Introduction
There are five types of germ cell tumours according
to clinical presentation, pathology and cytogenetics. Type I
tumours (teratomas and yolk sac tumours) are more frequent in
extragonadal sites than in the gonads; whereas type II tumours
(seminomas and non-seminomas) occur mainly in the gonads (1) Primary urinary bladder germ cell
tumours are exceedingly rare. The current report presents a case of
primary yolk sac tumour of the urinary bladder in a 31-year-old
female ex-ketamine abuser. The diagnosis of a yolk sac tumour at
this rare site can be difficult and biopsy alone is not reliable.
No previous studies have reported a correlation between chronic
ketamine abuse and urinary bladder malignancy development. Informed
consent was obtained from the patient.
Case report
In 2007, a 26-year-old female presented to the
Department of Urology of the Tuen Mun Hospital (Hong Kong, China)
with bilateral hydronephrosis. Since 2000, the patient had been
consuming 8–10 ketamine tablets daily from illicit sources.
Cystoscopy revealed cystitis and a biopsy showed florid reactive
changes in the urinary bladder associated with erosion involving
the urothelium, which underwent extensive intestinal metaplastic
changes. The patient defaulted follow-up examinations.
In January 2012, the patient presented again with
gross haematuria. Cystoscopy identified a 4-cm whitish mass at the
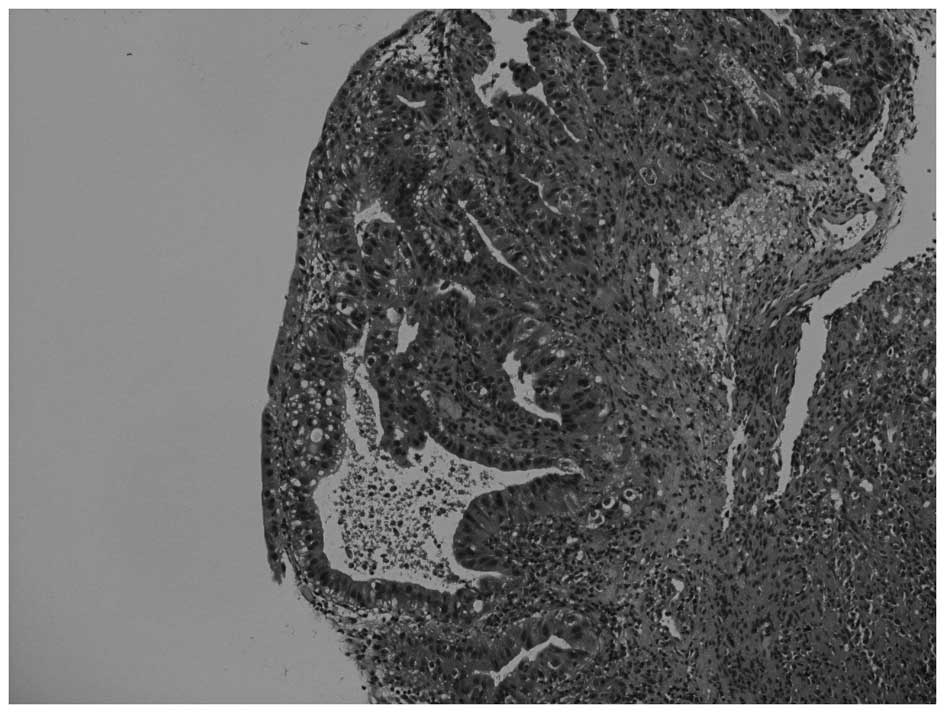
dome of the urinary bladder, with pathological features indicative
