Introduction
Squamous papillomas are benign epithelial tumors
that occur on the skin of the face and body and most often appear
in the mouth or genital regions. Squamous papilloma involving the
middle ear as a primary lesion is an extremely rare occurrence
(1). Few cases have been previously
reported in the English literature (2), hence, its etiology and
clinicopathological features remain unclear. Specific hypotheses
indicate that squamous papilloma lesions correlate with viral
infection, chronic inflammation, allergies or environmental
pollutants. Previous studies have shown that infection with human
papilloma virus (HPV) is involved in the occurrence of papillomas
in the head and neck region (3–6) and it
is known that Epstein-Barr virus (EBV) is carried by ~90% of the
adult population worldwide as a lifelong asymptomatic infection
(7).
Although no causal correlation has been established
between viral infections, including HPV and EBV, and the
development of middle ear squamous papilloma, it remains a
possibility that requires important consideration. The current
study reported four cases of primary middle ear squamous papilloma
and the results of HPV and EBV in situ hybridization (ISH).
The pathogenesis and diagnostic, therapeutic and prognostic aspects
of this tumor are also discussed in hope that the results of the
present study are useful for clarifying diagnostic and therapeutic
strategies for this type of papilloma and the involvement of HPV
and EBV infections.
Materials and methods
Patients
Between 2009 and 2012, four patients were treated at
the First Affiliated Hospital of Nanjing Medical University
(Nanjing, China) with an initial pathological diagnosis of squamous
papilloma of the middle ear. The records of these four patients
were retrospectively reviewed, including the clinical history,
treatment, follow-up, radiological data and pathology reports.
Paraffin-embedded tissue blocks from the middle ear of these
patients were recovered, sectioned and stained with hematoxylin and
eosin. To avoid interobserver variations, two pathologists reviewed
all pathological slides and were in agreement with the final
pathological reports. All lesions in this study were associated
with the middle ear and there was no evidence of prior papillomas
in the external auditory meatus or nasopharynx. The current study
was approved by the Institutional Review Board of the First
Affiliated Hospital of Nanjing Medical University. Written informed
consent was obtained from the patients.
ISH for HPV DNA
For detecting the presence of HPV, ISH was conducted
with a wide-spectrum digoxigenin-labeled probe (Triplex
International Biosciences Co. Ltd., Fuzhou, China) for common HPV
types according to the manufacturer’s instructions. The
wide-spectrum probe targets the genomic DNA of HPV types 5, 6, 8,
11, 16, 18, 26, 27, 30, 31, 33, 35, 39, 40, 41, 42, 43, 45, 47, 48,
51, 52, 53, 54, 55, 57, 58 and 59. Sections from the tissue blocks
were deparaffinized and rehydrated in graded alcohols and distilled
water. Target sample pretreatment was performed in a high-power
microwave oven. The hybridization reaction was detected by
incubation with an anti-digoxigenin antibody tagged with
horseradish peroxidase (POD), and diaminobenzidine (DAB) was
applied as the chromogen. Slides were counterstained with
hematoxylin and appropriate positive and negative controls were
included in each assay. Positive staining was defined as the
presence of dark brown granules in the nuclei of epithelial cells
at the site of hybridization.
ISH for EBV-encoded RNA (EBER)
EBER was detected by ISH with the EBER Detection kit
(Triplex International Biosciences Co. Ltd.) according to the
manufacturer’s instructions. Next, the sections were
deparaffinized, rehydrated and predigested with proteinase K and a
hybridization solution containing the digoxigenin-labeled EBER
nucleic acid probe was applied. Detection of the hybridized probe
was performed by application of anti-digoxigenin-POD and the
coloring reaction was performed with DAB. Sections were
counter-stained with hematoxylin and brown nuclear staining was
regarded as a positive hybridization signal.
Results
Clinical data
The clinical data of the four patients are
summarized in Table I. All patients
were male with ages ranging between 29 and 70 years, with variable
courses of disease ranging between 2 months and 50 years. Otorrhea
was the most frequent complaint, occurring in three of the four
patients. Other symptoms, including hearing loss (n=3), otalgia
(n=3), tinnitus (n=2) and aural fullness (n=2), were also observed.
