Introduction
Gastric cancer is one of the most common
malignancies in China and is a leading cause of cancer-related
mortality worldwide (1–3). Previous epidemiological studies have
shown that the average age of disease onset in females is usually
delayed by ~15 years compared with that in males. However, the
incidence following menopause in females is close to that in males
(4). Hormonal factors associated
with a greater exposure to estrogen and/or progesterone may be
associated with the decreased risk of gastric cancer (5). From a previous male cohort study of
patients with prostate cancer in Sweden, estrogen exposure resulted
in a decrease in the risk of gastric cancer (6). These observations suggested that
estrogen is pivotal in gastric cancer. However, the mechanism of
estrogen signaling in gastric carcinogenesis has not been well
established.
The endoplasmic reticulum is an organelle
responsible for protein folding and assembly, lipid and sterol
biosynthesis and free calcium storage. A number of biochemical,
physiological and pathological stimuli, such as those that cause
endoplasmic reticulum calcium depletion, altered glycosylation,
nutrient deprivation, oxidative stress or hypoxia, lead to
endoplasmic reticulum stress, in which numerous rescue responses,
including unfolded protein response (UPR), are triggered. To adapt
to stress conditions, the concerted action of three endoplasmic
reticulum transmembrane proteins, protein kinase RNA-like
endoplasmic reticulum kinase (PERK), inositol-requiring enzyme-1
(IRE1) and activating transcription factor (ATF) 6, are activated
and protect cells by an initial decrease in general protein
synthesis, promotion of protein folding via the induction of
chaperones [such as glucose-regulated protein 78 (GRP78)] and
prevention of accumulating misfolded proteins. However, if the
stress is severe or prolonged, distinct death signals may be
transduced during the UPR and cells undergo apoptosis (7,8).
Several previous studies have shown that in various tumors, such as
gastric cancer, whose cells experience increasing nutrient
starvation and hypoxia, endoplasmic reticulum stress is highly
induced and closely associated with cancer cell death mediated by
ATF4 and C/EBP homologous protein (CHOP) (9,10).
Furthermore, previous studies have found that
apoptosis induced by endoplasmic reticulum stress may be affected
by the estrogen signaling pathway. Estrogen may induce GRP78, which
has been found to correlate with cell viability and resistance to
paclitaxel and cisplatin in endometrial cancer (11). Administration of a small volume of
17β-estradiol (E2)prolonged the survival of rats by 3 h by
ameliorating endoplasmic reticulum stress (12). Isoflavones, that have a structure
similar to that of E2 and are capable of binding to estrogen
receptors with seven to eight times less binding affinity to
estrogen receptor (ER) α than to ER β, protected SH-SY5Y cells from
cell death by suppressing endoplasmic reticulum stress. This was
determined by decreased expression of GRP78 mRNA, spliced X-box
binding protein-1 mRNAs and CHOP (13). These findings suggested that low
concentrations of estrogen may protect cancer cells from apoptosis
induced by endoplasmic reticulum stress. In addition, they provide
marked explanations for previous epidemiological observations that
the incidence of gastric cancer following menopause in females
increases and is close to that in males, while estrogen exposure
results in a decrease in the risk of gastric cancer. However, the
molecular mechanism by which estrogen at low concentrations
protects gastric cancer cells from endoplasmic reticulum
stress-induced cell death remains unclear.
Therefore, the present study treated SGC7901 cells
with tunicamycin (TM), which is well known to induce endoplasmic
reticulum stress by inhibiting N-linked protein glycosylation
(14,15). Cells were then treated with TM plus
E2 at a nanomolar concentration (10−9 M). The
endoplasmic reticulum stress induced by TM was found to result in
apoptosis with the inhibition of Akt. In addition, the simultaneous
treatment of E2 with TM may protect SGC7901 cells from endoplasmic
reticulum stress-induced apoptosis by the Akt pathway.
Materials and methods
Antibodies and chemicals
The 17β-estradiol (E2) was purchased from
Sigma-Aldrich (St. Louis, MO, USA), rabbit anti-GRP78 was purchased
from Abcam (Cambridge, UK) and pAb against phospho-Akt at Ser473
(Ser473-Akt) was obtained from Cell Signaling Technology, Inc.
