Introduction
Although advances have been made in the surgical and
conservative therapy of head and neck squamous cell carcinoma
(HNSCC), the mortality rate from this disease has remained stable
over the last years (1). This is
mainly due to the development of therapy-resistant local and
regional recurrences (2).
Antineoplastic treatments, including chemotherapy or radiation, may
efficiently eradicate the majority of proliferating cells within
malignant tumors. However, there is increasing evidence that there
is a subpopulation of resistant tumor cells that are resistant to
these regimens. These cancer stem cells (CSCs) have distinct
features of somatic stem cells, including self-renewal,
proliferation and differentiation. Therefore, these cells are
essential and responsible for the initiation, but also the
maintenance and recurrence, of malignant disease (3). The CSC hypothesis has previously been
applied to HNSCC (4–6). Prince et al revealed that
CD44+ cancer cells, which typically comprise <10% of
the cells in a HNSCC tumor, but not CD44− cancer cells,
gave rise to new tumors in vivo(4). CD44+ cells in tumors of the
head and neck are therefore referred to as the CSCs of HNSCC.
Stromal cell-derived factor-1 (SDF-1), also known as
CXCL12, has variable effects on a plurality of cells (7). CXCR4 has been identified as its
corresponding receptor. The SDF-1-CXCR4 axis is postulated to be a
key pathway in the interaction between CSCs and the surrounding
supportive cells in the CSC niche, and this has mainly been shown
in the hematopoietic system (7,8).
SDF-1 is a multifunctional cytokine that is
expressed and secreted by several tissues, including endothelial
and stromal cells (9,10), which are one component of the bulk
of a HNSCC tumor. There is increasing evidence that the tumor
stroma also plays a significant role in terms of the response to
therapeutic interventions, including chemotherapy (11). SDF-1 has a single open reading frame
of 282 nucleotides that encodes a polypeptide of 93 amino acids.
The cytokine arises in two isoforms, SDF-1α (24–88 amino acids) and
SDF-1β (24–93 amino acids) by alternative splicing (10,12,13).
SDF-1 has been shown to be a potent chemoattractant for
hematopoietic progenitor cells (HPCs) and induces directional
locomotion and podia formation in HNSCC in a dose-dependent manner
(14). Therefore, SDF-1 is
considered to be one of the key regulators for HPC trafficking
between the peripheral blood circulation, bone marrow (9,10,15,16)
and in the CSC niche of HNSCC.
The analysis of the CSC niche theory, where CSCs are
in contact with their surrounding supportive cells, may provide
information regarding cell trafficking and the underlying
mechanisms of cancer, including tumor expansion, recurrence and
metastatic progress. The interaction between SDF-1 and its
receptor, CXCR4, may play a significant role in the CSC niche of
HNSCC and other malignant epithelial tumors.
The present study monitored the interaction between
the CD44+ UM-SCC-11A cell line and potentially
supportive microenvironmental cells as an in vitro model for
the stem cell niche in HNSCC. Fibrocytes, human umbilical vein
endothelial cells (HUVECs) and human microvascular vein endothelial
cells (HMVECs) served as potential counterparts to CSCs in this
model. The development of in vitro models that imitate
cellular interactions in cancer is essential for the evaluation of
potential therapeutic agents. The function of SDF-1 can be mimicked
by small peptide agonists, for example CTCE-0214 (10). These molecules have several
advantages over the natural substances, such as ease of
manufacturing. It is possible that such peptide agonists of SDF-1
comprise new strategies of therapeutical intervention in HNSCC. The
cancer stem cell theory requires confirmation by further
experiments.
Materials and methods
Cell lines and cell culture
The HNSCC cell line UM-SCC-11A was obtained from Dr
T E Carey (University of Michigan, Ann Arbor, MI, USA). The cell
line originates from a primary human HNSCC from the larynx of a
male patient who did not undergo treatment prior to the excision
(17). The cell culture of the
UM-SCC-11A cells was performed in Dulbecco’s modified Eagle’s
medium (DMEM; Fisher Scientific Co., Pittsburgh, PA, USA)
supplemented with 10% fetal calf serum (FCS) and antibiotics (Life
Technologies Inc., Gaithersburg, MD, USA).