All subjects received computed tomography (CT) scans of the
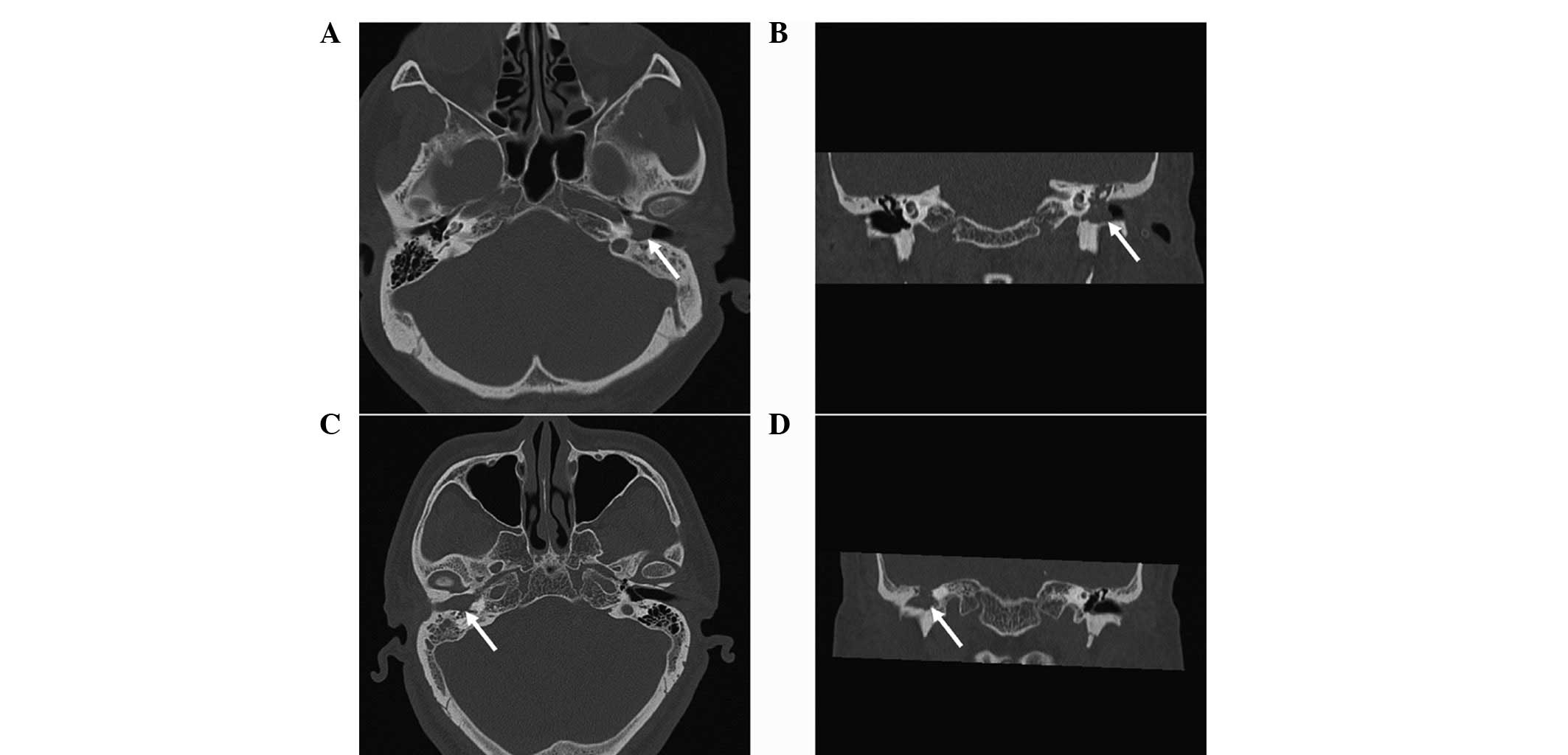
temporal bone prior to surgery and these scans showed soft tissue
density between all regions of the middle ear and the mastoid with
or without extension into the external auditory meatus (Fig. 1). Mild bone erosion of the
promontory was observed on the CT scan of case 1.
