confidence interval, 1.76–19.43
months) in the EGFR− patients. The difference was
statistically significant (P=0.01; Fig.
4).
Chemotherapy response prediction
Generally, when the chemotherapy response (complete
response, partial response and stable disease) and the EGFR status
were evaluated, there was no significant correlation (Table IV).
 | Table IVAssociation between chemotherapy
response and EGFR. |
Table IV
Association between chemotherapy
response and EGFR.
| EGFR | |
|---|
|
| |
|---|
| Negative | Positive | Total |
|---|
| No response | 4 | 3 | 7 |
| Response | 7 | 16 | 23 |
| Total | 11 | 19 | 30 |
Discussion
In the present study, EGFR expression was determined
to be 63.4%, of which 46.7% was cytoplasmic and 16.7% membranous.
This rate has been reported to range from 31–74% in the literature
(8,9). The main reason for this wide
distribution may be the lack of a standard for the evaluation of
EGFR. Although the cytoplasmic staining characteristic of EGFR has
been examined in certain studies, the assessments were made
according to its membranous staining status in the majority of the
studies. There are fewer studies that have evaluated membranous and
cytoplasmic staining together, as in the present study (9). Furthermore, technical reasons,
including the differences in the kits that are used, may affect the
results. The analyses were repeated according to the staining
proportions in the present study, as the literature on this subject
is unclear. However, the rates obtained are presented here.
In the present study, the proportion of patients
with stage IV disease in EGFR negative patients were higher but
statistically insignificant compared with the EGFR positive
patients at the time of diagnosis (P=0.058). Also, statistically
significant results were obtained for OS and PFS. The median
survival times from the time of diagnosis were 34.7 months in
patients with EGFR expression regardless of their stage and 10.6
months in patients without EGFR expression (P<0.01). The
association between EGFR expression and prognosis in the literature
is conflicting. Although it is associated with a poor prognosis in
numerous studies (10–16), there are also studies that have
shown EGFR expression to be associated with a good prognosis
(17–19). The survival rate of 89 patients with
post-operative gastric adenocarcinoma with membranous EGFR staining
was shown to be worse than for others by Gamboa-Dominquez et
al in 2004 (12).
EGFR expression using IHC and gene amplification
using fluorescence in situ hybridization (FISH) were
examined in patients with gastric cancer without distant metastases
who had undergone a D2 dissection and had been administered
adjuvant cisplatin and 5FU by Kim et al in 2009 (17). EGFR expression was observed to be
positive in 80.7% of patients with IHC, but this rate was
identified to be only 14% with FISH. There was no correlation
between the expression of EGFR and FISH positivity. Also, in this
study, as in the present study, positive correlation was not
detected between the clinicopathological features of the patients,
including EGFR expression and age, gender, stage or performance
status. In the multivariate analysis of the present study, it was
shown that low EGFR expression was an independent biomarker
predictive of a shorter OS and relapse.
Furthermore, the prognosis of Chinese patients with
familial gastric adenocarcinoma was investigated in a study by Ye
et al that was published in 2011 (18). In this study, although EGFR
expression in patients with gastric carcinoma was 49% (40/81), in
sporadic patients it was 30.9% (25/81). EGFR rates were identified
to be significantly higher in familial patients (P=0.011). Also in
subgroup analyses, the five-year survival rates were lower in the
familial patients with no EGFR expression (45 vs. 61%;
P=0.023).
In a study by Matsubara et al in 2008
(15), EGFR mRNA gene expression
levels in patients with advanced gastric cancer were examined. In
this study, it was determined that the median time until
progression for those who showed a higher level of EGFR expression
was longer when evaluating patients who were administered S1 as the
first-line treatment. Although the median time until progression
was 2.8 months in patients who showed less EGFR expression, it was
5.3 months in patients who showed more EGFR expression. However,
significant results were not detected in the patients who were
administered a cisplatin-based regimen for first-line treatment. In
the present study, the EGFR status was evaluated using IHC and the
prognosis of the patients with positive EGFR expression was good
regardless of their treatments.
