Introduction
Osteosarcoma is the most common type of bone-forming
malignant mesenchymal tumor, commonly present in the extremities of
adolescents and young adults (1).
Advances in chemotherapy and limb salvage surgery have improved the
survival rates since the 1970s, however, drug resistance and lung
metastases remain a great challenge for patients and clinicians
(2).
Epithelial-mesenchymal transition (EMT) is a
biological process in which epithelial cells lose their polarity
and cell-cell adhesion, instead assuming a mesenchymal cell
phenotype to gain increased migratory capacity and invasiveness.
EMT is essential for implantation, embryogenesis and organ
development, and is also involved in tissue regeneration and organ
fibrosis (3). Recent studies have
also shown that EMT is involved in cancer progression and
metastasis, which allows the conversion of early-stage tumors into
invasive malignancies. The loss of epithelial markers and the
upregulation of mesenchymal genes confers the carcinoma cells with
increased cell motility and invasive abilities (4–6).
Previous studies have demonstrated that EMT is
tightly associated with the biology of cancer stem cells (CSCs).
For example, CD44+CD24−/low cells are
considered to be breast CSCs, which exhibit an EMT phenotype as
characterized by the loss of E-cadherin expression and the gain of
vimentin expression (7). TGF-β is a
potential inducer of EMT, which causes the appearance of
CD24− cells from sorted pure CD24+ cell
populations, accompanied by increased mesenchymal marker expression
(8). However, the role of EMT in
mesenchymal-derived sarcomas remains unclear.
Telomerase is a ribonucleoprotein that maintains
integrity in the telomere regions, which shorten following each
replication cycle (9,10). Telomerase is a reverse transcriptase
enzyme composed of a catalytic component, human telomerase reverse
transcriptase (hTERT) and telomerase RNA component (11). Telomerase activity is undetectable
in the majority of human normal somatic cells. However, the
activity of telomerase is maintained in stem/progenitor cells in
self-renewal tissues (12). In
addition, telomerase activation has been observed in ~90% of all
human tumors, as well as in osteosarcoma, indicating that
telomerase plays a key role in cancer development (13–15).
The significance of the role of telomerase in stem
cells and cancer indicates that telomerase is more active in CSCs
and cells that undergo EMT or vice versa. Cancer cells with
stem-like and EMT properties exhibit higher telomerase activity. A
recent study has shown that hTERT promotes cancer metastasis and
recurrence by stimulating EMT and stemness of cancer cells
(16).
The present study separated MG63 cells into two
subgroups according to their telomerase activity and then
investigated the EMT properties of each subgroup. The aim was to
understand EMT in osteosarcoma and the correlation between EMT and
telomerase.
Materials and methods
Chemicals and reagents
RPMI 1640, fetal bovine serum (FBS) and
penicillin-streptomycin were obtained from Invitrogen Life
Technologies (Carlsbad, CA, USA). The lentiviral transcriptional
reporter vector, pGreenFire1-mCMV-EF1-Puro, was purchased from
System Biosciences (Mountain View, CA, USA). The following
antibodies for immunoblot and immunohistochemistry analysis were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA): Mouse monoclonal anti-E-cadherin, mouse monoclonal
anti-pan-cytokeratin, mouse monoclonal anti-vimentin and mouse
monoclonal anti-β-actin. The primary antibodies, rabbit polyclonal
anti-N-cadherin, rabbit polyclonal anti-desmin, rabbit polyclonal
anti-α-smooth muscle actin (SMA), mouse monoclonal anti-Twist1,
rabbit polyclonal anti-Twist2 and rabbit polyclonal anti-Zeb2 were
purchased from Abcam (Cambridge, MA, USA). The primary antibodies
rabbit monoclonal anti-Snail, rabbit monoclonal anti-Slug and
rabbit monoclonal anti-Zeb1 were purchased from Cell Signaling
Technology, Inc.
Cell culture
The human MG63 osteosarcoma cell line was purchased
from the Shanghai Institute for Biological Sciences of the Chinese
Academy of Sciences (Shanghai, China). The cells were cultured in
RPMI 1640 supplemented with 10% (vol/vol) FBS and 1% (vol/vol)
penicillin-streptomycin. The cells were propagated in a humidified
environment at 37ºC with 5% CO2 and 100% humidity. Cell
viability was determined using trypan blue stain (Invitrogen Life
Technologies). For sarcosphere culture, the cells were plated in
ultra-low attachment plates (Corning Inc., Corning, NY, USA) at a
density of 5,000 cells/ml in RPMI 1640 supplemented with B27
supplement (Invitrogen Life Technologies), 10 ng/ml human epidermal
growth factor (EGF; Sigma-Aldrich, St. Louis, MO, USA) and 10 ng/ml
human basic fibroblast growth factor (bFGF; Sigma-Aldrich). Fresh
aliquots of EGF and bFGF were added every other day. Following
culture for 14 days, colonies containing >50 cells were regarded
as sarcospheres and quantitated by inverted phase contrast
microscopy.
