Introduction
Head and neck squamous cell carcinoma, which
includes oral squamous cell carcinoma (OSCC), is the sixth most
prevalent malignancy worldwide (1,2). Due
to the poor prognosis of OSCC, the overall five-year survival rate
of patients following surgical resection has not improved markedly
during the past three decades (3).
The transcription factor NANOG is critical for the
regulation of cell fate in the inner cell mass during embryonic
development and pluripotency of embryonic stem cells (4–7).
Overexpression of NANOG protein has been previously found in a
variety of tumors, including breast cancer (8), colorectal cancer (9,10),
gastric carcinoma (11) and OSCC
(12,13). Previous studies report variable
NANOG expression, from undetectable to extremely high levels, in
OSCC samples. Furthermore, NANOG expression may be associated with
patient survival. Elevated NANOG expression has been found to be
associated with a poor prognosis, advanced stage and
medially-to-poorly differentiated OSCC (14,15).
Based on these observations, NANOG may be a useful prognosis
factor. However, the correlation among NANOG expression,
differentiation and metastasis in OSCC remains unclear.
In this study, NANOG expression in OSCC specimens
was examined by immunohistochemistry. Furthermore, the association
between NANOG expression and differentiation, metastatic potency
and resistance of OSCC to preoperative adjuvant therapy was
evaluated.
Materials and methods
Patients
Between 1997 and 2011, 60 patients with operable
oral cancer underwent surgery at the Department of Oral and
Maxillofacial Surgery (Osaka Dental University Hospital Hirakata,
Japan; Table I). This study follows
the tenets of the Declaration of Helsinki and was approved by the
ethics committee of Osaka Dental University Hospital (Osaka,
Japan). Informed consent was obtained from the patients. None of
the primary foci were subjected to preoperative adjuvant therapy
and, among 24 metastatic samples, 11 were from patients who
underwent preoperative adjuvant therapy. The constituents of the
adjuvant therapy are shown in Table
II. Tumors were evaluated histologically, based on the
International Union Against Cancer classification (16).
 | Table IClinicopathological factors in 60
patients with OSCC. |
Table I
Clinicopathological factors in 60
patients with OSCC.
| Variable |
Well-differentiated | Poorly
differentiated |
|---|
| Gender, n |
| Male | 18 | 18 |
| Female | 19 | 5 |
| Age, years |
| Mean | 65.6 | 63.5 |
| Range | 18–84 | 47–81 |
| Region, n |
| Tongue | 20 | 5 |
| Gingiva | 10 | 11 |
| Floor of oral
cavity | 2 | 6 |
| Buccal mucosa | 4 | 1 |
| Palate | 1 | 0 |
| T status, n |
| T1 | 11 | 5 |
| T2 | 17 | 12 |
| T3 | 8 | 3 |
| T4 | 1 | 3 |
| N status, n |
| N0 | 20 | 16 |
| N1 | 6 | 1 |
| N2a | 0 | 0 |
| N2b | 11 | 6 |
| N3 | 0 | 0 |
 | Table IIPreoperative adjuvant therapy
regimen. |
Table II
Preoperative adjuvant therapy
regimen.
| Patient no. | Differentiation
level | Regimen |
|---|
| 1 |
Well-differentiated | PEP + RT |
| 2 |
Well-differentiated | PEP + CDDP + TS-1 +
RT |
| 3 |
Well-differentiated | TS-1 + RT |
| 4 | Poorly
differentiated | PEP + RT |
| 5 | Poorly
differentiated | CDDP + 5-FU |
| 6 | Poorly
differentiated | TS-1 + RT |
| 7 | Poorly
differentiated | PEP + RT |
| 8 | Poorly
differentiated | CDDP + 5-FU + RT |
| 9 |
Well-differentiated | PEP + RT |
| 10 |
Well-differentiated | PEP + CDDP + RT |
| 11 |
Well-differentiated | PEP + CDDP + RT |
Immunohistochemistry
Tissue samples of oral cancers of various stages
from patients were fixed in 10% neutral-buffered formalin solution
immediately following resection and were embedded in paraffin.