Recently, the evaluation of the prognostic
significance of EGFR expression in gastric cancer using membranous
staining has also been investigated by Atmaca et al(19). EGFR positivity was not identified to
be a prognostic factor in this study, which evaluated 457 patients,
or in another study by Song et al(20).
Trastuzumab, which is a targeted therapy that is
added to chemotherapy in patients with advanced gastric cancer, has
been shown to be useful for treating HER2+ patients by
the TOGA study (5). In this study,
longer survival times were observed than were expected in the
patient group, which was administered only chemotherapy. This may
have been due to HER2 positivity. EGFR, which is a member of the
same family of receptor tyrosine kinases, may similarly be a good
prognostic marker in advanced gastric cancer. The prognostic and
predictive role of EGFR expression remains controversial and
contrasting opinions have been proposed in the literature.
Differences in the studies may be due to the lack of a standard
evaluation of EGFR expression in patients with gastric cancer.
Although the impact of the present study was low due
to a small sample size, a positive correlation was identified
between EGFR expression and survival. Determining the prognostic
parameters of new treatment strategies is significant for a gastric
carcinoma patient group that has a relatively low five-year
survival rate. In this disease, the use of targeted molecules in
the appropriate patients may increase the survival rates. In colon
and lung malignancies, receptor-dependent proteins, including
tyrosine kinases, and RAS status are extremely important, rather
than receptor expression. For these reasons, identifying the
association between the signal transduction pathways during the
development, progression and metastasis of gastric cancer may lead
to the development of new therapeutic strategies that may be used
against these targets.
References
|
1
|
Jemal A, Siegel R, Ward E, Murray T, Xu J,
Smigal C and Thun MJ: Cancer statistics, 2006. CA Cancer J Clin.
56:106–130. 2006. View Article : Google Scholar
|
|
2
|
Van Cutsem E, Moiseyenko VM, Tjulandin S,
et al: Phase III study of docetaxel and cisplatin plus fluorouracil
compared with cisplatin and fluorouracil as first-line therapy for
advanced gastric cancer: a report of the V325 Study Group. J Clin
Oncol. 24:4991–4997. 2006.
|
|
3
|
Al-Batran SE, Hartmann JT, Hofheinz R, et
al: Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel
(FLOT) for patients with metastatic adenocarcinoma of the stomach
or esophagogastric junction: a phase II trial of the
Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol.
19:1882–1887. 2008. View Article : Google Scholar
|
|
4
|
Cunningham D, Okines AF and Ashley S:
Capecitabine and oxaliplatin for advanced esophagogastric cancer. N
Engl J Med. 362:858–859. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bang YJ, Van Cutsem E, Feyereislova A, et
al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): a phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar
|
|
6
|
Voldborg BR, Damstrup L, Spang-Thomsen M
and Poulsen HS: Epidermal growth factor receptor (EGFR) and EGFR
mutations, function and possible role in clinical trials. Ann
Oncol. 8:1197–1206. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nicholsan RI, Gee JM and Harper ME: EGFR
and cancer prognosis. Eur J Cancer. 37(Suppl 4): S9–S15. 2001.
View Article : Google Scholar
|
|
8
|
Yonemura Y, Sugiyama K, Fujimara T, Kamata
T, Fushida S, Yamaguchi A, et al: Epidermal growth factor receptor
status and S-phase fractions in gastric carcinoma. Oncology.
46:158–161. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jang WI, Yang WI, Lee CI, Kim HS, Song KS,
Cho MY, Park JK and Shim YH: Immunohistochemical detection of p53
protein, c-erbB-2 protein, epidermal growth factor receptor protein
and proliferating cell nuclear antigen in gastric carcinoma. J
Korean Med Sci. 8:293–304. 1993. View Article : Google Scholar
|
|
10
|
García I, Vizoso F, Martín A, Sanz L,
Abdel-Lah O, Raigoso P and García-Muñiz JL: Clinical significance
of the epidermal growth factor receptor and HER2 receptor in
resectable gastric cancer. Ann Surg Oncol. 10:234–241.