 | Table IClinical observations of middle ear
squamous papilloma. |
Table I
Clinical observations of middle ear
squamous papilloma.
| Case no. (year) | Gender | Age, years | Presenting
symptoms | Localization | Premalignant
change | Treatment | Follow-up,
months |
|---|
| 1 (2009) | M | 70 | Otorrhea, HL and
otalgia | Left middle ear,
extending into the inner ear and EAM | + | Subtotal temporal
bone resection | NED, 36 |
| 2 (2010) | M | 50 | Otorrhea, otalgia and
aural fullness | Left middle ear | + | Partial temporal bone
resection | NED, 28 |
| 3 (2012) | M | 29 | Otorrhea, HL and
tinnitus | Left middle ear,
extending into the EAM | − | Radical
tympanomastoidectomy | NED, 12 |
| 4 (2012) | M | 60 | HL, otalgia and
tinnitus | Right middle ear,
extending into the EAM | − | Radical
tympanomastoidectomy | NED, 6 |
Clinical management of these patients was based
primarily on the preoperative imaging observations and
intraoperative frozen-section examinations. Papillomas associated
with premalignant changes were found in the frozen sections of
cases 1 and 2 and in these cases, extended resections of the lesion
(subtotal or partial temporal bone resection) had been performed.
Radical tympanomastoidectomy was used in the other two cases to
guarantee radical resection of the neoplasms. All patients
recovered well postoperatively and were discharged as scheduled. To
date, the patients have been followed for between 6 and 36 months
without evidence of recurrent disease, clinically and
radiologically.
Pathological observations
Histological examination of these cases confirmed
the diagnosis of squamous papilloma. In addition, premalignant
changes, including carcinoma in situ and high grade squamous
intraepithelial neoplasia, were observed in the sections of cases 1
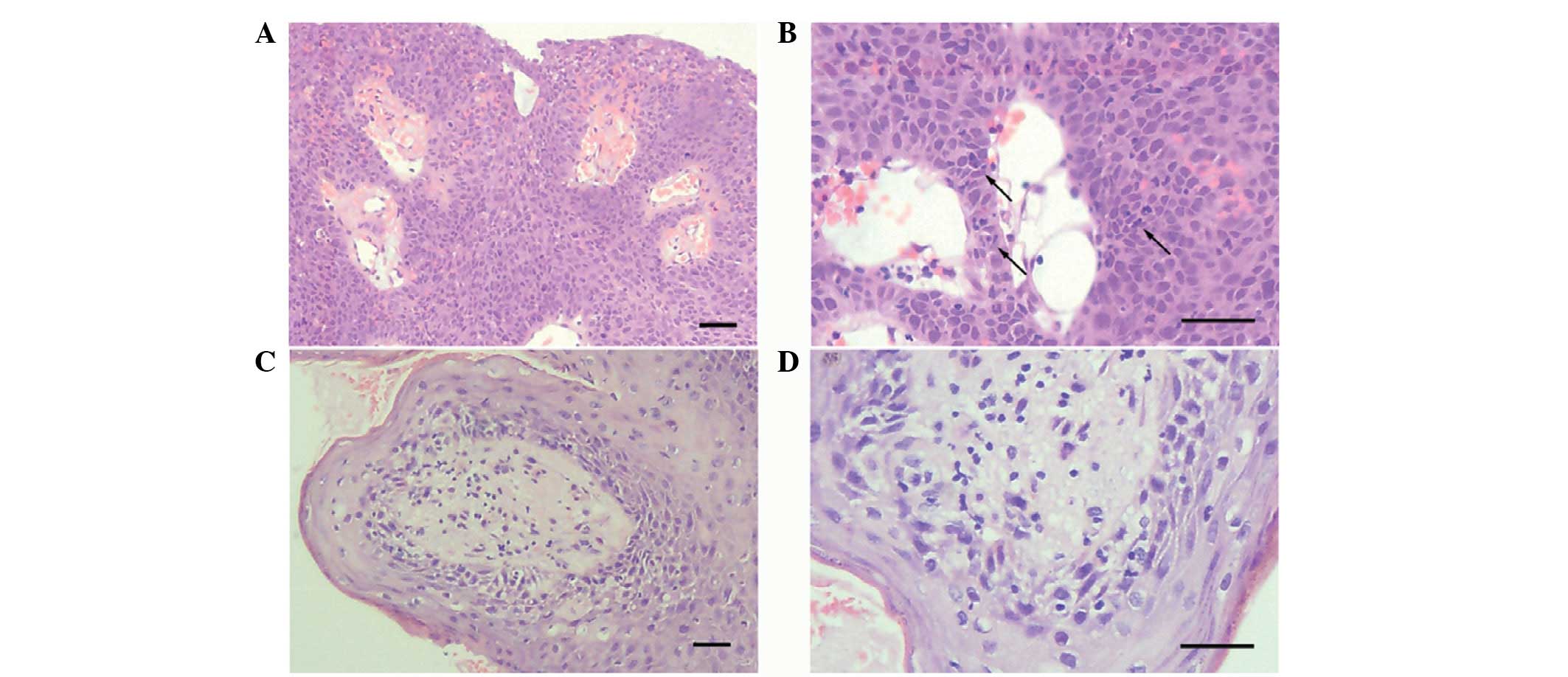