Sections of 4-μm thickness were cut and mounted on silane-coated
glass slides. The sections were deparaffinized in d-limonene and
dehydrated in a graded ethanol series. Antigen retrieval was
performed by autoclaving at 121°C for 15 min in Tris-EDTA buffer
(pH 9.0). Endogenous peroxidase activity was blocked by incubation
with 3% H2O2 for 10 min and nonspecific
reactions were blocked by incubation with blocking solution
(Nacalai Tesque, Inc., Kyoto, Japan) for 10 min. The tissue
sections were incubated with goat anti-NANOG polyclonal antibody
(1:300; Abnova, Taipei, Taiwan) at room temperature for 1 h. Tissue
sections were then incubated with anti-goat IgG
peroxidase-conjugated micropolymer (Vector Laboratories,
Burlingame, CA, USA) at room temperature for 30 min and visualized
by incubation with 3,3′-diaminobenzidine tetrahydrochroride liquid
system (Dako, Tokyo, Japan) at room temperature for 5 min. The
sections were counterstained with hematoxylin and observed by light
microscopy (Olympus Corporation, Tokyo, Japan).
Evaluation of slides
NANOG protein immunoreactivity was evaluated by two
independent pathologists who had no knowledge of the patient’s
clinicopathological factors and outcomes. Nuclear expression of
NANOG protein was scored semiquantitatively by the combination of
intensity (1, weak staining; 2, moderate staining; and 3, strong
staining) and the proportion of positively stained tumor cells per
1,000 tumor cells in high-power fields (1, <25%; 2, 25–50%; 3,
51–75%; and 4, >75%). The sum of the staining intensity and
percentage of positive tumor cell scores was graded as follows: +,
2–3; ++, 4–5; and +++, 6–7. There were no discrepancies between the
two pathologists in the overall interpretation of the
immunohistochemistry results.
Statistical analysis
Mann-Whitney U tests were performed using the SPSS
software (version 13.0; SPSS, Inc., Chicago, IL, USA) to identify
statistically significant differences between samples. Data are
presented as the mean ± SD. P<0.05 was considered to indicate a
statistically significant difference.
Cell culture
Human SAS, HSC-3 and HSC-4 OSCC cell lines (RIKEN
BioResource Center, Ibaraki, Japan) were cultured in DMEM
supplemented with 10% fetal calf serum (both Invitrogen Life
Technologies, Carlsbad, CA, USA) at 37°C in a humidified atmosphere
of 95% air and 5% CO2. Cell monolayers were prepared by
plating on 10-cm cell culture dishes (Asahi Glass, Tokyo,
Japan).
Western blotting
Proteins were resolved in RIPA Buffer [150 mM NaCl,
1.0% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS and 50 mM
Tris-HCl (pH 8.0)] and separated by 10% SDS-PAGE. A rabbit
anti-NANOG antibody (Abcam, Cambridge, UK) was used as the primary
antibody and peroxidase-linked ECL anti-rabbit IgG (GE Healthcare
Japan, Tokyo, Japan) was used as the secondary antibody. ECL plus
(GE Healthcare Japan) was used as the substrate for western
blotting.
Immunofluorescence staining
Cultured cells were fixed with 3.5% formaldehyde,
permeabilized with 0.2% Triton X-100 and blocked with Image-iT™ FX
Signal Enhancer (Invitrogen Life Technologies). Rabbit anti-NANOG
antibody (Abcam) was used as the primary antibody. Next, Alexa
Fluor 594-conjugated IgG (Molecular Probes, Eugene, OR, USA) was
used as the secondary antibody. Following incubation with the
antibodies, SlowFade® Gold antifade reagent with
4′,6-diamidino-2-phenylindole (Invitrogen Life Technologies) was
added and coverslips were mounted. The specimens were observed
using a laser scanning confocal microscope (FV10i-DOC; Olympus
Corporation).