2003.PubMed/NCBI
|
|
11
|
Galizia G, Lieto E, Orditura M, Castellano
P, Mura AL, Imperatore V, Pinto M, Zamboli A, De Vita F and
Ferraraccio F: Epidermal growth factor receptor (EGFR) expression
is associated with a worse prognosis in gastric cancer patients
undergoing curative surgery. World J Surg. 31:1458–1468. 2007.
View Article : Google Scholar
|
|
12
|
Gamboa-Dominguez A, Dominguez-Fonseca C,
Quintanilla-Martinez L, Reyes-Gutierrez E, Green D, Angeles-Angeles
A, Busch R, Hermannstädter C, Nährig J, Becker KF, Becker I, Höfler
H, Fend F and Luber B: Epidermal growth factor receptor expression
correlates with poor survival in gastric adenocarcinoma from
Mexican patients: a multivariate analysis using a standardized
immunohistochemical detection system. Mod Pathol. 17:579–587. 2004.
View Article : Google Scholar
|
|
13
|
Lieto E, Ferraraccio F, Orditura M,
Castellano P, Mura AL, Pinto M, Zamboli A, De Vita F and Galizia G:
Expression of vascular endothelial growth factor (VEGF) and
epidermal growth factor receptor (EGFR) is an independent
prognostic indicator of worse outcome in gastric cancer patients.
Ann Surg Oncol. 15:69–79. 2008. View Article : Google Scholar
|
|
14
|
Kim MA, Lee HS, Lee HE, Jeon YK, Yang HK
and Kim WH: EGFR in gastric carcinomas: prognostic significance of
protein overexpression and high gene copy number. Histopathology.
52:738–746. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsubara J, Yamada Y, Nakajima TE, Kato
K, Hamaguchi T, Shirao K, Shimada Y and Shimoda T: Clinical
significance of insulin-like growth factor type 1 receptor and
epidermal growth factor receptor in patients with advanced gastric
cancer. Oncology. 74:76–83. 2008. View Article : Google Scholar
|
|
16
|
Terashima M, Kitada K, Ochiai A, Ichikawa
W, Kurahashi I, Sakuramoto S, Sano T, Imamura H and Sasako M;
ACTS-GC Group. Impact of expression of human epidermal growth
factor receptors EGFR and ERBB2 on survival in patients with stage
II/III gastric cancer. Clin Cancer Res. 18:5992–6000. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim JS, Kim MA, Kim TM, Lee SH, Kim DW, Im
SA, Kim TY, Kim WH, Yang HK, Heo DS, Bang YJ, Lee KU, Choe KJ and
Kim NK: Biomarker analysis in stage III-IV (M0) gastric cancer
patients who received curative surgery followed by adjuvant
5-fluorouracil and cisplatin chemotherapy: epidermal growth factor
receptor (EGFR) associated with favourable survival. Br J Cancer.
100:732–738. 2009. View Article : Google Scholar
|
|
18
|
Ye YW, Dong RZ, Zhou Y, Du CY, Wang CM, Fu
H and Shi YQ: Prognostic analysis of familial gastric cancer in
Chinese population. J Surg Oncol. 104:76–82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Atmaca A, Werner D, Pauligk C, Steinmetz
K, Wirtz R, Altmannsberger HM, Jäger E and Al-Batran SE: The
prognostic impact of epidermal growth factor receptor in patients
with metastatic gastric cancer. BMC Cancer. 12:5242012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song HS, Do YR, Kim IH, Sohn SS and Kwon
KY: Prognostic significance of immunohistochemical expression of
EGFR and C-erbB-2 oncoprotein in curatively resected gastric
cancer. Cancer Res Treat. 36:240–245. 2004. View Article : Google Scholar : PubMed/NCBI
|