of adenocarcinoma (Fig. 1). A
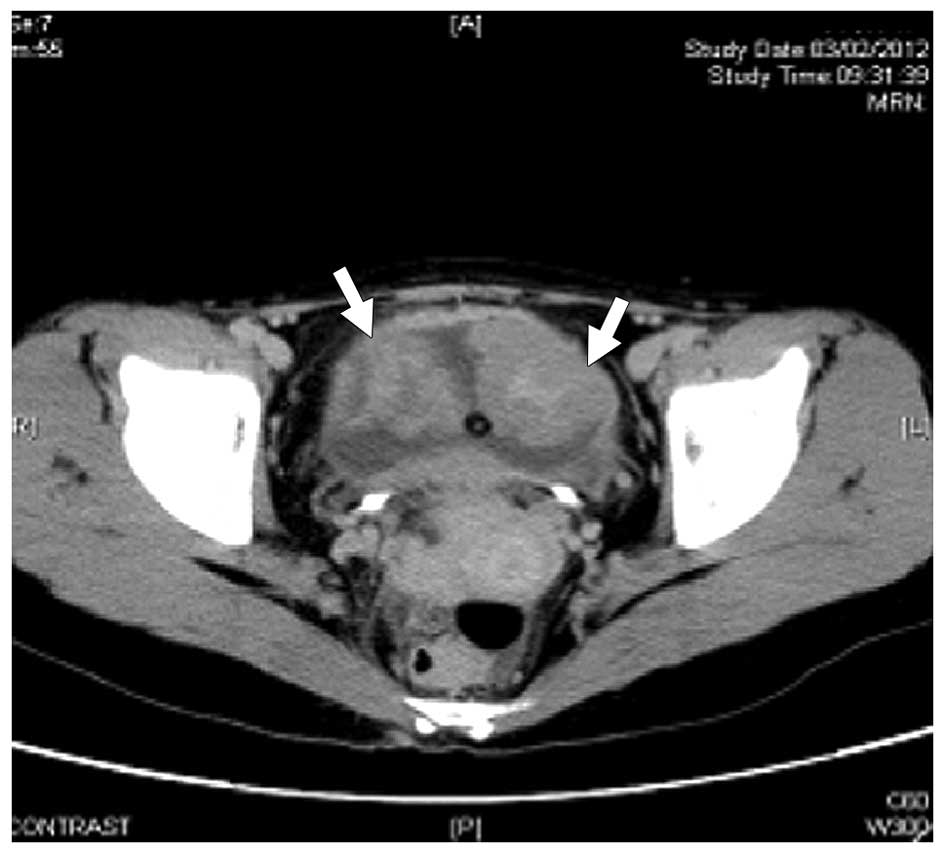
computed tomography (CT) scan of the abdomen was performed on
February 3, 2012, which demonstrated masses within the urinary
bladder (Fig. 2). A transurethral
resection of the bladder tumours was subsequently performed on
January 31, 2012. Pathological examination showed a
muscle-invasive, poorly-differentiated carcinoma with a clear cell
component. The patient underwent a radical cystectomy and T-pouch
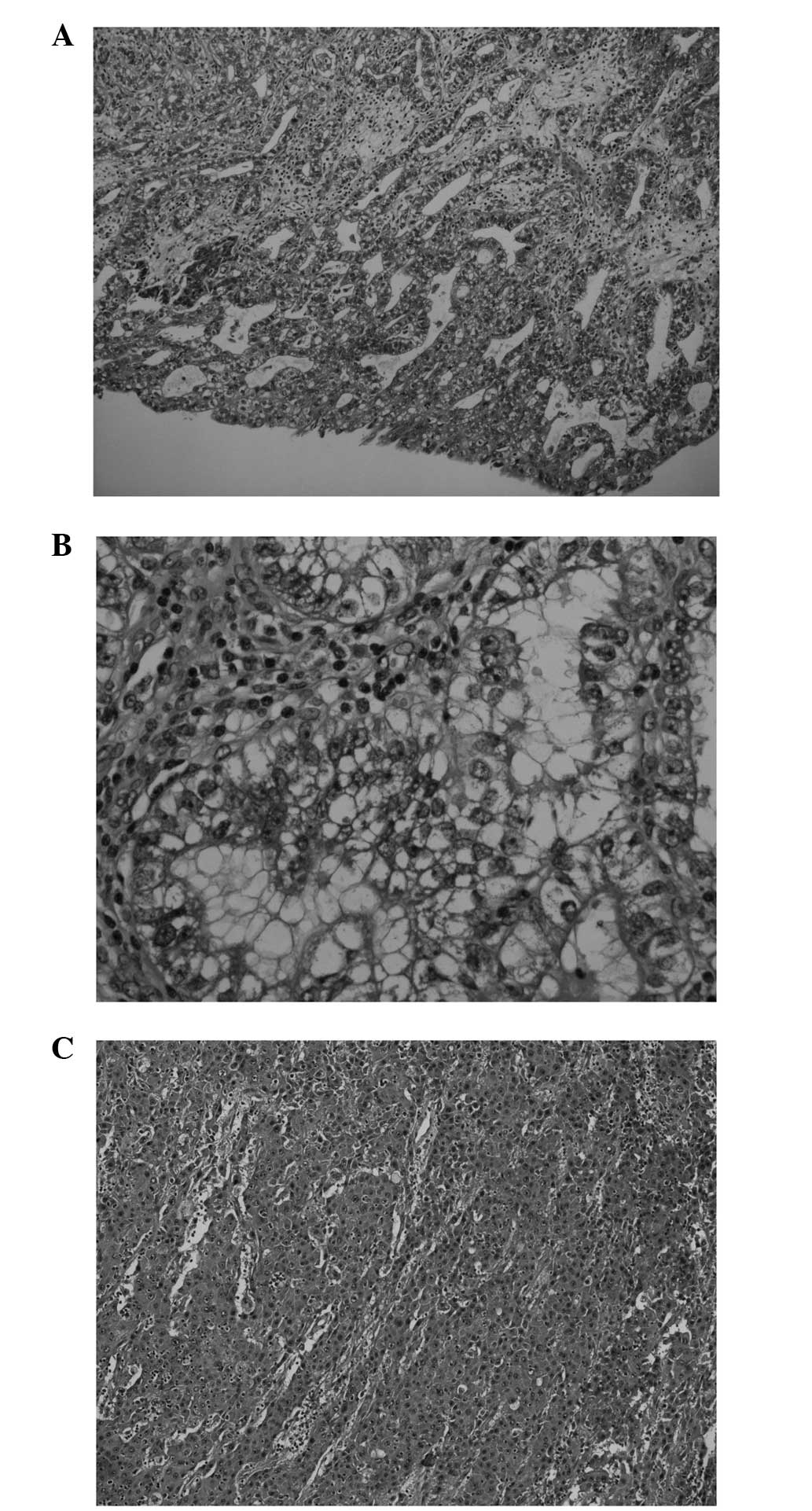
orthotopic substitution cystoplasty on February 21, 2012. Two
similar 5-cm tumours were present, with various histological
morphologies in different areas, featuring reticular, glandular,