and 2. Representative histopathological images of the middle ear
squamous papillomas and premalignant changes are shown in Fig. 2. Squamous papilloma exhibits as
fibrovascular axes covered with pluristratified keratinized
epithelium and a clear margin with no cytological features of
malignancy in exophytic papillary projections. Premalignant change
is shown by high-grade dysplasia of the epithelial cells without
penetration through the basement membrane in papillary fronds.
HPV and EBV detection
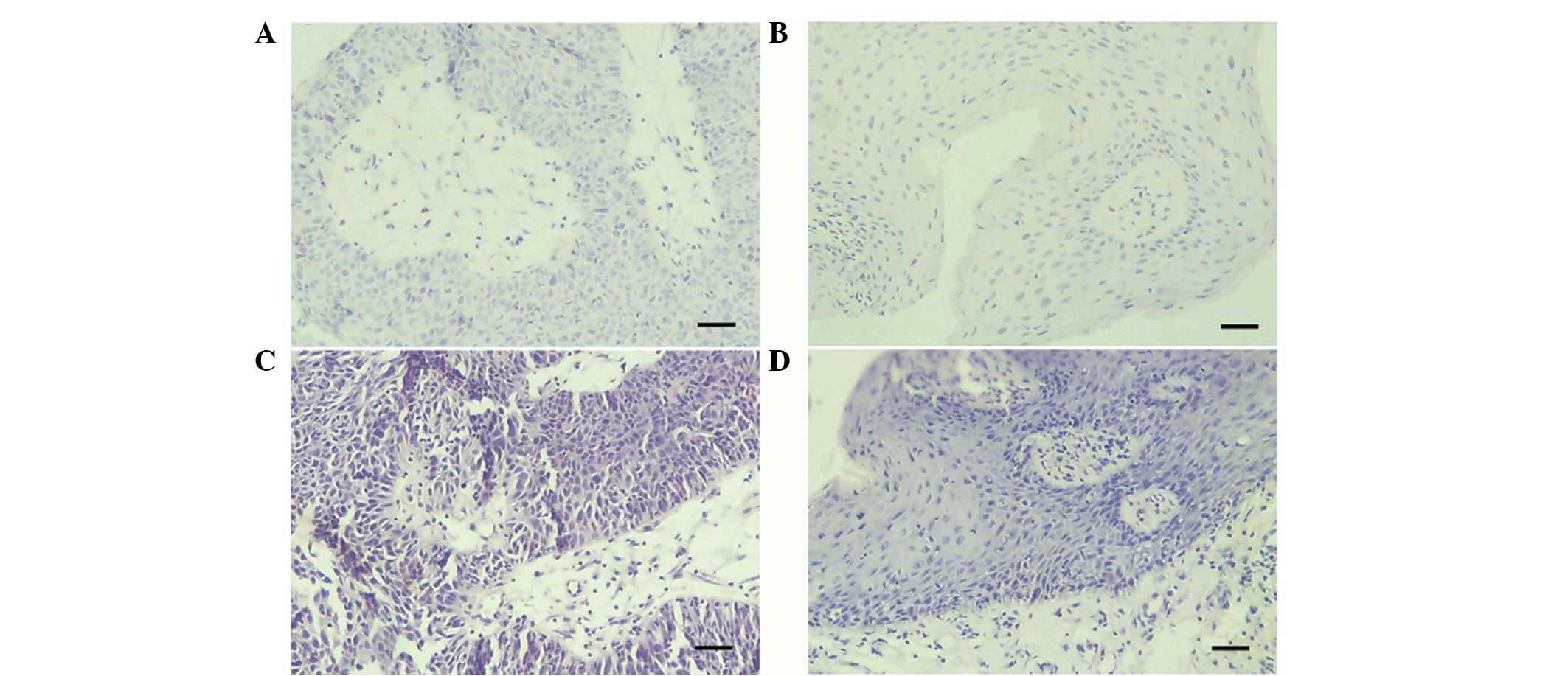
HPV and EBV ISH showed no detectable HPV or EBV
genomes in any of the four cases with middle ear squamous papilloma
(Fig. 3).
Discussion
Squamous papilloma may, in theory, occur anywhere on
the epidermis and mucosa, but in the head and neck region, it is
most commonly found in the mouth and throat. Primary middle ear
squamous papilloma is an exceedingly rare temporal bone neoplasm
and an Ovid Medline search using the search terms ‘squamous
papilloma’ and ‘middle ear/temporal bone’ returned only two cases
in the English literature (2,8). The
data in the previous literature consisted only of case reports and
the clinical features and pathogenesis of the tumor are vague due
to the low frequency of the disease. The present study presented
four patients with primary middle ear squamous papilloma.
Premalignant changes were observed in two of these cases and the
results of ISH showed that HPV and EBV are not present in these
lesions. Based on these results, we hypothesize that HPV and EBV
infection may not play a role in the pathogenesis of middle ear
squamous papilloma.
The etiology and pathogenesis of papillomas of the
middle ear remain unknown. CT scans of the current patients did not
detect lesions in the sinonasal cavity, nasopharynx or pharyngeal
opening of the auditory tube. In addition, none of the patients had