Results
NANOG protein expression in OSCC patients
and OSCC cell lines
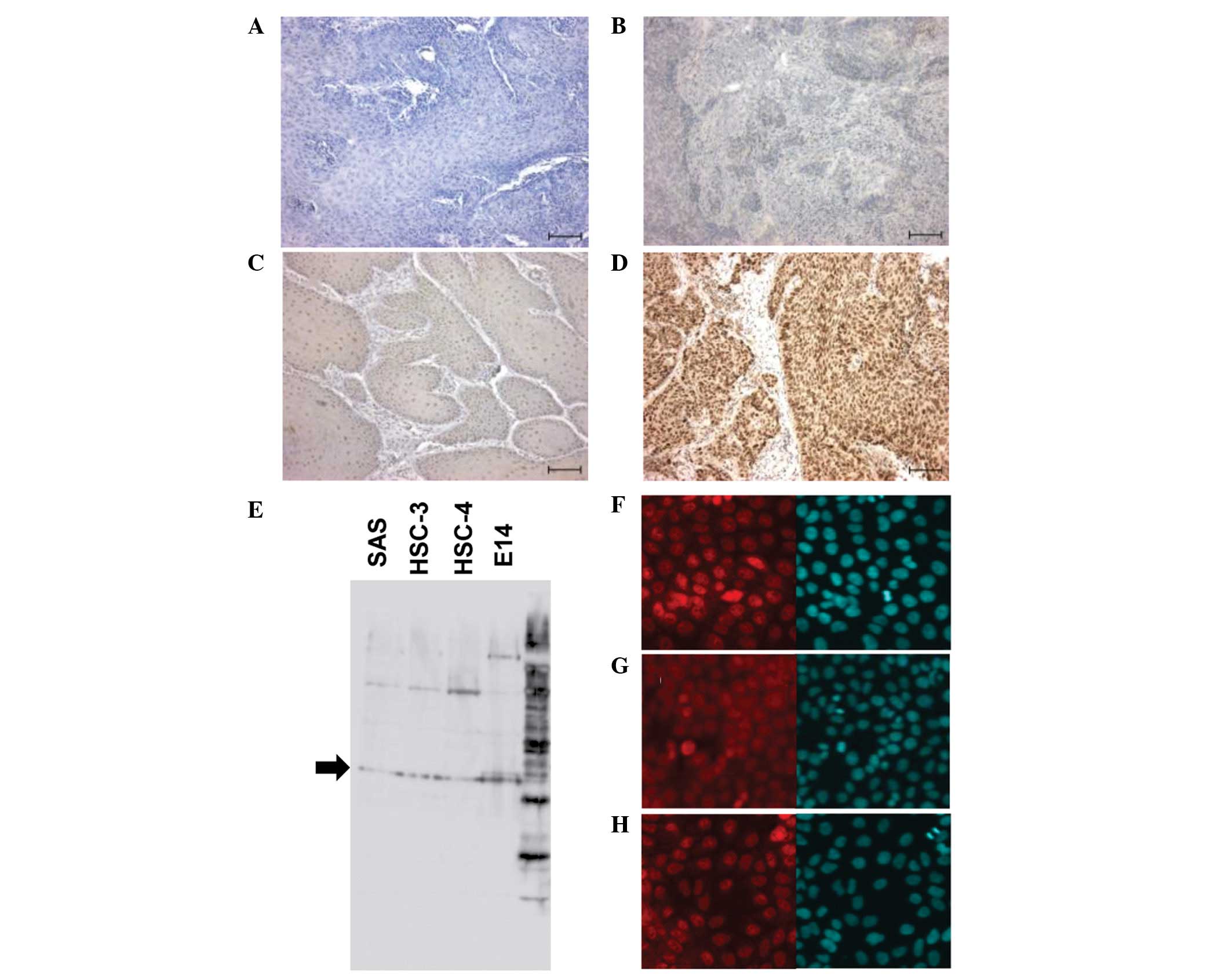
NANOG protein was clearly stained in the nuclei of
cells at various levels in OSCC specimens. Among 60
paraffin-embedded OSCC tissues of primary focus, eight cases
(13.3%) were negative (−), 15 (25%) showed weak expression (+), 22
(36.7%) showed moderate expression (++) and 15 (25%) showed strong
expression (+++). Representative cases of the different NANOG
protein expression levels are shown in Fig. 1A–D. To confirm the expression of
NANOG in OSCC cell lines, NANOG protein levels were analyzed in
SAS, HSC-3 and HSC-4 cells derived from tongue SCCs by western
blotting and immunofluorescence staining. NANOG protein was
detectable at the same levels in all three cell lines (Fig. 1E–H).