Lentiviral-reporter vector construction
and infection
The hTERT promoter region (1.5 kb) (17–19)
was cloned into the multiple cloning site of lentiviral vector,
pGreenFire1-mCMV-EF1-Puro. HEK293 T cells were transfected with the
constructs and then the viral supernatants were obtained. MG63
cells were infected with the viral supernatant and selected using
puromycin (5 μg/ml) for one week to obtain stable reporter
cells.
Flow cytometry
The stable reporter MG63 cells were harvested using
fresh 0.25% trypsin solution and resuspended in phosphate-buffered
saline (PBS). The cells were maintained on ice prior to analysis.
Green fluorescent protein (GFP) expression was assessed and the
cells were sorted into telomerase-positive (TELpos) and
telomerase-negative (TELneg) subpopulations according to their GFP
expression status, using a Becton-Dickinson FACSort (San Jose, CA,
USA).
Telomeric repeat amplification protocol
(TRAP)-ELISA
Telomerase activity was determined using the
TeloTAGGG PCR ELISA PLUS kit (Roche Diagnostics GmbH, Mannheim,
Germany), according to the manufacturer’s instructions. In brief,
the cells were lysed and used for TRAP reaction. PCR products were
then immobilized and detected. The absorbance of the samples was
measured at 450 nm. Heat-treated cell extract was used as the
negative control and a DNA template with the same sequence as a
telomerase product with 8 telomeric repeats was used as a positive
control.
Immunocytofluorescence staining and
microscopy
The sorted cells were seeded on square coverslips in
six-well plates for 24 h to allow them to attach. Subsequently, the
cells were fixed, permeated and blocked. The cells were then
incubated with anti-vimentin antibody (diluted at 1:200) overnight
at 4ºC. Phycoerythrin-labeled secondary antibody (Invitrogen Life
Technologies) was applied for 1 h at room temperature. The cells
were counterstained with DAPI and washed with PBS following each
step of the staining procedure. Coverslips were mounted using
ProLong Gold antifade reagent (Invitrogen Life Technologies).
Images were captured using a Zeiss LSM 510 Meta confocal microscope
(Carl Zeiss AG, Oberkochen, Germany). The long and short axes of
cells were measured using the Zeiss LSM Image Examiner software
(Carl Zeiss AG), and the long/short axis ratio was determined by
counting 100 cells per experiment.
Quantitative PCR
Total RNA was isolated and reverse transcribed.
Quantitative (q)PCR was then performed using an ABI 7900 System
(Applied Biosystems, Inc., Foster City, CA, USA) in the presence of
SYBR Green. The primer sequences used for the PCR are listed in
Table I. Target sequences were
amplified at 95ºC for 10 min, followed by 40 cycles of 95ºC for 15
sec and 60ºC for 1 min. GAPDH was used as an endogenous
normalization control. All assays were performed in triplicate. The
fold change in mRNA expression was determined according to the
2ΔΔCt method.
 | Table IPrimer sequences used for qPCR. |
Table I
Primer sequences used for qPCR.
| Gene | Primer sequence |
|---|
| hTERT |
| Forward |
5′-GGAGCAAGTTGCAAAGCATTG-3′ |
| Reverse |
5′-TCCCACGACGTAGTCCATGTT-3′ |
| Twist1 |
| Forward |
5′-GCAGGACGTGTCCAGCTC-3′ |
| Reverse |
5′-CTGGCTCTTCCTCGCTGTT-3′ |
| Twist2 |
| Forward |
5′-GCAAGAAGTCGAGCGAAGAT-3′ |
| Reverse |
5′-GCTCTGCAGCTCCTCGAA-3′ |
| Snail |
| Forward |
5′-GAGGCGGTGGCAGACTAG-3′ |
| Reverse |
5′-GACACATCGGTCAGACCAG-3′ |
| Slug |
| Forward |
5′-CATGCCTGTCATACCACAAC-3′ |
| Reverse |
5′-GGTGTCAGATGGAGGAGGG-3′ |
| Zeb1 |
| Forward |
5′-CGAGTCAGATGCAGAAAATGAGCAA-3′ |
| Reverse |
5′-ACCCAGACTGCGTCACATGTCTT-3′ |
| Zeb2 |
| Forward |
5′-GGCGCAAACAAGCCAATCCCA-3′ |
| Reverse |
5′-TTCACTGGACCATCTACAGAGGCTT-3′ |
| GAPDH |
| Forward |
5′-GAAGGCTGGGGCTCATTTG-3′ |
| Reverse |
5′-AGGGGCCATCCACAGTCTTC-3′ |
Western blot analysis
Cell lysates were extracted using
radioimmunoprecipitation assay lysis buffer containing protease
inhibitor cocktail (Sigma-Aldrich). Protein concentrations were
determined using the bicinchoninic acid method (Sigma-Aldrich).