The HUVECs (Promocell, Heidelberg, Germany) were
cultured in Endothelial Cell Growth Medium (C-22010; Promocell)
supplemented with additives (C-39215; 0.4% endothelial cell growth
supplement/heparin (ECGS/H), 2% FCS+0.1 ng/ml epidermal growth
factor+1 μg/ml hydrocortisone+1 ng/ml basic fibroblast factor;
Promocell). The HMVECs (Clonetics Corp., San Diego, CA, USA) were
cultured in Endothelial Cell Growth Medium MV (C-22020; Promocell)
with additives (C-39225; 0,4% ECGS/H+5% FCS+10 ng/ml epidermal
growth factor+1 μg/ml hydrocortisone; Promocell). The fibrocytes
were obtained from the skin of a patient at the Department of
Otorhinolaryngology Head and Neck Surgery, University Medical
Centre Mannheim (Mannheim, Germany) who was administered radiation.
The fibrocytes were raised in DMEM high glucose with additives
(C-71210; 10% FCS+2% 200 mM L-glutamin+1% pen/strep/Fungizone;
Promocell). Written informed consent was obtained from the patient.
The culture of the HUVECs, HMVECs and fibrocytes was performed in
gelatine-coated culture flasks. Confluent monolayers were passaged
by trypsin. The cell culture of all the cell lines was performed at
37°C in a 5% CO2 fully humidified atmosphere.
Enzyme-linked immunosorbent assay
(ELISA)
The transmission of SDF-1 by the cell lines was
measured using a human SDF ELISA kit (R&D Systems, Wiesbaden,
Germany). A monoclonal antibody against soluble SDF-1 was adsorbed
to the microwells in 96-well microtiter plates. The samples,
including standards of known SDF-1 concentrations and the samples
that were tested, were pipetted into the wells. During the first
incubation, the SDF-1 antigen was added to wells. Subsequent to
being washed, a biotinylated monoclonal antibody that was specific
for SDF-1 was incubated and the streptavidin-peroxidase enzyme was
added. Following the incubation period and washing to remove all
the unbound enzyme, a substrate solution was added, which catalyzed
a reaction on the bound enzyme and induced a colored reaction
product. The intensity of this colored product was directly
proportional to the concentration of SDF-1 that was present in the
samples.
Immunofluorescence labeling
To detect the expression of CD44 as a CSC marker in
the UM-SCC-11A line and of CD105 in the fibrocytes, HMVECs and
HUVECs, the cells were incubated with CD44/CD105 antibody (mouse
monoclonal; 1:100; Abcam, Cambridge, UK) in order to observe the
cell membrane staining for 1 hour at 37°C, followed by incubation
with a second biotinylated antibody (anti-mouse, 1:100) for 30 min.
Following further washing steps with phosphate-buffered saline, the
cells were treated with streptavidin-Cy3
(1:1,000)/Streptavidin-Alexia 488 (1:500) (Jackson ImmunoResearch
Inc., West Grove, PA, USA) for 30 min at room temperature. The cell
nuclei were stained by DAPI. Immunofluorescence labeling using
CD44/CD105 was used to prepare the cells for an evaluation of
cell-cell-interaction and podia formation.
Fluorescence microscopy
The analysis of the cell morphology, cell-cell
interaction and podia formation was performed as follows. The
CD44+ UM-SCC-11A cells (with green fluorescence using
Alexia 488) and CD105+ fibrocytes, HMVECs and HUVECs
(with red fluorescence via Cy 3) were seeded in DMEM (Fisher
Scientific Co.), supplemented with 10% FCS and antibiotics (Life
Technologies Inc.) and incubated to promote podia formation and
contact. Following this, the cell morphology was assessed using
fluorescence microscopy subsequent to the cells being fixed.
Migration assay
Chemotaxis was assessed using an in vitro
two-chamber transwell assay. The fibrocytes, HUVECs or HMVECs were
added to the lower section of the transwell chamber (8.0 μm pore
size, 6.5 mm diameter inserts; Costar Inc., Union City, CA, USA).
Equal cell numbers of UM-SCC-11A were seeded in the upper chamber
in medium that did not contain SDF-1. After 24 h, the transwells
were removed and the cells that had migrated through the micropores
were counted. UM-SCC-11A cells are adherent cells. When migrated
through pores of the upper well of the migration assay, they do not
drop to the bottom of the lower well. They remain adherent to the
bottom of the upper well, which makes it difficult to count them.
However, they form a cell-ring on the bottom that may be colored
and the width may be measured as a indicator of cell count. A total
of four experiments were performed.