papillary, microcystic, solid and hepatoid patterns (Fig. 3). The tumour cells showed
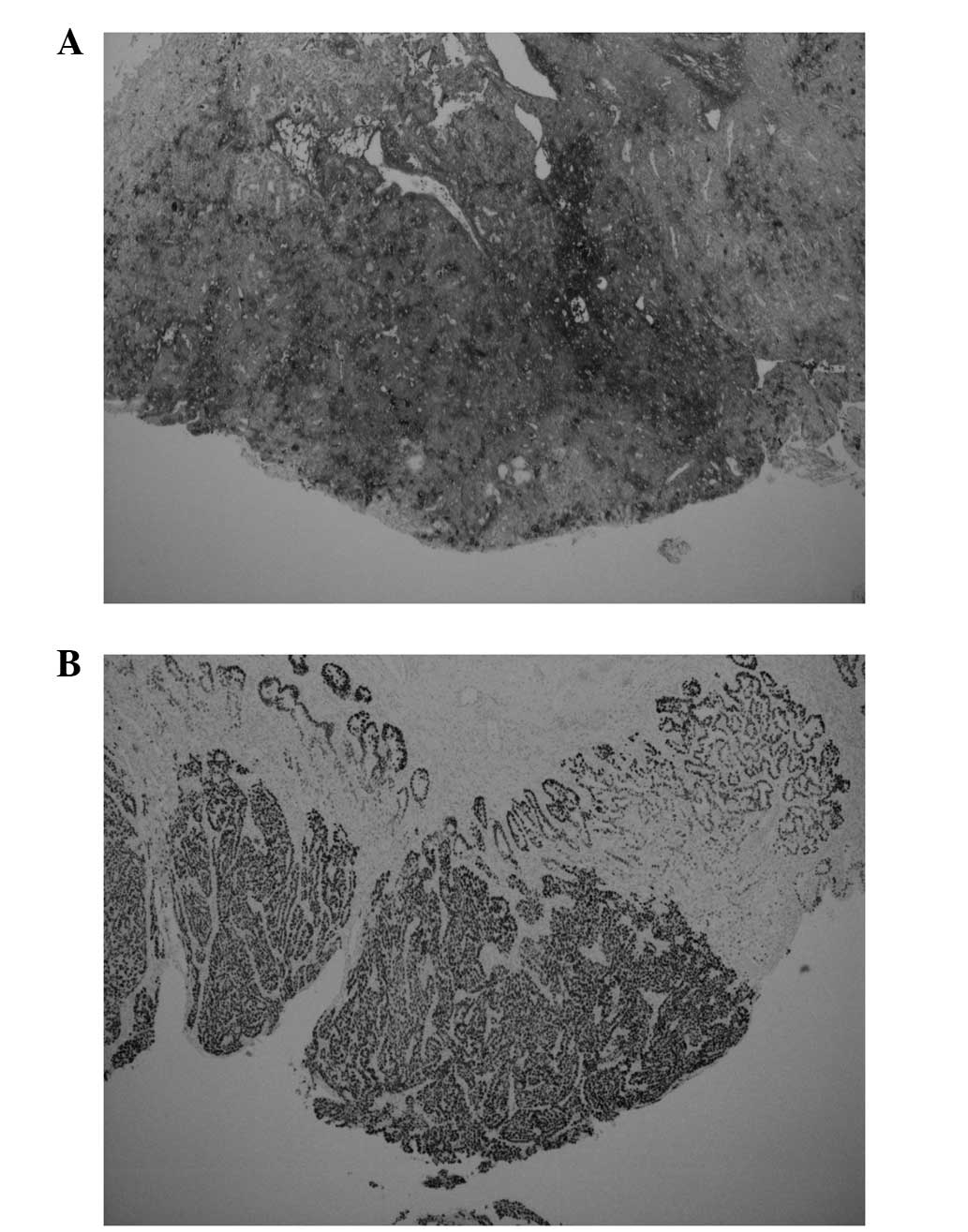
immunohistochemical reactivity for antibodies against MNF116,
α-fetoprotein (αFP) and Sal-like protein 4 (Fig. 4), but not against EMA, HCG,
placental alkaline phosphatase, CD30 and octamer-binding
transcription factor 3/4. The final pathological diagnosis was of a
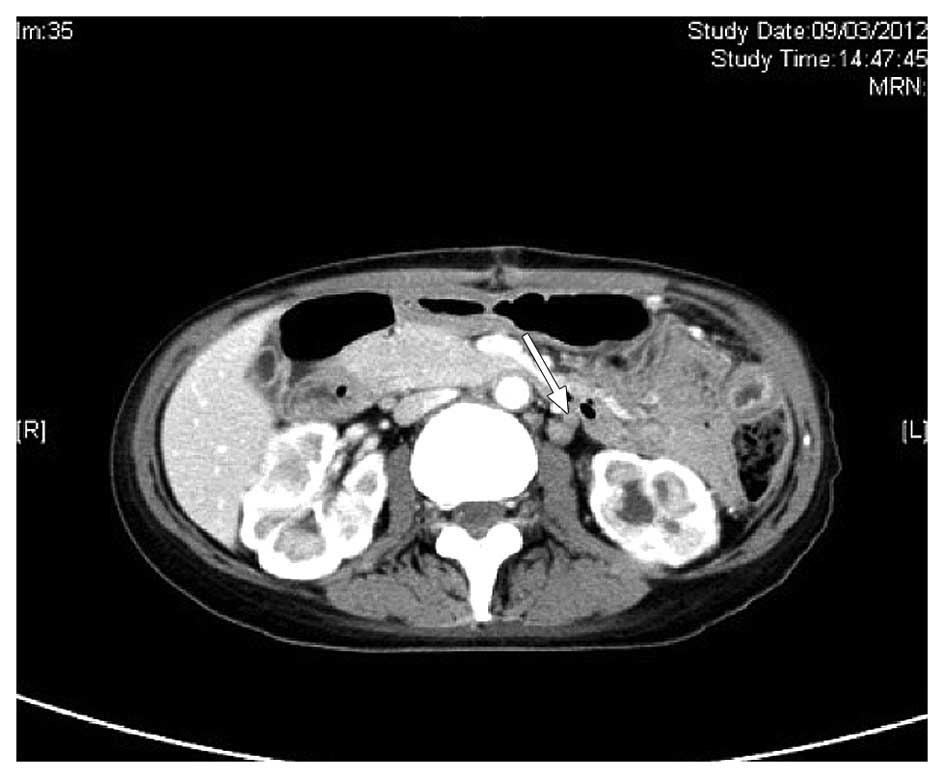
yolk sac tumour. A CT scan of the abdomen and pelvis on March 9,
2012, demonstrated several enlarged paraaortic lymph nodes of ≤1.5
cm in size (Fig. 5). No sites of
suspicious ovarian primary or other disease involvement were
identified. An examination performed by a gynaecologist did not
reveal a primary gynaecological site of disease.