Cell lysates containing 40 μg protein were loaded and separated on
10% SDS-PAGE gels and subsequently transferred to polyvinylidene
fluoride membranes (Invitrogen Life Technologies). The membranes
were blocked and incubated at 4ºC overnight with primary antibodies
diluted in 5% (w/v) skimmed milk powder in Tris-Buffered Saline
with Tween 20 (Invitrogen Life Technologies). The membranes were
then washed and incubated with secondary antibody at 1:5,000
dilutions for 1 h at room temperature. The membranes were again
washed and developed using enhanced chemiluminescence substrate
(Sigma-Aldrich).
Wound healing assay
When adherent cells reached 80% confluence, a
scratch was made using a 200-μl pipette tip. The cells were further
incubated for 12 h and photomicrographs were captured under a phase
contrast microscope (Nikon Eclipse TE2000-U; Nikon Instruments Co.,
Ltd., Shanghai, China).
Transwell cell invasion assay
Assays were performed using a Matrigel-coated
Transwell invasion assay plate (Corning Inc.). Assayed cells were
placed in the upper chamber (1×105 cells/well) in
serum-free RPMI 1640. The lower chambers were filled with RPMI 1640
medium with 10% FBS. Upon the termination of the assay (24 h), the
inserts were removed and the inner side was wiped with cotton
swabs. The filters were stained with Harris’s hematoxylin solution
(Sigma-Aldrich) and peeled off following washing and mounting the
slides. The migrated cells were counted under a light microscope
(Nikon Eclipse TE2000-U; Nikon Instruments Co., Ltd.).
Statistical analyses
Each experiment was performed independently a
minimum of three times. Data are presented as the mean ± SD. A
two-tailed Student’s t-test was used to estimate intergroup
differences if not otherwise stated. P<0.05 was considered to
indicate a statistically significant difference.
Results