a past history of papillomas of the external ear canal or relevant
surgical procedures. Thus, this infers that the tumors in the
present cases did not arise in the adjacent area and become
incidentally involved with the middle ear via the eustachian tube
or the external acoustic meatus. Therefore, this indicates that the
tumors were of multicentric primary origin. Furthermore, inverted
papilloma, an additional type of papilloma, rarely occurs in the
middle ear (9). Ectopic migration
of ectodermal tissue to the middle ear, viruses, chronic
inflammation, allergies and carcinogenic exposure have all been
considered as possible etiological factors for this neoplasm
(9,10). Thus, it is reasonable to conclude
that these factors may also be potential causes for the onset of
squamous papilloma in the middle ear. Among these factors, viral
and HPV infections, in particular, have long been considered to
stimulate the development of squamous papilloma in the squamous
epithelial cells of the skin.
There is growing evidence to support the association
of HPV with the pathogenesis of squamous papillomas, including
genital warts and recurrent childhood respiratory papillomatosis
(11,12). HPV encompasses a group of
double-stranded DNA viruses of the papovavirus subgroup A and a
number of HPV subtypes are associated with lesions of the head and
neck. High-risk HPV has been identified in middle ear carcinomas
(13), but the correlation between
HPV infection and papillomas in the temporal bone remains unknown.
We hypothesize that HPV infection is involved in the occurrence of
middle ear squamous papilloma in a manner similar to such tumors in
the genital regions. Detection of viral presence by ISH has
demonstrated a valuable method for determining diagnoses and has
led to new insights into viral and neoplastic diseases (14,15).