Statistical analysis
All results are presented as the means ± SD.
Student’s t-test (two-tailed distribution, two-sample equal
variance) was used to estimate the probability of the differences.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of CD44 in the UM-SCC-11A
cells
Immunofluorescence labeling of UM-SCC-11A was
performed. CD44, as a stem cell marker in HNSCC, was visualized as
green fluorescence by immunofluorescence labeling using Alexia 488.
In the UM-SCC-11A cells, an intense green fluorescence signal of
all the cells was detected by marking CD44. CD44 was mainly
expressed on the cell surface in all the samples that were stained,
which allowed the evaluation of the cell-cell interaction and podia
formation of the UM-SCC-11A cells towards the potentially
supportive cell types that were used in the experiments
(fibrocytes, HUVECs and HMVECs).
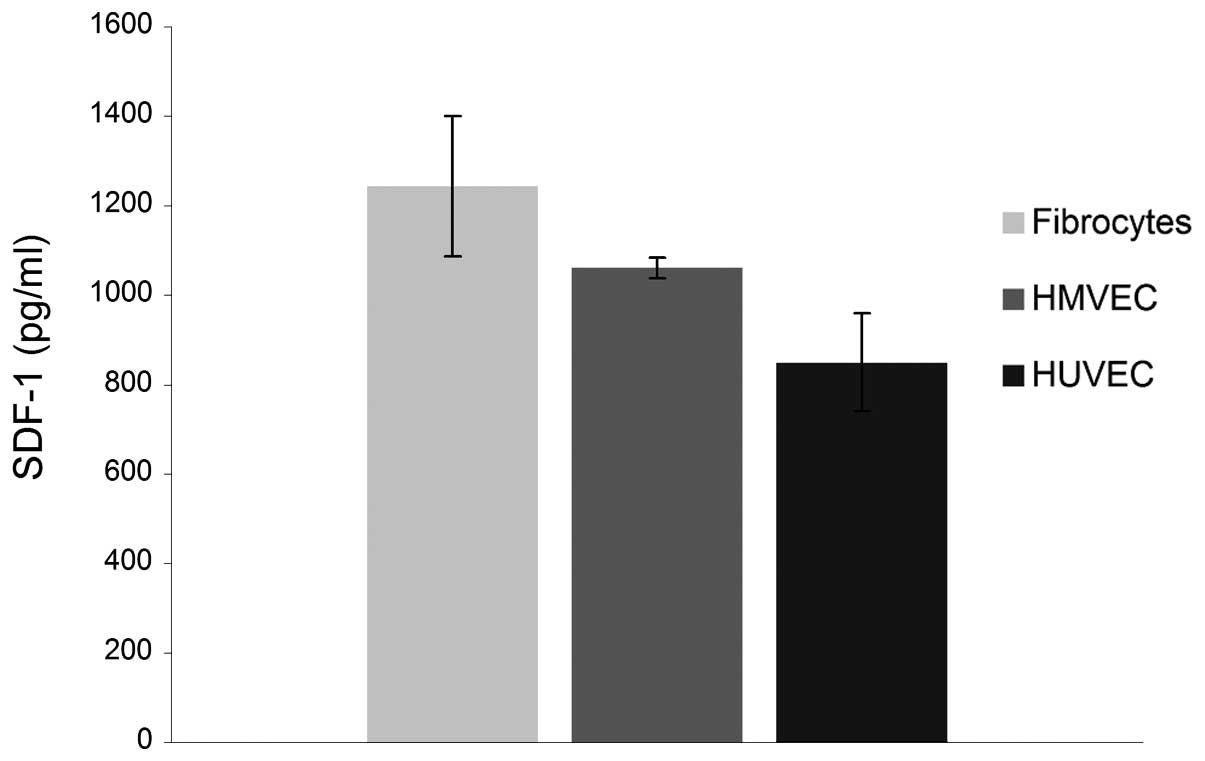
Transmission of SDF-1 by supportive cell
lines
An ELISA analysis was performed to measure the
transmission of SDF-1 by fibrocytes, HMVECs and HUVECs. The level
of SDF-1 that was secreted into the culture medium by the
fibrocytes, HMVECs and HUVECs is shown in Fig. 1. The highest concentration was
observed in the fibrocytes, followed by the HMVECs. The lowest
concentration of SDF-1 was observed in the HUVECs. The values were
determined as mfibro, 1243.3±156.2 pg/ml and
mHMVEC, 1061.4±23.2 pg/ml; mHUVEC,
849.6±110.9 pg/ml (Fig. 1).