High NANOG protein expression in poorly
differentiated OSCC and metastatic foci of OSCC
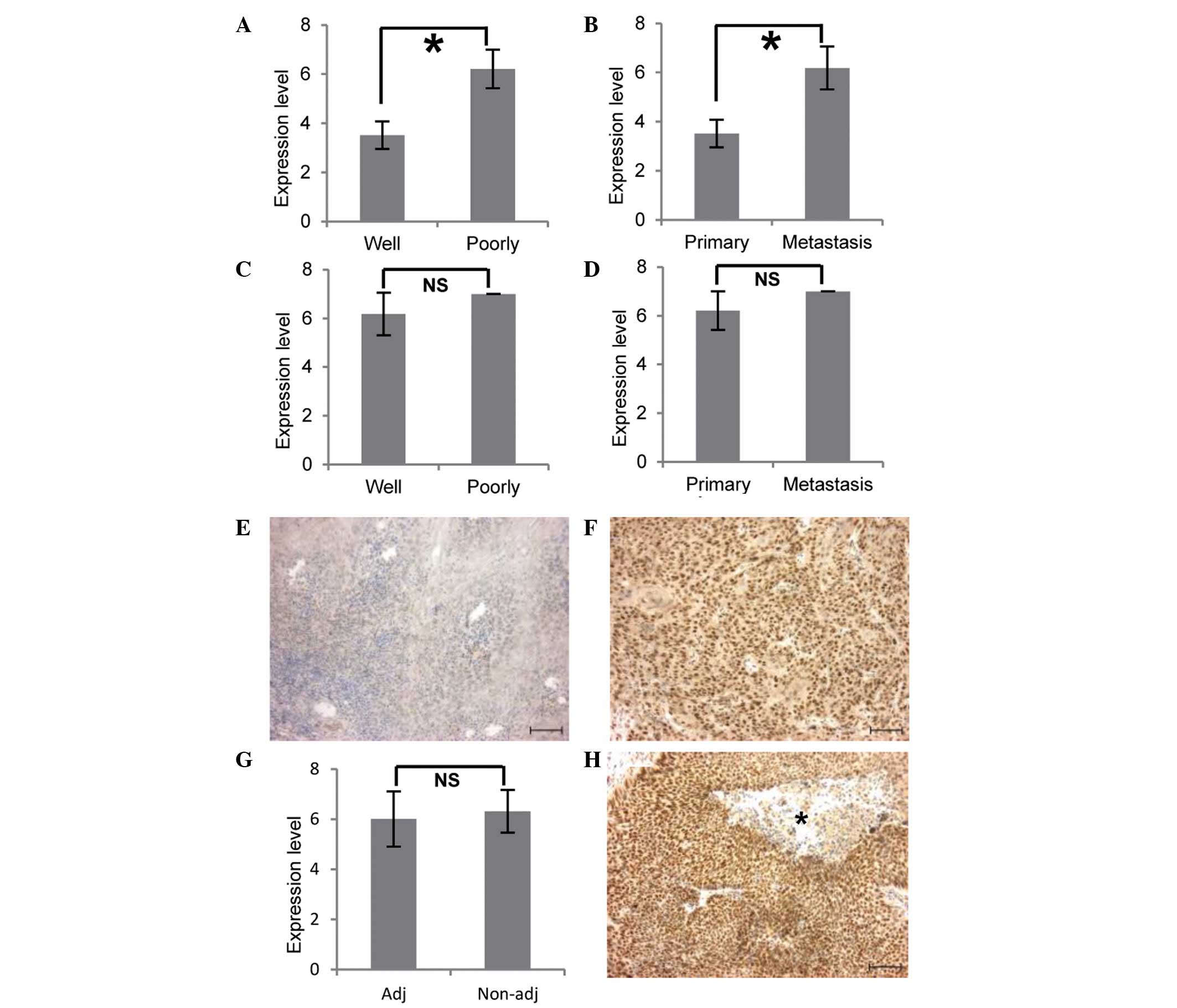
NANOG protein expression levels were higher in
primary foci of poorly differentiated OSCC than in those of
well-differentiated OSCC (P<0.01; Table III; Fig. 2A). However, NANOG expression did not
correlate with gender, region or T (stage of primary tumor) and N
(stage of lymph node metastasis) status (P>0.05; Table III). In well-differentiated OSCC,
NANOG expression in metastatic foci was elevated in comparison with
its level in primary foci (P<0.01; Table III; Fig. 2B). Representative cases are shown in
Fig. 2E and F. However, in
metastatic foci, there was no significant association between NANOG
expression and differentiation levels (P>0.05; Table III; Fig. 2C). Similarly, among primary and
metastatic foci of poorly differentiated OSCC, no significant
differences according to NANOG expression were identified
(P>0.05; Table III; Fig. 2D).