The serum αFP level was found to be raised (1,028
ng/ml) on March 13, 2012, but fell to 45.9 ng/ml on April 17, 2012,
prior to undergoing chemotherapy. In view of the impaired renal
function (48.9 ml/min creatinine clearance, according to the
Cockcroft-Gault formula), the patient was treated with the
combination chemotherapy JEB regimen (day 1, area under curve 5
mg/ml/min carboplatin, intravenously; days 1–5, 100
mg/m2 etoposide, intravenously; days 1, 8 and 15, 30 mg
bleomycin, intravenously; and the regimen was repeated every 21
days). Two cycles of chemotherapy were administered between April
and May 2012. The first cycle of chemotherapy was complicated by
grade 3 mucositis and neutropenic sepsis. Moreover, the second
cycle was complicated by appendicitis with intra-abdominal abscess
formation requiring laparotomy, intensive care and prolonged
post-operative management. It was then decided to terminate the
chemotherapy. The patient was put on close clinical surveillance,
including regular serum tumour marker analysis and CT scan
monitoring. The final CT scan, on July 23, 2012, showed no interval
change of the paraaortic lymph nodes. The patient’s nadir serum αFP
level following chemotherapy was 14.1 ng/ml on August 9, 2012.
Discussion
Primary germ cell tumours of the urinary bladder are
extremely rare. In the present review of the literature, <10
cases had been previously reported (2–7). To
the best of our knowledge, the current case is the first reported
primary yolk sac tumour of the urinary bladder in adulthood.
The hypotheses for the development of extragonadal
germ cell tumours include the following: i) Failure of the
primitive germ cells to complete the normal migration along the
urogonadal ridges; ii) germ cell tumours undergo reverse migration;
iii) germ cell tumours are the metastatic deposits from occult
gonadal primaries; and iv) germ cell tumours result from the germ
cells distributed to other organs physiologically for function
(1,8,9).
The accurate pathological diagnosis of germ cell
tumours and the distinction from non-germ cell tumours is critical,
as the majority of cases of germ cell tumours are potentially
curable, particularly in young patients. Diagnosing a yolk sac
tumour may be difficult since a yolk sac tumour may assume a
variety of architectural patterns, including microcystic, solid,
myxomatous, papillary, polyvesicular vitelline, alveolar,
glandular, hepatoid and intestinal (10,11).
These may explain the diagnoses of adenocarcinoma and invasive
carcinoma in the present patient’s previous pathological
examinations. The presence of the particularly characteristic
histological Schiller-Duval body, which consists of arrays of
neoplastic cells surrounding a central vessel in a glomeruloid
appearance, aided the diagnosis (12–14).
Beside classical histological features, immunohistochemical study
is essential for determining the correct diagnosis. This is based
on the relatively specific immunohistochemical profiles carried by
the various types of germ cell tumour (Table I) (12,14–18).
 | Table IImmunohistochemical study of germ cell
tumours. |
Table I
Immunohistochemical study of germ cell
tumours.
| Type of germ cell
tumour | Reactivity |
|---|
|
Seminoma/dysgerminoma | PLAP, c-kit and
Oct3/4 |
| Spermatocytic
seminoma | c-kit and PLAP |
| Embryonal
carcinoma | MNF116 (cytokeratin),
CD30, Oct3/4 and SALL4 |
| Yolk sac tumour | αFP and SALL4 |
| Choriocarcinoma | MNF116
(cytokeratin) |
The patient’s medical history in 2007 identified a
dysplastic process occurring in the urinary bladder. However, in
the present review of the literature, the correlation between
ketamine abuse and the formation of a urinary bladder malignancy
was shown to have not previously been documented. The chronic
recreational use of ketamine and its associated urinary system
issues has become a global issue (19–21).
The new medical entity ‘ketamine-induced ulcerative cystitis’
originated from a publication by Shahani et al(21) in 2007. Common symptoms include
frequent urination, urge incontinence and painful haematuria. The
method of production and composition of illicit ketamine is
unclear, and the chemicals and metabolites responsible for the
pathogenesis are not well known. Cystoscopic observations may
reveal features of cystitis and ulceration, characterised by
granulation tissue formation and fibrosis in the epithelium and
lamina propria (21). Although
there is eosinophilic infiltration, the overall pathology of
chemical cystitis is distinct from that of eosinophilic cystitis,
which has been reported to be associated with transitional cell
carcinoma (22).