Podia formation of UM-SCC-11A cells and
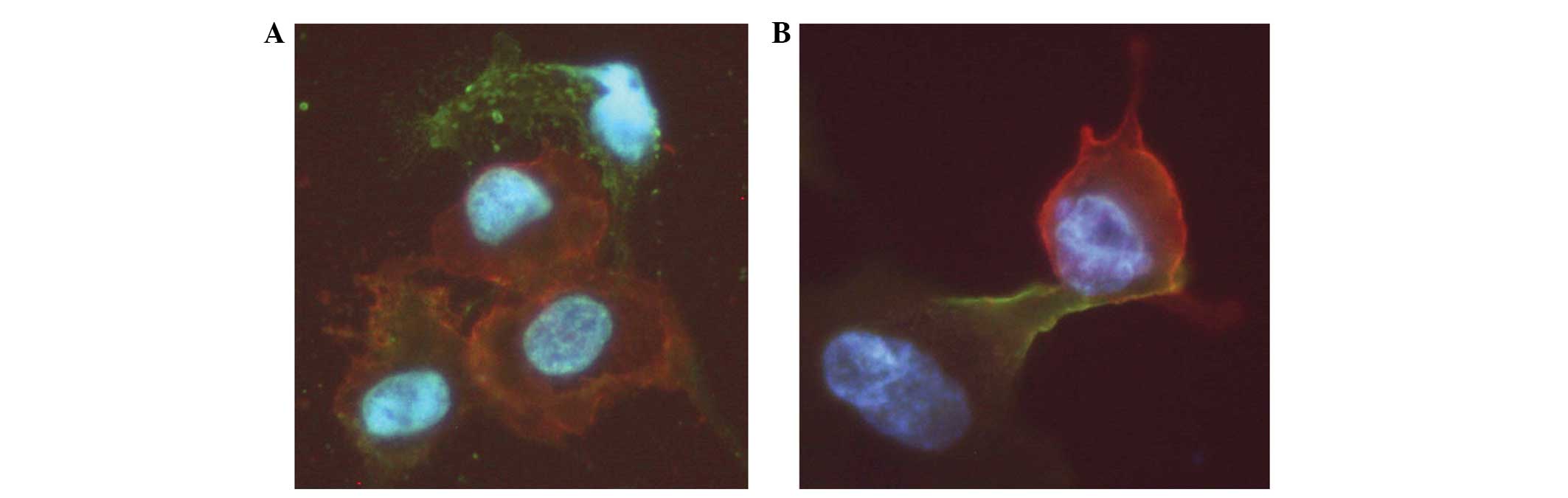
cell-cell interaction with supportive cell lines
Polarization and podia formation are prerequisites
for the directional locomotion of cells. The present study analyzed
podia formation and cell-cell interaction between CD44+
UM-SCC-11A cells and CD105+ fibrocytes, HMVECs and
HUVECs as a model for the CSC niche of HNSCC. For this purpose,
fluorescence labeling of the UM-SCC-11A cells (CD44−
Alexia 488, green fluorescence) and of the fibrocytes, HMVECs and
HUVECs (CD105− Cy 3, red fluorescence) was performed.
Direct cell-cell interaction was observed in terms of podia
formation and adhesion of the UM-SCC-11A cells to the supportive
stromal cell types. The cell-cell interactions were observed as
broadly based cell contacts (Fig.
3A). However, digitiform podia formations of the UM-SCC-11A
cells towards the supportive stromal cells were also identified
(Fig. 3B).
Migration of UM-SCC-11A cells towards
supportive cell lines
The chemotaxis of the CD44+ UM-SCC-11A
cells towards the SDF-1-expressing stromal cells was analyzed using
a transwell migration assay. The CD44+ HNSCC UM-SCC-11A
cell line was used as a model for CSCs, and their migration towards
potentially supportive microenvironmental cells was evaluated. All
the cell types that were tested secreted various concentrations of
SDF-1 into the culture medium (mfibro, 1243.3±156.2
pg/ml; mHMVEC, 1061.4±23.2 pg/ml and mHUVEC,
849.6±110.9 pg/ml; Fig. 1).