 | Table IIICorrelation between NANOG expression
and clinicopathological factors in 60 patients with OSCC. |
Table III
Correlation between NANOG expression
and clinicopathological factors in 60 patients with OSCC.
| | | Expression, n | |
|---|
| | |
| |
|---|
| Variable | Negative, n | Positive, n | + | ++ | +++ | P-value |
|---|
| Total patients | 8 | 52 | 15 | 22 | 15 | |
| Gender |
| Male | 4 | 32 | 10 | 8 | 14 | NS |
| Female | 4 | 20 | 5 | 14 | 1 | |
| Region |
| Tongue | 0 | 25 | 9 | 12 | 4 | NS |
| Gingiva | 5 | 16 | 4 | 6 | 6 | |
| Floor of oral
cavity | 1 | 7 | 1 | 1 | 5 | |
| Buccal mucosa | 2 | 3 | 0 | 3 | 0 | |
| Palate | 0 | 1 | 1 | 0 | 0 | |
| T status |
| T1 | 1 | 15 | 3 | 9 | 3 | NS |
| T2 | 4 | 25 | 9 | 9 | 7 | |
| T3 | 1 | 10 | 3 | 4 | 3 | |
| T4 | 2 | 2 | 0 | 0 | 2 | |
| N status |
| N1 | 0 | 5 | 0 | 2 | 3 | NS |
| N2a | 0 | 0 | 0 | 0 | 0 | |
| N2b | 0 | 8 | 0 | 1 | 7 | |
| N3 | 0 | 0 | 0 | 0 | 0 | |
| Primary tumor |
|
Well-differentiated | 4 | 33 | 15 | 18 | 0 | P<0.01 |
| Poorly
differentiated | 4 | 19 | 0 | 4 | 15 | |
| Metastasis |
|
Well-differentiated | 0 | 11 | 0 | 3 | 8 | NS |
| Poorly
differentiated | 0 | 2 | 0 | 0 | 2 | |
| Recieved adjuvant
therapy | 0 | 11 | 0 | 4 | 7 | NS |
| No adjuvent
therapy | 0 | 13 | 0 | 3 | 10 | |
High NANOG expression is maintained in
metastatic foci with preoperative adjuvant therapy
To investigate the association between NANOG
expression and preoperative adjuvant therapy in OSCC, NANOG levels
in metastatic lymph nodes were compared between patients who
received preoperative adjuvant therapy and those who did not. There
was no significant difference between the two groups (P>0.05;
Table III; Fig. 2G). Moreover, OSCC cells (except for
those in necrotic tissue) in metastatic lymph nodes subjected to
adjuvant therapy expressed NANOG at high levels (Fig. 2H).
Discussion
Our results show that the nuclei of cancer cells in
the majority of OSCC samples (86.7%) were NANOG-positive. NANOG
protein expression levels were higher in poorly differentiated OSCC
than in well-differentiated OSCC, and NANOG was detected in all
nuclei of OSCC cell lines examined. Furthermore, regardless of
preoperative adjuvant therapy, NANOG expression in metastatic foci
was extremely high. Although a number of the primary foci (13.3%)
were negative for NANOG expression, all corresponding metastatic
foci expressed high levels of NANOG.