hTERT promoter reporter divides MG63
cells into two subpopulations
The MG63 cells were transduced with the hTERT
promoter reporter and selected for stably transduced cells. The
expression of GFP was assessed by fluorescence microscopy and flow
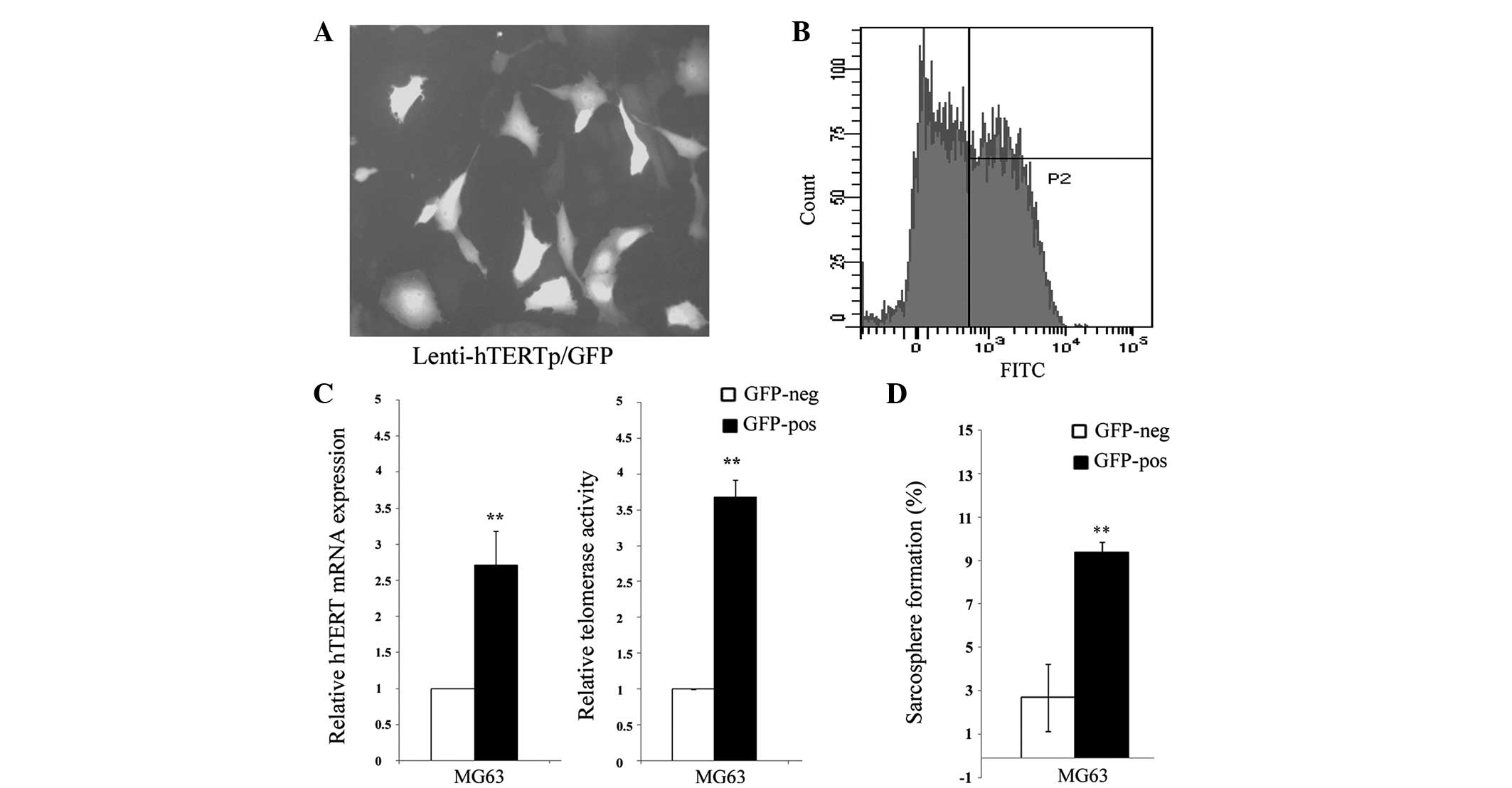
cytometry. The hTERT promoter was found to be heterogeneous among
individual cells (Fig. 1A) and was
sorted into two subpopulations by fluorescence-activated cell
sorting, according to the hTERT promoter activity (Fig. 1B). It was further confirmed that
these GFP-positive cells exhibit a significantly higher expression
of hTERT and telomerase activity (Fig.
1C). Thereafter, the two different cell populations were named
TELpos and TELneg, respectively. In addition, it was found that the
sarcosphere formation capacity in the TELpos MG63 cells was greatly
enhanced compared with the TELneg cells (Fig. 1D).
TELpos cells are similar to
mesenchymal-like cells
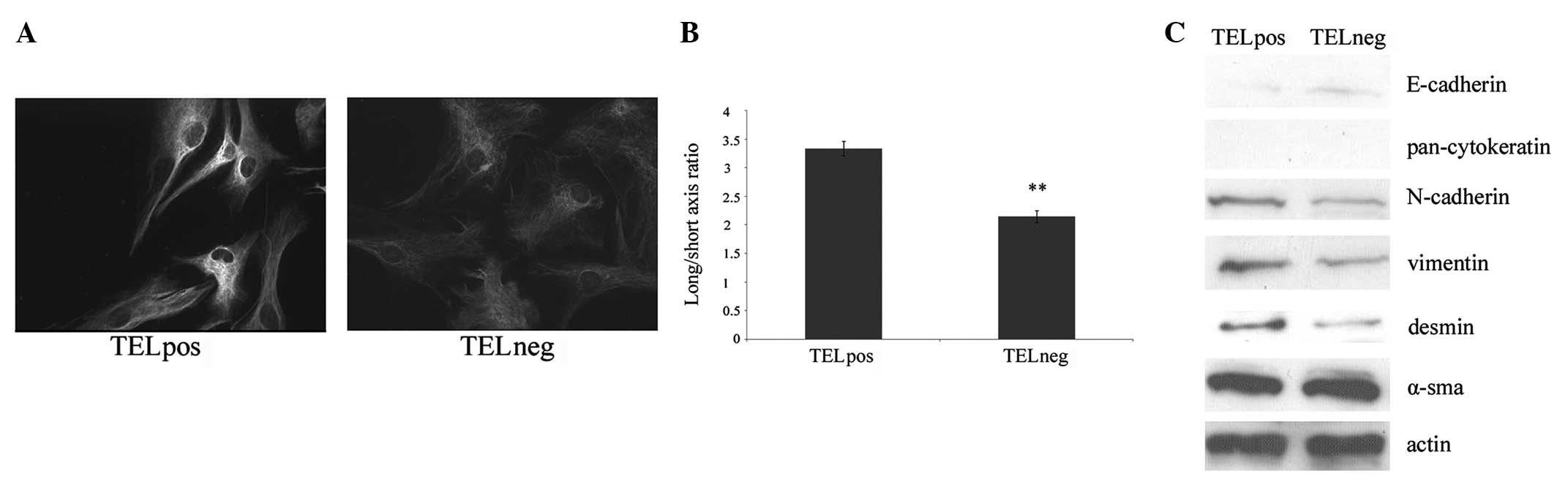
The sorted TELpos and TELneg cells were stained
using vimentin by immunocytochemistry. Vimentin expression was
found to be higher in the TELpos cells compared with the TELneg
cells (Fig. 2A). EMT is often
accompanied by morphological alteration from a rounded phenotype to
a spindle shape. Overall, the MG63 cells were spindle-shaped,
however, round cells were also identified. Therefore, the
long/short axis ratio was evaluated to assess the morphological
differences. The TELpos cells were found to have an average ratio
of 3.34, which is higher than that in the TELneg cells (average
ratio, 2.14; Fig. 2B). In addition,
the expression of common epithelial and mesenchymal markers was
examined by western blot analysis. The epithelial marker,
E-cadherin, was found to be increased in the TELneg cells, while
cytokeratin was not detectable in the two groups.