According to this, the migration of the UM-SCC-11A cells towards
the supportive cells was increased by a higher supply of SDF-1
(contrfibro, 315.23±61.55 μm; mfibro,
477.73±143.7 μm; Pfibro=0.003; contrHMVEC,
123.41±66,68 μm; mHMVEC, 249.04±111,95 μm;
PHMVEC=0.004; contrHUVEC, 189.7±93.26 μm;
mHUVEC, 260.82±161.58 μm; Fig. 2). A significantly larger amount of
UM-SCC-11A cells migrated was towards the differentiated fibrocytes
compared with the HMVECs or HUVECs (Pfibro/HMVEC=
2.12E-11; Pfibro/HUVEC=2.28E-5; Fig. 2).
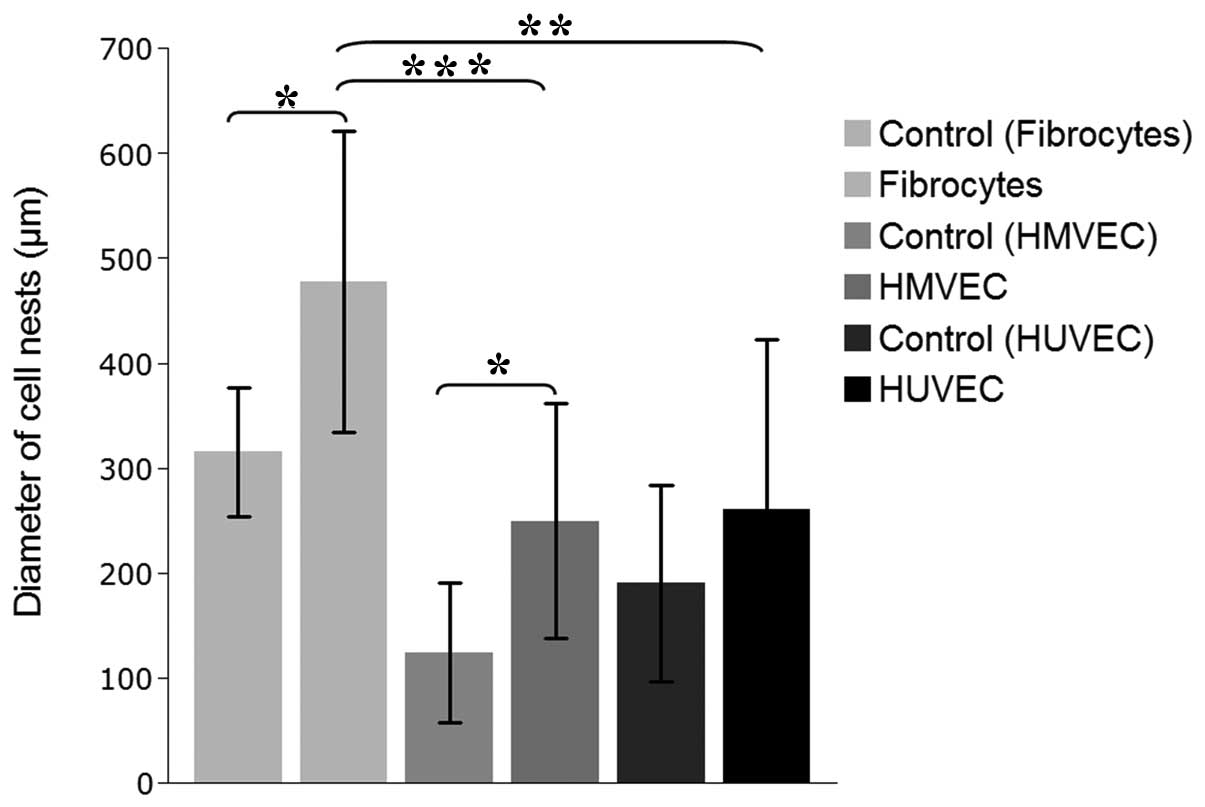 | Figure 2Migration of UM-SCC-11A cells towards
the supportive microenvironmental stromal cells. The migration of
the CD44+ UM-SCC-11A towards the supportive stromal
cells was increased by a higher supply of SDF-1. The values are
presented as the mean ± standard deviation. contrfibro,
315.23±61.55 μm; mfibro, 477.73±143.7 μm;
*Pfibro=0.003; contrHMVEC,
123.41±66.68 μm; mHMVEC, 249.04±111.95 μm;
*PHMVEC=0.004; contrHUVEC,
189.7±93.26 μm; mHUVEC, 260.82±161.58 μm. The amount of
UM-SCC-11A migrating was significantly higher towards the
differentiated fibrocytes compared with the HMVECs or HUVECs
(***Pfibro/HMVEC=2.12E-11;
**Pfibro/HUVEC=2.28E-5). SDF-1, stromal
cell-derived factor-1; CSC, cancer stem cell; HMVEC, human
microvascular vein endothelial cell; HUVEC, human umbilical vein
endothelial cell. |
Discussion
The stem cell theory has become increasingly
significant in tumor biology, particularly with regard to tumor
development, progression and metastasis (18). Therefore, the CSC theory has
provided new ideas and considerations for research and therapeutic
options for malignant diseases (19). Currently, the presence of CSCs may
not only be identified in hematological malignancies, but also in
solid tumor entities (4,20). The CSC theory in solid tumors, such
as HNSCC, has dramatic consequences. To date, therapeutic
interventions, including surgery or chemoradiation, have been
directed towards the bulk of the tumor without focusing on the
small amount of specialized tumor cells, which have the facilities
of self-renewal, differentiation and unlimited proliferation.