Previous studies have shown that almost all tumors
are heterogeneous (17–21). Cancer stem cells (CSCs), a small
subpopulation of tumor cells, are the main factor in the
initiation, growth, metastasis (14) and resistance of chemotherapy of
tumors (22), therefore, cancer
tends to recur. NANOG protein has been reported to be important in
various tumor types, including OSCC (50% of primary foci and 66.7%
of metastatic foci express NANOG protein) (14), colorectal cancer (20% of tumors)
(10) and gastric carcinoma (10% of
tumors) (11). In the present
study, overexpression of NANOG was detected in poorly
differentiated OSCC. Well-differentiated OSCC consists of numerous
differentiated cells in the central region of the tumor and the
majority undifferentiated tumor cells expressing NANOG exist at the
fringe of focus. Immunofluorescence staining showed that OSCC cell
lines expressed NANOG protein at the same level. These data
indicate that NANOG is expressed not only in CSCs, but also in a
large proportion of OSCC cells that are undifferentiated and highly
proliferative. Previous studies indicate that NANOG promotes
dedifferentiation of p53-deficient mouse astrocytes into brain
cancer stem-like cells (23) and
blocks differentiation, indicating that, in addition to its
importance in CSCs, NANOG plays a significant role in maintaining
the non-differentiation or proliferation of OSCC cells. Although
the sensitivity of the present immunohistochemistry technique was
higher than that in previous studies, specific samples were
negative and all were confirmed to express the cell cycle marker
Ki-67 (data not shown). These data indicate that NANOG is
associated with proliferation, independently of the cell cycle, in
undifferentiated OSCC cells, including CSCs. In OSCC patients with
primary foci in which there was no expression of NANOG, metastatic
foci markedly expressed NANOG. As aforementioned, NANOG-negative
tumors may contain a limited number of undifferentiated OSCC cells,
including CSCs. A previous study showed that high expression of
NANOG was associated with metastasis (14). Therefore, in NANOG-negative
patients, CSCs expressing NANOG in early stage primary foci
metastasize and form the secondary tumor. Thereafter,
NANOG-positive undifferentiated cancer cells may be maintained in
metastatic foci and disappear from primary foci.
Sentinel lymph node biopsy, which is commonly used
to aid breast cancer and melanoma staging, is effective in the
diagnosis of OSCC metastasis (24).
Immunohistochemistry is required to identify micrometastases and
isolated tumor cells (25). The
present study indicates that assessment of NANOG protein levels may
be useful in sentinel lymph node biopsy.
In the present study, metastatic foci, with or
without preoperative adjuvant therapy, showed extremely high
expression of NANOG, although necrotic tissues were present within
tumors in metastatic lymph nodes subjected to adjuvant therapy.
These data indicate that specific tumor cells were necrotized by
preoperative adjuvant therapy and that surviving NANOG-positive
tumor cells proliferated. A previous study showed that preoperative
adjuvant therapy for oral cancer did not significantly improve the
survival rate despite the primary local control rate being improved
(26). NANOG expression is
positively associated with chemoresistance of OSCC (12,13),
and CSCs express high levels of NANOG and exhibit high levels of
chemoresistance (22). Thus, it is
possible that specific tumor cells that did not express NANOG
underwent cell death, while undifferentiated tumor cells, including
CSCs overexpressing NANOG, survived and continued to proliferate in
patients who underwent preoperative adjuvant therapy.
The results of the present study demonstrate that
undifferentiated cancer cells overexpressing NANOG are important
for metastatic OSCC. Therefore, we hypothesize that targeting NANOG
protein may be a useful strategy for the treatment of OSCC
metastasis.
References
|
1
|
Chen YJ, Lin SC, Kao T, et al: Genome-wide
profiling of oral squamous cell carcinoma. J Pathol. 204:326–332.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pentenero M, Gandolfo S and Carrozzo M:
Importance of tumor thickness and depth of invasion in nodal
involvement and prognosis of oral squamous cell carcinoma: a review
of the literature. Head Neck. 27:1080–1091. 2005. View Article : Google Scholar
|
|
3
|
Myers JN, Elkins T, Roberts D and Byers
RM: Squamous cell carcinoma of the tongue in young adults:
increasing incidence and factors that predict treatment outcomes.
Otolaryngol Head Neck Surg. 122:44–51. 2000. View Article : Google Scholar
|
|
4
|
Chambers I, Colby D, Robertson M, et al:
Functional expression cloning of Nanog, a pluripotency sustaining
factor in embryonic stem cells. Cell. 113:643–655. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mitsui K, Tokuzawa Y, Itoh H, et al: The
homeoprotein Nanog is required for maintenance of pluripotency in
mouse epiblast and ES cells. Cell. 113:631–642. 2003. View Article : Google Scholar
|
|
6
|
Cavaleri F and Schöler HR: Nanog: a new
recruit to the embryonic stem cell orchestra. Cell. 113:551–552.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan G and Thomson JA: Nanog and
transcriptional networks in embryonic stem cell pluripotency. Cell
Res. 17:42–49. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ezeh UI, Turek PJ, Reijo RA and Clark AT:
Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are
expressed in both seminoma and breast carcinoma. Cancer.