Mesenchymal-markers, including N-cadherin, vimentin and desmin,
were upregulated in the TELpos cells compared with the TELneg
cells. α-SMA was detected in the two groups, but no significant
difference was found (Fig. 2C).
TELpos cells exhibit increased expression
of EMT driver genes
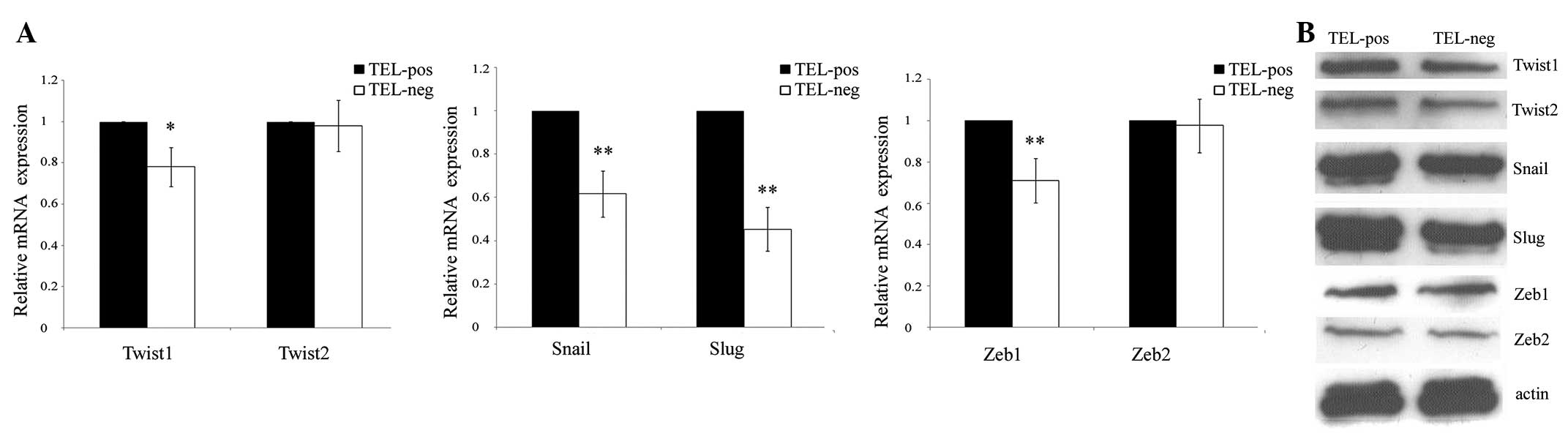
The EMT process is regulated by developmental
transcriptional factors, which repress epithelial marker
expression, but induce the expression of mesenchymal markers. The
correlation between hTERT expression and these transcriptional
factors, including Twist1/2, Snail, Slug and ZEB1/2, was
investigated. In total, 4 out of 6 genes were found to be
significantly upregulated in the TELpos cells by qPCR, in which
Slug exhibited the highest average fold increase of 2.2. Twist2 and
ZEB2 did not show any differences between the two cell groups
(Fig. 3A). These results were
confirmed by western blot analysis (Fig. 3B) and demonstrated that the high
expression of hTERT is a predictor of an EMT-related gene
signature.