However, the correct combination of markers that should be used to
isolate the CSC in HNSCC remains unclear. The combination of
markers appears to be different for different types of tumors
(4,20,21).
Furthermore, the presence of CSCs in HNSCC is postulated. Prince
et al revealed that CD44+ cells, compared with
CD44− cells, were able to engraft a new HNSCC tumor in
the mouse model (4). According to
results of the study by Prince et al, CD44+ was
sufficient to isolate cells with CSC properties out of the bulk of
a HNSCC tumor. Following updated research, ALDH1 is another marker
that has been postulated as a CSC marker in HNSCC (22). In the present study, CD44 was used
as a CSC marker. In a previous study, a high expression of CD44,
particularly at the invasive front of the tumor, was identified in
HNSCC tissue samples (23), where
the tumor cells were in contact with their surrounding cells,
including the stromal and endothelial cells (24). The localization of CSC candidates at
the border of the tumor is feasible, as this is where invasion and
tumor growth occurs. Invasiveness and metastasis of a tumor also
depends on the capacity to cut and rebuild the extracellular matrix
(25,26). Malignant cells infiltrate healthy
tissue by degrading components of the extracellular matrix,
breaking down vessel borders and therefore generating metastases in
distant organs. A number of tumor types have been shown to involve
the presence of matrix metalloproteinases, including HNSCC
(24,26,27).
The SDF-1-CXCR4 axis is involved in several aspects
of tumor progression, including angiogenesis, metastasis and
survival (28). The
microenvironment of the bone marrow has been considered to support
the survival, differentiation and proliferation of hematopoietic
progenitor cells (29), as well as
malignant progenitor cells of the hematopoietic system, including
those of B-cell acute lymphoblastic leukemia (30). The pathway that includes the
SDF-1-CXCR4 axis is postulated to be responsible for the retention
of lymphoid and myeloid leukemia cells in the bone marrow (30,31).
The significance of the SDF-1-CXCR4 axis is well-discussed in the
hematopoietic system. However, to the best of our knowledge, the
present study was the first to show that HNSCC cells also
demonstrate podia formation as a pre-condition for locomotion using
dose-dependent migration towards an SDF-1 gradient (14). In summary, the SDF-1-CXCR4 axis may
also play a crucial role in the development, progress, invasion and
metastasis of HNSCC and may be an essential pathway in the
interaction between CSCs in HNSCC and the surrounding supportive
niche.
In previous studies, CXCR4 was identified in the
tumor nests of HNSCC, but not in the surrounding stroma of the CSC
niche (23). Clatot et al
revealed that the intratumoral level of SDF-1 correlated with
survival in HNSCC (32). By
contrast, the concentration of SDF-1 in the peripheral blood of
HNSCC patients was not observed to differ in comparison with
healthy donors (14). The latter
findings are consistent with the results of the present study,
which suggest that the SDF-1-CXCR4 axis may be involved in the CSC
niche within the tumor, but not in the periphery of the blood
system. In previous studies, and in the present in vitro
model of the stem cell niche, we have shown that polarization and
the formation of filopodia may be increased in the CD44+
CXCR4+ HNSCC UM-SCC-11A cell line in a dose-dependent
manner using SDF-1 (10,14). This effect may be attributed to the
cytoskeleton rearrangements of actin-containing protrusions
(10,33) and may be influenced by extracellular
factors, including matrix metalloproteinases (33,34).