(Danvers, MA, USA). pPERK (Thr 981) and anti-β-actin (C4)
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). TM was purchased from Alexis Biochemical
Corp. (San Diego, CA, USA), dissolved in DMSO at a concentration of
3 mM and stored at −20°C. Bicinchoninic acid protein detection kit,
goat anti-mouse peroxidase-conjugated secondary antibody,
chemiluminescent substrate kit and polyvinylidene difluoride (PVDF)
membranes were purchased from Pierce Biotechnology, Inc. (Rockford,
IL, USA). RIPA buffer and enhanced chemiluminescence reagents were
purchased from Beyotime Institute of Biotechnology (Haimen,
China).
Cell culture and treatment
The human gastric adenocarcinoma cell line, SGC7901,
was obtained from the Cell Center of Basic Medicine, Chinese
Academy of Medical Sciences (Beijing, China). Cells were cultured
in RPMI-1640 containing 10% fetal calf serum, at 37°C in a 5%
CO2 atmosphere. To study the effect of TM on endoplasmic
reticulum stress, the cells were treated with TM at various
concentrations. In addition, to explore the protective potential of
E2 in endoplasmic reticulum stress-induced apoptosis, various
concentrations of E2 were administered in the TM-treated cells and
the same concentrations of DMSO and alcohol were used as vehicle
control.
Cellular viability using WST-1 test
Viability of SGC7901 cells treated with TM and
cotreated with TM plus E2 was measured using a WST-1 cell counting
kit (Beyotime Institute of Biotechnology) according to the
manufacturer’s instructions. SGC7901 cells were seeded in 96-well
culture plates in the media for 48 h and treated with various
concentrations of TM, with and without various concentrations of
E2, for 48 h. Corresponding controls with analogous concentrations
of DMSO and alcohol were performed in parallel. The cells were then
incubated with WST-1 reagent for 1 h at 37°C. The absorbance at 450
nm was monitored and the reference wavelength was set at 630 nm.
The percentage viability of cells was calculated by comparison with
that of control cells (16).
Western blot analysis
Western blot analysis was performed according to the
methods previously established (17,18)