TELpos cells are more prone to migration
and invasion
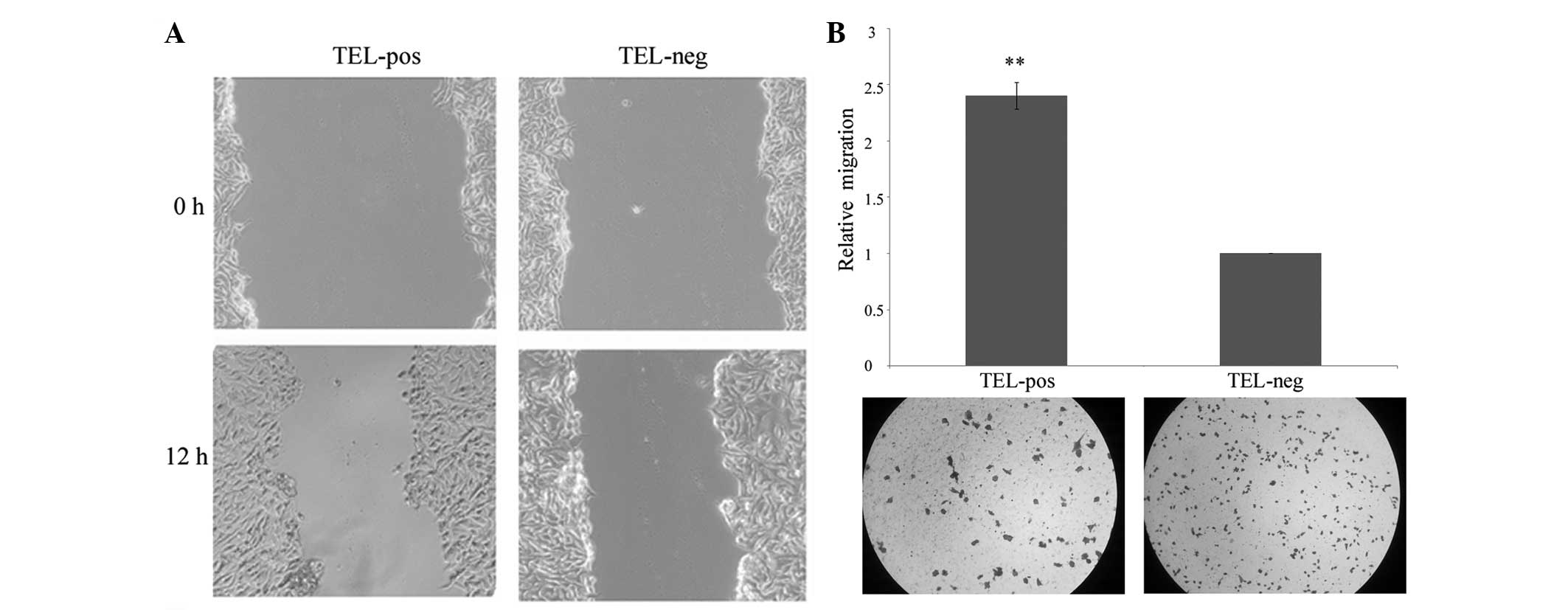
The difference in migratory and invasive capacity
was investigated between the two cell populations. The TELpos cells
exhibited significantly increased cell migration compared with the
TELneg cells (Fig. 4A). The
invasion potential through the Matrigel of the TELpos cells was
also enhanced, with an average fold increase of 2.4 (Fig. 4B). This demonstrated that the high
expression of hTERT correlates with increased cell motility.
Discussion
Mesenchymal-to-epithelial transition (MET) is the
reverse biological process to EMT, and has been shown to inhibit
cancer progression. Silencing of the autocrine motility
factor/phosphoglucose isomerase results in the MET of breast
cancer, human lung fibrosarcoma and osteosarcoma cells, with
reduced malignancy (20–22). However, whether the concept of EMT
also applies to osteosarcoma remains unclear. The present study
provided evidence that there is heterogeneity among osteosarcoma
cells with regard to their EMT status, and demonstrated that hTERT
promoter activity is a predictor for EMT characteristics.
The most commonly used assay for telomerase activity
determination is the TRAP assay. hTERT is the core reverse
transcriptase in telomerase holoenzyme, and the telomerase activity
has been reported to correlate with the expression of hTERT.
Therefore, hTERT expression levels are also used as an equivalent
to cellular telomerase activity (23–26),
however, these are not suitable for single cell analysis. Previous
studies have shown that telomerase activity is not detectable in
approximately two-thirds of osteosarcoma samples. Considering the
evidence that heterogeneity widely exists in the tumor, we assume
that in these TELneg samples, only a minority of the cells
exhibited telomerase activity, which may lead to a false negative
result. In addition, the previously described assays are not
suitable for further functional confirmation. The present study
constructed a lentiviral hTERT promoter-reporter to reflect the
hTERT expression level and therefore, the telomerase activity. The
results confirmed differential telomerase activity by determining
the expression of hTERT and via the TRAP assay. Next, the MG63 cell
population was divided into TELpos and TELneg subgroups for further
experiments.