In general, podia formation is believed to interact with cell
adhesion to the microenvironment (10). Evidence that podia formation and
adhesion to the CSC niche plays a role in HNSCC CD44+
cells may improve the understanding of these interactions and offer
insight into new strategies for cancer-directed therapy in HNSCC
using small molecule agonists or antagonists of SDF-1 (10). These may be used to interfere with
the CSC niche and cause the inhibition or prevention of tumor
invasion and metastasis. Further experiments are required to expand
and specify the cell-cell interactions in the CSC niche of solid
tumors. It may be possible to develop strategies of therapy that
are aimed at CSCs or the SDF-1-CXCR4 axis.
Acknowledgements
The authors would like to thank Petra Prohaska for
excellent technical support.
References
|
1
|
Boehm A, Wichmann G, Mozet C and Dietz A:
Current therapy options in recurrent head and neck cancer. HNO.
58:762–769. 2010.(In German).
|
|
2
|
Ho AS, Kraus DH, Ganly I, Lee NY, Shah JP
and Morris LG: Decision making in the management of recurrent head
and neck cancer. Head Neck. Mar;2013.(Epub ahead of print).
|
|
3
|
Soltanian S and Matin MM: Cancer stem
cells and cancer therapy. Tumour Biol. 32:425–440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prince ME, Sivanandan R, Kaczorowski A, et
al: Identification of a subpopulation of cells with cancer stem
cell properties in head and neck squamous cell carcinoma. Proc Natl
Acad Sci USA. 104:973–978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang P, Zuo H, Ozaki T, Nakagomi N and
Kakudo K: Cancer stem cell hypothesis in thyroid cancer. Pathol
Int. 56:485–489. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Allegra E and Trapasso S: Cancer stem
cells in head and neck cancer. Onco Targets Ther. 5:375–383. 2012.
View Article : Google Scholar
|
|
7
|
Hattermann K and Mentlein R: An infernal
trio: the chemokine CXCL12 and its receptors CXCR4 and CXCR7 in
tumor biology. Ann Anat. 195:103–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suárez-Álvarez B, López-Vázquez A and
López-Larrea C: Mobilization and homing of hematopoietic stem
cells. Adv Exp Med Biol. 741:152–170. 2012.
|
|
9
|
Dar A, Goichberg P, Shinder V, et al:
Chemokine receptor CXCR4-dependent internalization and resecretion
of functional chemokine SDF-1 by bone marrow endothelial and
stromal cells. Nat Immunol. 6:1038–1046. 2005. View Article : Google Scholar
|
|
10
|
Faber A, Roderburg C, Wein F, et al: The
many facets of SDF-1alpha, CXCR4 agonists and antagonists on
hematopoietic progenitor cells. J Biomed Biotechnol.
2007:260652007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hale MD, Hayden JD and Grabsch HI:
Tumour-microenvironment interactions: role of tumour stroma and
proteins produced by cancer-associated fibroblasts in chemotherapy
response. Cell Oncol (Dordr). 36:95–112. 2013. View Article : Google Scholar
|
|
12
|
De La Luz Sierra M, Yang F, Narazaki M, et
al: Differential processing of stromal-derived factor-1alpha and
stromal-derived factor-1beta explains functional diversity. Blood.
103:2452–2459. 2004.
|
|
13
|
Shirozu M, Nakano T, Inazawa J, et al:
Structure and chromosomal localization of the human stromal
cell-derived factor 1 (SDF1) gene. Genomics. 28:495–500. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Faber A, Hoermann K, Stern-Straeter J,
Schultz DJ and Goessler UR: Functional effects of SDF-1α on a
CD44(+) CXCR4(+) squamous cell carcinoma cell line as a model for
interactions in the cancer stem cell niche. Oncol Rep. 29:579–584.
2013.
|
|
15
|
Chute JP: Stem cell homing. Curr Opin
Hematol. 13:399–406. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Petit I, Goichberg P, Spiegel A, et al:
Atypical PKC-zeta regulates SDF-1-mediated migration and
development of human CD34+ progenitor cells. J Clin Invest.