104:2255–2265. 2005. View Article : Google Scholar
|
|
9
|
Meng HM, Zheng P, Wang XY, et al:
Overexpression of nanog predicts tumor progression and poor
prognosis in colorectal cancer. Cancer Biol Ther. 9:295–302. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu F, Dai C, Zhang R, Zhao Y, Peng S and
Jia C: Nanog: a potential biomarker for liver metastasis of
colorectal cancer. Dig Dis Sci. 57:2340–2346. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsuoka J, Yashiro M, Sakurai K, et al:
Role of the stemness factor sox2, oct3/4, and nanog in gastric
carcinoma. J Surg Res. 174:130–135. 2012. View Article : Google Scholar
|
|
12
|
Tsai LL, Yu CC, Chang YC, Yu CH and Chou
MY: Markedly increased Oct4 and Nanog expression correlates with
cisplatin resistance in oral squamous cell carcinoma. J Oral Pathol
Med. 40:621–628. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bourguignon LY, Earle C, Wong G, Spevak CC
and Krueger K: Stem cell marker (Nanog) and Stat-3 signaling
promote MicroRNA-21 expression and chemoresistance in
hyaluronan/CD44-activated head and neck squamous cell carcinoma
cells. Oncogene. 31:149–160. 2012. View Article : Google Scholar
|
|
14
|
Chiou SH, Yu CC, Huang CY, et al: Positive
correlations of oct-4 and nanog in oral cancer stem-like cells and
high-grade oral squamous cell carcinoma. Clin Cancer Res.
14:4085–4095. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Q, Shi S, Yen Y, Brown J, Ta JQ and
Le AD: A subpopulation of CD133(+) cancer stem-like cells
characterized in human oral squamous cell carcinoma confer
resistance to chemotherapy. Cancer Lett. 289:151–160. 2010.
|
|
16
|
Sobin LH and Wittekind C: International
Union Against Cancer TNM classification of malignant tumors. 5th
edition. Wiley-Liss Publications; Hoboken, NJ: 1997
|
|
17
|
Dalerba P, Cho RW and Clarke MF: Cancer
Stem Cells: Models and Concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Clarke MF, Dick JE, Dirks PB, et al:
Cancer stem cells - perspectives on current status and future
directions: AACR Workshop on cancer stem cells. Cancer Res.
66:9339–9344. 2006. View Article : Google Scholar
|
|
19
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cell. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Costea DE, Tsinkalovsky O, Vintermyr OK,
Johannessen AC and Mackenzie IC: Cancer stem cells - new and
potentially important targets for the therapy of oral squamous cell
carcinoma. Oral Dis. 12:443–454. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Locke M, Heywood M, Fawell S and Mackenzie
IC: Retention of intrinsic stem cell hierarchies in
carcinoma-derived cell lines. Cancer Res. 65:8944–8950. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Izumiya M, Kabashima A, Higuchi H, et al:
Chemoresistance is associated with cancer stem cell-like properties
and epithelial-to-mesenchymal transition in pancreatic cancer
cells. Anticancer Res. 32:3847–3853. 2012.PubMed/NCBI
|
|
23
|
Moon JH, Kwon S, Jun EK, et al:
Nanog-induced dedifferentiation of p53-deficient mouse astrocytes
into brain cancer stem-like cells. Biochem Biophys Res Commun.
412:175–181. 2011.PubMed/NCBI
|
|
24
|
Chen SL, Iddings DM, Scheri RP and Bilchik
AJ: Lymphatic mapping and sentinel node analysis: current concepts
and applications. CA Cancer J Clin. 56:292–309. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Trivedi NP, Ravindran HK, Sundram S, et
al: Pathologic evaluation of sentinel lymph nodes in oral squamous
cell carcinoma. Head Neck. 32:1437–1443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kayahara H, Okuda M, Terakado N, Shintani
S and Hamakawa H: Non-randomized clinical study comparing
chemotherapy plus radiotherapy with radiotherapy alone in
neoadjuvant therapy for oral cancer. Gan To Kagaku Ryoho.
29:911–916. 2002.(In Japanese).
|