In the current study, a wide-spectrum probe for HPV DNA was applied
to paraffin-embedded papilloma tissues, but all four cases of
squamous papilloma were found to be HPV-negative.
The HPV infection rate in middle ear squamous
papilloma has not been previously reported and only a few studies
have investigated the presence of HPV infection in middle ear
inverted papilloma. Previous attempts to identify HPV in the tissue
sections of seven cases of primary temporal inverted papilloma have
been described in previous studies (16–18).
In five cases, inverted papilloma was studied with ISH, and in two
cases with a combination of ISH and PCR assay. None of the cases
evaluated with ISH were confirmed to be HPV-positive and HPV DNA
type 6 was identified by PCR assay in one of the two cases in which
it was used (18). The results of
the current study are consistent with these ISH results and as a
sensitive and wide-spectrum of HPV testing was conducted and found
no evidence for the presence of HPV, we conclude that HPV infection
is not involved in the occurrence of middle ear squamous
papilloma.
There were certain limitations to the examination of
HPV in the present study, the most relevant of which was that the
sensitivity of ISH appeared to be low compared with that of
liquid-phase amplification (10).
Thus, if HPV exists in the tissues at low levels, the virus may
escape detection by the ISH methods used in the current study. More
sensitive methods, including type-specific PCR for HPV, are
required in future experiments to determine any role for HPV in the
pathogenesis and behavior of middle ear squamous papilloma.
Furthermore, although the ISH experiments performed in the present
study yielded negative results for HPV infection, there is the
possibility of infection with other HPV types. The wide-spectrum
probe used detects a number of HPV types simultaneously, including
the main types of HPV that have been reported in a variety of head
and neck papillomas. However, it does not cover HPV types 2, 3, 10
and 28, which have been shown to be present in other specific
cutaneous lesions (19).
EBV is a virus of the herpes family that infects the
majority of the human population. This virus infects B cells of the
immune system and epithelial cells. There is evidence that
infection with EBV is associated with a higher risk of particular
forms of cancer and may function as an oncogenic agent (20). Thus, the association of EBV with
premalignant changes, including those observed in the four patients
of the current study, is worthy of investigation. Furthermore,
previous studies have shown that ≤68% of inverted Schneiderian
papillomas have tested positive for the presence of EBV (7) and it has been reported that specific
cases of non-inverted-type papilloma are also associated with EBV
(21). These observations indicate
that EBV may be a causative agent of papillomas, but, at present,
no results are available with regard to the presence of EBV in
middle ear squamous papilloma. In the current study, the squamous
papilloma samples obtained from the patients tested negative for
EBER despite the notion that EBER ISH is more sensitive than other
tests for detecting EBV in tissues (22). Therefore, it is hypothesized that
EBV is not associated with the pathologies of this neoplasm.
The symptoms of middle ear squamous papilloma
resemble other neoplasms involving the middle ear, including
chronic otitis media with cholesteatoma or granulation tissue.
Otorrhea and hearing loss are the most common complaints and the
majority of cases, including the two previously reported and four
present cases, have a clear history of otorrhea with antibiotic
treatment that did not resolve symptoms and generally, worsened
with time. This is consistent with the assumption that middle ear
mucosa metaplasia, due to chronic inflammation, also induces the
development of squamous papilloma (2). This tumor may be detected by CT or
surgical observations, but an accurate diagnosis must be made
pathologically. As squamous papilloma has specific histological
features, it must be easily distinguishable from other soft tissue
neoplasms in the middle ear, including cholesteatomas and adenomas.
Therapy for this tumor is mainly by surgery or biopsy prior to
surgery and intraoperative frozen-section examination is currently
recommended to exclude malignant disease. Other papillomas in the
head and neck areas have a high recurrence rate and the possibility
of malignant transformation if not completely excised, therefore,
middle ear squamous papilloma has also been predicted to have this
trend. Radical tympanomastoidectomy or temporal bone resection must
be considered as the initial treatment to guarantee radical
resection of the lesion.