Despite their rarity, extragonadal yolk sac tumours
mainly affect children and young females (9–11,13).
The mediastinum and retroperitoneum are the most common
extraovarian primary sites. Less common sites may include the
omentum, vagina and brain (10–12,17).
These tumours are highly aggressive, harbouring the tendency for
early lymphatic and haematological metastasis to distant sites
(11,17). Long-term survival rates,
specifically for extragonadal yolk sac tumours, are not well known.
The International Germ Cell Cancer Collaborative Group data,
determined the 5-year survival rate for mediastinal non-seminoma
(i.e. poor risk group) as 48% (23), and a large case series from the
German group of extragonadal germ cell tumours showed similar
survival rates (8,24,25).
Extragonadal germ cell tumours have been managed under the same
principle as their primary gonadal counterparts, using treatment
comprised of systemic chemotherapy together with local
treatment, including surgery and radiotherapy. Adjunct
chemotherapy following local surgical treatment is recommended by
the majority of studies if the disease is operable upfront;
cisplatin-based regimens are widely used (8–11,13,24–27).
Regimens, including bleomycin, etoposide and cisplatin (BEP) and
vinblastine, ifosfamide and cisplatin (VIP), are the most commonly
adopted. In addition, high-dose chemotherapy followed by autologous
bone marrow transplantation is used (8,24,28).
To date, the evidence has not been sufficient to determine the
optimal chemotherapy duration for extragonadal germ cell tumours,
including yolk sac tumours. Four cycles of cisplatin-based
combination chemotherapy is the most prevalent treatment regime
(8,11,12,24,26,27),
and factors taken into account prior to treatment include patient
age, performance status, organ function (particularly the lungs and
kidneys), histology, serum marker levels, the location of the
primary tumour and the sites of the metastases (23,28,29).
In conclusion, the diagnosis of a yolk sac tumour
may be challenging in sites of rare occurrence. A high level of
clinical suspicion is required, particularly in young patients.
Prior to the availability of new therapeutic agents, systemic
cisplatin-based chemotherapy remains the standard of care for
extragonadal germ cell tumours.
References
|
1
|
Oosterhuis JW, Stoop H, Honecker F and
Looijenga LH: Why human extragonadal germ cell tumours occur in the
midline of the body: old concepts, new perspectives. Int J Androl.
30:256–263. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuyumcuoğlu U and Kale A: Unusual
presentation of a dermoid cyst that derived from the bladder dome
presenting as subserosal leiomyoma uteri. Clin Exp Obstet Gynecol.
35:309–310. 2008.
|
|
3
|
Taylor G, Jordan M, Churchill B and Mancer
K: Yolk sac tumor of the bladder. J Urol. 129:591–594.
1983.PubMed/NCBI
|
|
4
|
Tinkler SD, Roberts JT, Robinson MC and
Ramsden PD: Primary choriocarcinoma of the urinary bladder: a case
report. Clin Oncol (R Coll Radiol). 8:59–61. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fowler AL, Hall E and Rees G:
Choriocarcinoma arising in transitional cell carcinoma of the
bladder. Br J Urol. 70:333–334. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yokoyama S, Hayashida Y, Nagahama J,
Nakayama I, Kashima K and Ogata J: Primary and metaplastic
choriocarcinoma of the bladder. A report of two cases. Acta Cytol.
36:176–182. 1992.PubMed/NCBI
|
|
7
|
Huang HY, Ko SF, Chuang JH, Jeng YM, Sung
MT and Chen WJ: Primary yolk sac tumor of the urachus. Arch Pathol
Lab Med. 126:1106–1109. 2002.PubMed/NCBI
|
|
8
|
Bokemeyer C, Hartmann JT, Fossa SD, et al:
Extragonadal germ cell tumors: relation to testicular neoplasia and
management options. APMIS. 111:49–63. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ait Benali H, Lalya L, Allaoui M, et al:
Extragonadal mixed germ cell tumor of the right arm: description of
the first case in the literature. World J Surg Oncol.