115:168–176. 2005.PubMed/NCBI
|
|
17
|
Grenman R, Burk D, Virolainen E, Wagner
JG, Lichter AS and Carey TE: Radiosensitivity of head and neck
cancer cells in vitro. A 96-well plate clonogenic cell assay for
squamous cell carcinoma. Arch Otolaryngol Head Neck Surg.
114:427–431. 1988. View Article : Google Scholar
|
|
18
|
Spandidos DA: A unified theory for the
development of cancer. Biosci Rep. 6:691–708. 1986. View Article : Google Scholar
|
|
19
|
Podberezin M, Wen J and Chang CC: Cancer
stem cells: A review of potential clinical applications. Arch
Pathol Lab Med. Nov;2012.(Epub ahead of print).
|
|
20
|
La Porta CA: Thoughts about cancer stem
cells in solid tumors. World J Stem Cells. 4:17–20. 2012.PubMed/NCBI
|
|
21
|
Grosse-Gehling P, Fargeas CA, Dittfeld C,
et al: CD133 as a biomarker for putative cancer stem cells in solid
tumours: limitations, problems and challenges. J Pathol.
229:355–378. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Clay MR, Tabor M, Owen JH, et al:
Single-marker identification of head and neck squamous cell
carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck.
32:1195–1201. 2010. View Article : Google Scholar
|
|
23
|
Faber A, Goessler UR, Hoermann K, et al:
SDF-1-CXCR4 axis: cell trafficking in the cancer stem cell niche of
head and neck squamous cell carcinoma. Oncol Rep. 29:2325–2331.
2013.PubMed/NCBI
|
|
24
|
Sterz CM, Kulle C, Dakic B, et al: A
basal-cell-like compartment in head and neck squamous cell
carcinomas represents the invasive front of the tumor and is
expressing MMP-9. Oral Oncol. 46:116–122. 2010. View Article : Google Scholar
|
|
25
|
Nakajima M, Gohji K, Fabra A, Fidler IA
and Tsuruo T: Regulation of tumor metastasis and extracellular
matrix degradative enzyme production by microenvironments. Gan To
Kagaku Ryoho. 20:380–386. 1993.(In Japanese).
|
|
26
|
Faber A, Sauter A, Hoedt S, et al:
Alteration of MMP-2 and −14 expression by imatinib in HPV-positive
and -negative squamous cell carcinoma. Oncol Rep. 28:172–178.
2012.
|
|
27
|
Schultz JD, Rotunno S, Erben P, et al:
Down-regulation of MMP-2 expression due to inhibition of receptor
tyrosine kinases by imatinib and carboplatin in HNSCC. Oncol Rep.
25:1145–1151. 2011.
|
|
28
|
Teicher BA and Fricker SP: CXCL12
(SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 16:2927–2931.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Metzen E, Jin F, Brockmeier U and
Otterbach F: New insight into the SDF-1/CXCR4 axis in a breast
carcinoma model: hypoxia-induced endothelial SDF-1 and tumor cell
CXCR4 are required for tumor cell intravasation. Mol Cancer Res.
10:1021–1031. 2012. View Article : Google Scholar
|
|
30
|
Purizaca J, Meza I and Pelayo R: Early
lymphoid development and microenvironmental cues in B-cell acute
lymphoblastic leukemia. Arch Med Res. 43:89–101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Juarez J, Dela Pena A, Baraz R, et al:
CXCR4 antagonists mobilize childhood acute lymphoblastic leukemia
cells into the peripheral blood and inhibit engraftment. Leukemia.
21:1249–1257. 2007. View Article : Google Scholar
|
|
32
|
Clatot F, Picquenot JM, Choussy O, et al:
Intratumoural level of SDF-1 correlates with survival in head and
neck squamous cell carcinoma. Oral Oncol. 47:1062–1068. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Geutskens SB, Andrews WD, van Stalborch
AM, et al: Control of human hematopoietic stem/progenitor cell
migration by the extracellular matrix protein Slit3. Lab Invest.
92:1129–1139. 2012. View Article : Google Scholar
|
|
34
|
Ghosh MC, Makena PS, Gorantla V, Sinclair
SE and Waters CM: CXCR4 regulates migration of lung alveolar
epithelial cells through activation of Rac1 and matrix
metalloproteinase-2. Am J Physiol Lung Cell Mol Physiol.
302:L846–L856. 2012. View Article : Google Scholar : PubMed/NCBI
|