Notably, middle ear squamous papilloma in the
present cases occurred predominantly in males between the fifth and
seventh decades of life, which is similar to the demographic
features of head and neck papillary squamous cell carcinoma
(23,24). Papillomas associated with
premalignant changes were found in two cases of the present study,
but definite squamous cell carcinoma was not observed in any of the
cases. Therefore, we conclude that this tumor has a tendency for
malignant transformation. By contrast, the association of sinonasal
inverted papilloma with squamous cell carcinoma is well recognized
and varies between 5 and 53%, with an overall average value of 10%
(25). It is difficult to obtain
definitive information from the literature with regard to the
incidence and prognosis of middle ear squamous papilloma associated
with carcinoma due to the rarity of this tumor. To the best of our
knowledge, the cases presented in the current study are the first
reported of this tumor accompanied with premalignant changes. For
these patients, radical surgery was sufficient and there was no
requirement for postoperative irradiation. In the follow-up periods
of >2 years, no recurrence was observed. In spite of this,
long-term postoperative follow-up for middle ear squamous papilloma
cases is recommended for the purposes of detecting recurrence and
monitoring for malignant transformation. As two cases in the
present study had a long history of otorrhea, we hypothesize that
chronic inflammatory stimulation, not HPV and EBV infection, is a
causative agent of premalignant changes. It is entirely possible,
however, that infection by other viruses or specific unidentified
causes may also be involved in the occurrence of malignant
transformation.
In the present study, four cases of middle ear
squamous papilloma have been reported. Although this tumor is
exceedingly rare, it is possible that its incidence is under
recognized. Commonly, these papillomas develop in males of
~60-years of age and otorrhea is the most frequent complaint.
Premalignant changes were observed in two of the present cases and
ISH of HPV and EBV in all four cases was found to be negative. The
present results indicate that chronic inflammatory stimulation, not
HPV and EBV infection, is involved in the occurrence of this tumor
and its malignant transformation. Radical surgery and long-term
postoperative follow-up are recommended due to its malignant and
recurrent potential. The current study was limited to a small
number of cases and the etiology of this disease remains unclear.
Further genetic investigations with newly identified cases are
required to clarify the pathogenesis of this disease.
Acknowledgements
The current study was supported by a grant from the
Jiangsu Health Administration of China (no. LJ201120)
References
|
1
|
Xia MY, Zhu WY, Lu JY, Lu Q and Chen L:
Ultrastructure and human papillomavirus DNA in papillomatosis of
external auditory canal. Int J Dermatol. 35:337–339. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cahali S, da Silva FB, Machado MC, da
Silva DA, Reforeme OM and Cahali MB: Middle ear squamous papilloma:
report of a case and literature review. Braz J Otorhinolaryngol.
71:396–398. 2005.(In Portuguese).
|
|
3
|
Gaffey MJ, Frierson HF, Weiss LM, Barber
CM, Baber GB and Stoler MH: Human papillomavirus and Epstein-Barr
virus in sinonasal Schneiderian papillomas. An in situ
hybridization and polymerase chain reaction study. Am J Clin
Pathol. 106:475–482. 1996.
|
|
4
|
Pou AM, Weems J, Deskin RW, Nason R and
Payne DA: Molecular characterization of mutations in patients with
benign and aggressive recurrent respiratory papillomatosis: a
preliminary study. Ann Otol Rhinol Laryngol. 113:180–186. 2004.
View Article : Google Scholar
|
|
5
|
Carneiro TE, Marinho SA, Verli FD,
Mesquita AT, Lima NL and Miranda JL: Oral squamous papilloma:
clinical, histologic and immunohistochemical analyses. J Oral Sci.
51:367–372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oliveira LR, Ribeiro-Silva A, Ramalho LN,
Simões AL and Zucoloto S: HPV infection in Brazilian oral squamous
cell carcinomapatients and its correlation with clinicopathological
outcomes. Mol Med Rep. 1:123–129. 2008.
|
|
7
|
Sham CL, To KF, Chan PK, Lee DL, Tong MC
and van Hasselt CA: Prevalence of human papillomavirus,
Epstein-Barr virus, p21 and p53 expression in sinonasal inverted
papilloma, nasal polyp, and hypertrophied turbinate in Hong Kong
patients. Head Neck. 34:520–533. 2012. View Article : Google Scholar
|
|
8
|
de Santos Torres SAM, Castro TW, Bento RF
and Lessa HA: Middle ear papilloma. Braz J Otorhinolaryngol.