and cultured cells were directly lysed with a RIPA buffer. The
protein concentration was measured using the bicinchoninic acid kit
according to the manufacturer’s instructions. Next, proteins were
separated by 10% SDS-polyacrylamide gel electrophoresis and
transferred to PVDF membranes. The membranes were blocked with 5%
non-fat milk dissolved in TBS Tween-20 [50 mM Tris-HCl (pH 7.6),
150 mM NaCl and 0.2% Tween-20] for 1 h and probed with primary
antibodies at 4°C overnight. The blots were then incubated with
anti-mouse or -rabbit IgG conjugated to horseradish peroxidase
(1:5,000) for 1 h at 37°C. The blots were visualized with enhanced
chemiluminescence and quantitatively analyzed using the TotalLab
analysis software (Nonlinear USA Inc., Durham, NC, USA).
Statistical analysis
Data are expressed as means ± standard deviation and
were analyzed using SPSS 12.0 statistical software (SPSS Inc.,
Chicago, IL, USA). The one-way analysis of variance procedure
followed by the least significant difference post hoc test were
used to analyze the differences among groups.
Results
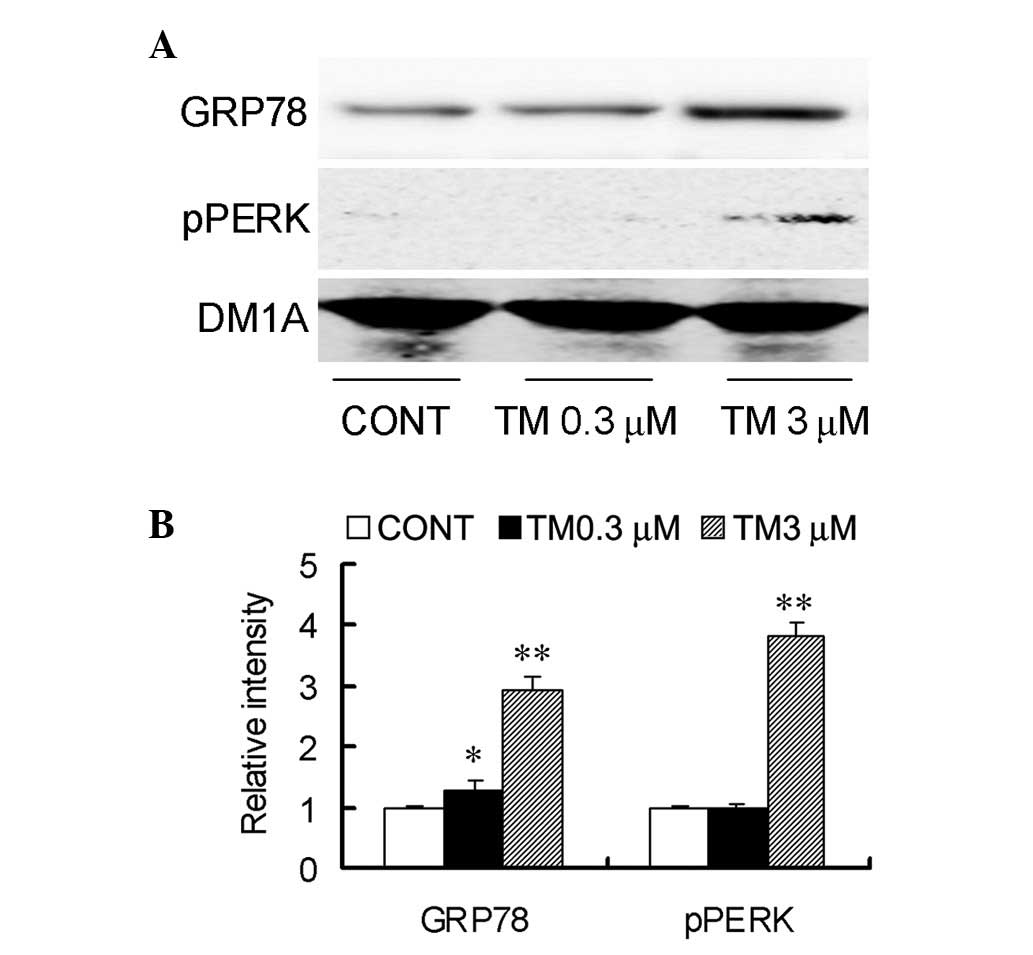
TM induces endoplasmic reticulum stress
in SGC7901 cells
To produce an endoplasmic reticulum stress model
in vitro, SGC7901 cells were treated with TM at
concentrations of 0.3 and 3 μM for 24 h. Next, the expression of
GRP78 and pPERK, the ER stress markers, were detected. Treatment
with the two concentrations of TM were found to increase the
protein levels of GRP78, whereas the increased levels of pPERK were
only detected at 3 μM (Fig. 1).
These results confirmed the in vitro induction of
endoplasmic reticulum stress by TM.
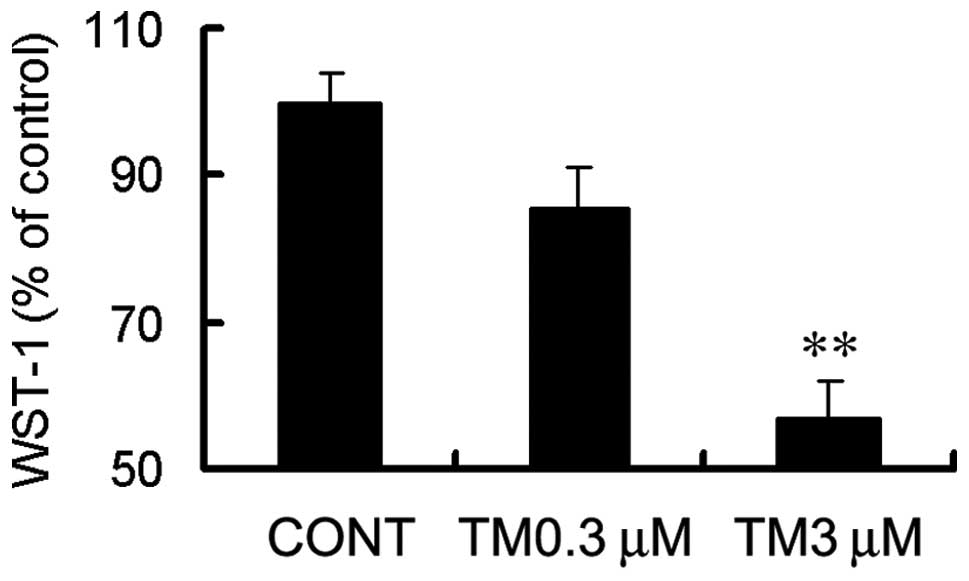
Endoplasmic reticulum stress induced by
TM results in apoptosis
TM is a nucleoside antibiotic that leads to
apoptosis, by inhibiting the N-glycosylation of target asparagine
residues in the luminal domains of proteins (19). Using a viability assay (WST-1 test),
TM treatment at a concentration of 3 μM was found to result in
evident cytotoxicity (Fig. 2) and 3
μM TM treatment for 24 h increased the production of 17- and 19-kDa
activated caspase-3 (Fig. 4).