CSCs are considered to be involved in the initiation
and progression of malignant tumors, and it has been confirmed by a
number of previous studies that CSCs also exist in osteosarcoma.
CSCs not only have the capacity of self-renewal and multipotent
differentiation, but also contribute to metastasis and drug
resistance (27,28). Considering the close connection
between CSC and EMT, the present study tested whether different
telomerase activity levels are associated with stem cell-like
properties. TELpos MG63 cells were found to exhibit a significantly
higher capacity to form sarcospheres in vitro compared with
TELneg cells, which indicated an important role for hTERT in CSC
self-renewal. Consistent with the results of the current study, it
has been previously shown that CSCs exhibit abundant amounts of
telomerase activity and that disruption of telomerase suppresses
the self-renewal of CSCs (29). In
addition, it has been found that, in gastric cancer, hTERT may
regulate the activity of the canonical wnt pathway to modulate
cancer stem-cell activity and EMT properties (16).
Experimentally, the activation of hTERT is a
prerequisite for cellular immortalization and malignant
transformation (12). In clinical
studies, hTERT is expressed in 30% of primary osteosarcoma tumors
and hTERT positivity is associated with tumor recurrence and
decreased overall survival (13).
The most well-defined mechanism for the requirement of telomerase
is that tumor cells require telomerase to maintain telomere length.
In addition, previous studies have shed light on the multiple
biological functions during carcinogenesis independent of
telomere-based activity. hTERT may induce the expression of
vascular endothelial growth factor (30). Furthermore, overexpression of hTERT
in normal stem cells may enhance their mobilization and
proliferation, which is achieved by activation of the canonical wnt
pathway (31). A novel hTERT
function has been reported in gastric cancer, in which ectopically
overexpressed hTERT promotes EMT and stemness, whereas knockdown by
siRNA suppresses EMT and stemness (16). The observations of the current study
also support the hypothesis that hTERT is involved in the process
of the EMT of osteosarcoma. Firstly, TELpos cells exhibit a higher
expression of mesenchymal markers, but a lower expression of
epithelial markers compared with TELneg cells. Secondly, TELpos
cells exhibit an increased long/short axis ratio, which indicates a
mesenchymal phenotype. Thirdly, TELpos cells exhibit a higher
expression of EMT-driver genes, including Snail, Slug, Twist and
ZEB, compared with TELneg cells. Finally, TELpos cells are more
likely to migrate and invade than TELneg cells. Collectively, the
present study results revealed that osteosarcoma expresses a number
of EMT-related genes, and these genes are heterogeneously expressed
among individual cells. In addition, indirect evidence has been
provided indicating that hTERT is involved in regulating the EMT
program, which is different from its ability to maintain telomere
length.
Consequently, therapies targeting telomerase may aid
the elimination of EMT and CSCs, thereby preventing cancer
progression. Future studies are required to evaluate the efficacy
of telomerase inhibitors in the prevention of cancer recurrence,
metastasis and drug resistance.
Acknowledgements
The present study was supported in part by grants
from the Hubei Natural Science Foundation of China (no. 2011CHB039)
and Fundamental Research Funds for the Central Universities (no.
T201230205).
References
|
1
|
Savage SA and Mirabello L: Using
epidemiology and genomics to understand osteosarcoma etiology.
Sarcoma. 2011:5481512011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar
|
|
3
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013.PubMed/NCBI
|
|
5
|
Bastid J: EMT in carcinoma progression and
dissemination: facts, unanswered questions, and clinical
considerations. Cancer Metastasis Rev. 31:277–283. 2012. View Article : Google Scholar
|
|
6
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: an emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
May CD, Sphyris N, Evans KW, Werden SJ,
Guo W and Mani SA: Epithelial-mesenchymal transition and cancer
stem cells: a dangerously dynamic duo in breast cancer progression.
Breast Cancer Res. 13:2022011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morel AP, Lievre M, Thomas C, Hinkal G,
Ansieau S and Puisieux A: Generation of breast cancer stem cells
through epithelial-mesenchymal transition. PLoS One. 3:e28882008.
View Article : Google Scholar
|
|
9
|
Bacchetti S and Counter C: Telomeres and
telomerase in human cancer (review). Int J Oncol. 7:423–432.