73:4312007.
|
|
9
|
Zhou H, Chen Z, Li H and Xing G: Primary
temporal inverted papilloma with premalignant change. J Laryngol
Otol. 125:206–209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Filippis C, Marioni G, Tregnaghi A,
Marino F, Gaio E and Staffieri A: Primary inverted papilloma of the
middle ear and mastoid. Otol Neurotol. 23:555–559. 2002.PubMed/NCBI
|
|
11
|
Chelimo C, Wouldes TA, Cameron LD and
Elwood JM: Risk factors for and prevention of human
papillomaviruses (HPV), genital warts and cervical cancer. J
Infect. 66:207–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Derkay CS and Wiatrak B: Recurrent
respiratory papillomatosis: a review. Laryngoscope. 118:1236–1247.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsai ST, Li C, Jin YT, Chao WY and Su IJ:
High prevalence of human papillomavirus types 16 and 18 in
middle-ear carcinomas. Int J Cancer. 71:208–212. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Speel EJ, Hopman AH and Komminoth P:
Amplification methods to increase the sensitivity of in situ
hybridization: play card(s). J Histochem Cytochem. 47:281–288.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Syrjänen S, Syrjänen K, Mäntyjärvi R,
Collan Y and Kärjä J: Human papillomavirus DNA in squamous cell
carcinomas of the larynx demonstrated by in situ DNA hybridization.
ORL J Otorhinolaryngol Relat Spec. 49:175–186. 1987.PubMed/NCBI
|
|
16
|
Wenig BM: Schneiderian-type mucosal
papillomas of the middle ear and mastoid. Ann Otol Rhinol Laryngol.
105:226–233. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roberts WH, Dinges DL and Hanly MG:
Inverted papilloma of the middle ear. Ann Otol Rhinol Laryngol.
102:890–892. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Marioni G, Altavilla G, Busatto G,
Blandamura S, De Filippis C and Staffieri A: Detection of human
papillomavirus in temporal bone inverted papilloma by polymerase
chain reaction. Acta Otolaryngol. 123:367–371. 2003. View Article : Google Scholar
|
|
19
|
de Koning MN, Khoe LV, Eekhof JA, et al:
Lesional HPV types of cutaneous warts can be reliably identified by
surface swabs. J Clin Virol. 52:84–87. 2011.
|
|
20
|
Tseng CJ, Pao CC, Tseng LH, et al:
Lymphoepithelioma-like carcinoma of the uterine cervix: association
with Epstein-Barr virus and human papillomavirus. Cancer. 80:91–97.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mizugaki Y, Sugawara Y, Shinozaki F and
Takada K: Detection of Epstein-Barr virus in oral papilloma. Jpn J
Cancer Res. 89:604–607. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsai ST, Jin YT, Mann RB and Ambinder RF:
Epstein-Barr virus detection in nasopharyngeal tissues of patients
with suspected nasopharyngeal carcinoma. Cancer. 82:1449–1453.
1998. View Article : Google Scholar
|
|
23
|
Suarez PA, Adler-Storthz K, Luna MA,
El-Naggar AK, Abdul-Karim FW and Batsakis JG: Papillary squamous
cell carcinomas of the upper aerodigestive tract: a
clinicopathologic and molecular study. Head Neck. 22:360–368. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Russell JO, Hoschar AP and Scharpf J:
Papillary squamous cell carcinoma of the head and neck: a
clinicopathologic series. Am J Otolaryngol. 32:557–563. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Batsakis JG and Suarez P: Schneiderian
papillomas and carcinomas: a review. Adv Anat Pathol. 8:53–64.
2001. View Article : Google Scholar : PubMed/NCBI
|