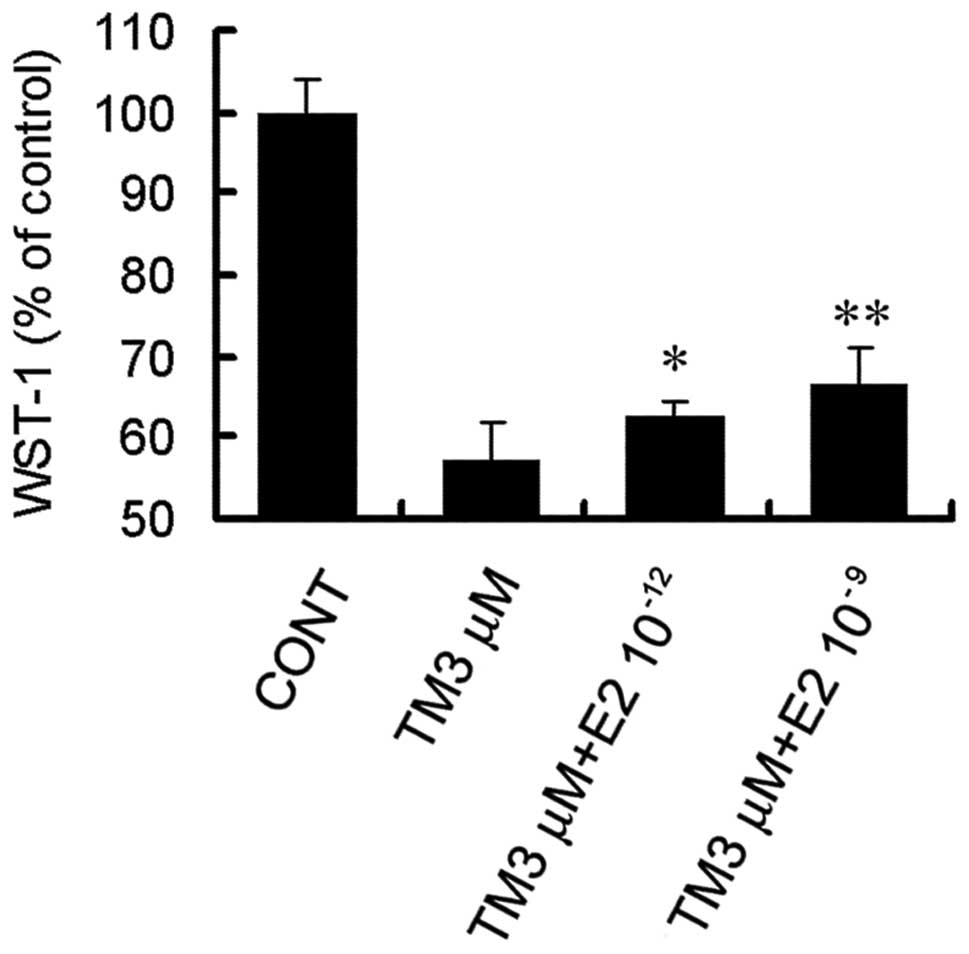
E2 protects SCG7901 cells against
apoptosis induced by endoplasmic reticulum stress
To determine the effect of E2 on endoplasmic
reticulum stress-induced cytotoxicity, cells were cotreated with 3
μM TM and various concentrations of E2 for 48 h. E2 significantly
attenuated cytotoxicity at the two concentrations of
10−12 and 10−9 M (Fig. 3). E2 at 10−9 M was found
to have a significant effect and was selected for further
experiments.