1995.PubMed/NCBI
|
|
10
|
Meyerson M: Role of telomerase in normal
and cancer cells. J Clin Oncol. 18:2626–2634. 2000.PubMed/NCBI
|
|
11
|
Gomez DE, Armando RG, Farina HG, et al:
Telomere structure and telomerase in health and disease (review).
Int J Oncol. 41:1561–1569. 2012.PubMed/NCBI
|
|
12
|
Kim NW, Piatyszek MA, Prowse KR, et al:
Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sanders RP, Drissi R, Billups CA, Daw NC,
Valentine MB and Dome JS: Telomerase expression predicts
unfavorable outcome in osteosarcoma. J Clin Oncol. 22:3790–3797.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shay JW and Bacchetti S: A survey of
telomerase activity in human cancer. Eur J Cancer. 33:787–791.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hiyama E, Hiyama K, Yokoyama T and Shay
JW: Immunohistochemical detection of telomerase (hTERT) protein in
human cancer tissues and a subset of cells in normal tissues.
Neoplasia. 3:17–26. 2001. View Article : Google Scholar
|
|
16
|
Liu Z, Li Q, Li K, et al: Telomerase
reverse transcriptase promotes epithelial-mesenchymal transition
and stem cell-like traits in cancer cells. Oncogene. 32:4203–4213.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wick M, Zubov D and Hagen G: Genomic
organization and promoter characterization of the gene encoding the
human telomerase reverse transcriptase (hTERT). Gene. 232:97–106.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takakura M, Kyo S, Kanaya T, et al:
Cloning of human telomerase catalytic subunit (hTERT) gene promoter
and identification of proximal core promoter sequences essential
for transcriptional activation in immortalized and cancer cells.
Cancer Res. 59:551–557. 1999.
|
|
19
|
Cong YS, Wen J and Bacchetti S: The human
telomerase catalytic subunit hTERT: organization of the gene and
characterization of the promoter. Hum Mol Genet. 8:137–142. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Niinaka Y, Harada K, Fujimuro M, et al:
Silencing of autocrine motility factor induces
mesenchymal-to-epithelial transition and suppression of
osteosarcoma pulmonary metastasis. Cancer Res. 70:9483–9493. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Funasaka T, Hu H, Yanagawa T, Hogan V and
Raz A: Down-regulation of phosphoglucose isomerase/autocrine
motility factor results in mesenchymal-to-epithelial transition of
human lung fibrosarcoma cells. Cancer Res. 67:4236–4243. 2007.
View Article : Google Scholar
|
|
22
|
Funasaka T, Hogan V and Raz A:
Phosphoglucose isomerase/autocrine motility factor mediates
epithelial and mesenchymal phenotype conversions in breast cancer.
Cancer Res. 69:5349–5356. 2009. View Article : Google Scholar
|
|
23
|
Kirkpatrick KL, Clark G, Ghilchick M,
Newbold RF and Mokbel K: hTERT mRNA expression correlates with
telomerase activity in human breast cancer. Eur J Surg Oncol.
29:321–326. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saretzki G, Petersen S, Petersen I, Kolble
K and von Zglinicki T: hTERT gene dosage correlates with telomerase
activity in human lung cancer cell lines. Cancer Lett. 176:81–91.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ito H, Kyo S, Kanaya T, Takakura M, Inoue
M and Namiki M: Expression of human telomerase subunits and
correlation with telomerase activity in urothelial cancer. Clin
Cancer Res. 4:1603–1608. 1998.PubMed/NCBI
|
|
26
|
Shigeishi H, Sugiyama M, Tahara H, et al:
Increased telomerase activity and hTERT expression in human
salivary gland carcinomas. Oncol Lett. 2:845–850. 2011.
|
|
27
|
Gibbs CP Jr, Levings PP and Ghivizzani SC:
Evidence for the osteosarcoma stem cell. Curr Orthop Pract.
22:322–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Siclari VA and Qin L: Targeting the
osteosarcoma cancer stem cell. J Orthop Surg Res. 5:782010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marian CO, Cho SK, McEllin BM, et al: The
telomerase antagonist, imetelstat, efficiently targets glioblastoma
tumor-initiating cells leading to decreased proliferation and tumor
growth. Clin Cancer Res. 16:154–163. 2010. View Article : Google Scholar
|
|
30
|
Kirkpatrick KL, Newbold RF and Mokbel K:
The mRNA expression of hTERT in human breast carcinomas correlates
with VEGF expression. J Carcinog. 3:12004. View Article : Google Scholar
|
|
31
|
Park JI, Venteicher AS, Hong JY, et al:
Telomerase modulates Wnt signalling by association with target gene
chromatin. Nature. 460:66–72. 2009. View Article : Google Scholar : PubMed/NCBI
|