10:692012.PubMed/NCBI
|
|
10
|
Furtado LV, Leventaki V, Layfield LJ,
Lowichik A, Muntz HR and Pysher TJ: Yolk sac tumor of the thyroid
gland: a case report. Pediatr Dev Pathol. 14:475–479. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim SW, Park JH, Lim MC, Park JY, Yoo CW
and Park SY: Primary yolk sac tumor of the omentum: a case report
and review of the literature. Arch Gynecol Obstet. 279:189–192.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pasternack T, Shaco-Levy R, Wiznitzer A
and Piura B: Extraovarian pelvic yolk sac tumor: case report and
review of published work. J Obstet Gynaecol Res. 34:739–744. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dede M, Pabuccu R, Yagci G, Yenen MC,
Goktolga U and Gunhan O: Extragonadal yolk sac tumor in pelvic
localization. A case report and literature review. Gynecol Oncol.
92:989–991. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moran CA, Suster S and Koss MN: Primary
germ cell tumors of the mediastinum: III. Yolk sac tumor, embryonal
carcinoma, choriocarcinoma and combined nonteratomatous germ cell
tumors of the mediastinum - a clinicopathologic and
immunohistochemical study of 64 cases. Cancer. 80:699–707. 1997.
View Article : Google Scholar
|
|
15
|
Hoei-Hansen CE, Kraggerud SM, Abeler VM,
Kaern J, Rajpert-De Meyts E and Lothe RA: Ovarian dysgerminomas are
characterised by frequent KIT mutations and abundant expression of
pluripotency markers. Mol Cancer. 6:122007. View Article : Google Scholar
|
|
16
|
Cao D, Humphrey PA and Allan RW: SALL4 is
a novel sensitive and specific marker for metastatic germ cell
tumors, with particular utility in detection of metastatic yolk sac
tumors. Cancer. 115:2640–2651. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gupta R, Mathur SR, Arora VK and Sharma
SG: Cytologic features of extragonadal germ cell tumors: a study of
88 cases with aspiration cytology. Cancer. 114:504–511. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang F, Liu A, Peng Y, et al: Diagnostic
utility of SALL4 in extragonadal yolk sac tumors: an
immunohistochemical study of 59 cases with comparison to
placental-like alkaline phosphatase, alpha-fetoprotein and
glypican-3. Am J Surg Pathol. 33:1529–1539. 2009. View Article : Google Scholar
|
|
19
|
Venyo A and Benatar B: A Review of the
Literature on Ketamine-Abuse-Uropathy. WebmedCentral Urology.
3:WMC0030482012.
|
|
20
|
Chu PS, Kwok SC, Lam KM, et al: ‘Street
ketamine’-associated bladder dysfunction: a report of ten cases.
Hong Kong Med J. 13:311–313. 2007.
|
|
21
|
Shahani R, Streutker C, Dickson B and
Stewart RJ: Ketamine associated ulcerative cystitis: a new clinical
entity. Urology. 69:810–812. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Itano NM and Malek RS: Eosinophil cystitis
in adults. J Urol. 165:805–807. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
No authors listed. International Germ Cell
Consensus Classification: a prognostic factor-based staging system
for metastatic germ cell cancers. International Germ Cell Cancer
Collaborative Group. J Clin Oncol. 15:594–603. 1997.PubMed/NCBI
|
|
24
|
Bokemeyer C, Nichols CR, Droz JP, et al:
Extragonadal germ cell tumors of the mediastinum and
retroperitoneum: results from an international analysis. J Clin
Oncol. 20:1864–1873. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Corti F, Sarnacki S, Pattec C, et al:
Prognosis of malignant sacrococcygeal germ cell tumours according
to their natural history and surgical management. Surg Oncol.
21:e31–e37. 2012.PubMed/NCBI
|
|
26
|
Pliarchopoulou K and Pectasides D:
First-line chemotherapy of non-seminomatous germ cell tumors
(NSGCTs). Cancer Treat Rev. 35:563–569. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de La Motte Rouge T, Pautier P, Duvillard
P, et al: Survival and reproductive function of 52 women treated
with surgery and bleomycin, etoposide, cisplatin (BEP) chemotherapy
for ovarian yolk sac tumor. Ann Oncol. 19:1435–1441.
2008.PubMed/NCBI
|
|
28
|
Riese MJ and Vaughn DJ: Chemotherapy for
patients with poor prognosis germ cell tumors. World J Urol.
27:471–476. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hartmann JT, Nichols CR, Droz JP, et al:
Prognostic variables for response and outcome in patients with
extragonadal germ-cell tumors. Ann Oncol. 13:1017–1028. 2002.
View Article : Google Scholar : PubMed/NCBI
|