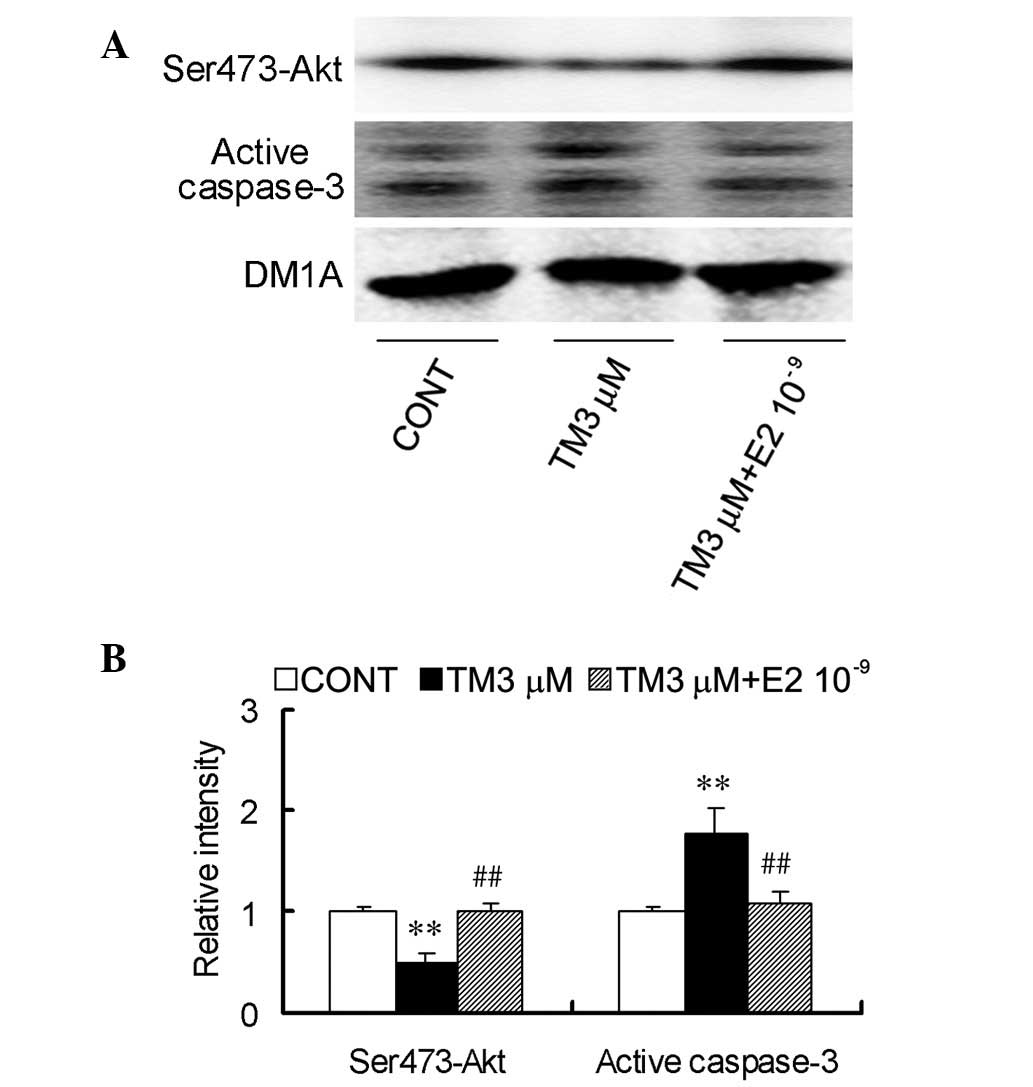
To further confirm the protective effect of E2 on
endoplasmic reticulum stress-induced apoptosis, protein levels of
the cleavage of procaspase-3 to active caspase-3 fragments were
measured. The production of 17- and 19-kDa activated caspase-3 was
found to decrease following cotreatment with 3 μM TM and
10−9 M E2 for 24 h (Fig.
4).
E2 protects SGC7901 cells from
endoplasmic reticulum stress-induced apoptosis by the Akt
pathway
Akt, a serine/threonine protein kinase that
regulates the balance between cell survival and apoptosis, has been
previously reported to be involved in endoplasmic reticulum
stress-induced apoptosis. It was tested whether TM affects the
activation-associated phosphorylation of Ser473 of Akt and whether
E2 protects SGC7901 cells from endoplasmic reticulum stress-induced
apoptosis by the Akt pathway. Treatment with 3 μM TM was found to
greatly decrease Ser473-Akt immunoreactivity (Fig. 4) and cotreatment with
10−9 M E2 counteracted the inhibitory effect of TM on
Akt, causing an increase in Ser473-Akt (Fig. 4).
Overall, these results indicated that E2 is able to
counteract endoplasmic reticulum stress-induced inactivation of Akt
to block signaling to caspase-3.
Discussion
In the present study, the induction of endoplasmic
reticulum stress by treatment with TM was found to induce apoptosis
with the inhibition of Akt. Simultaneous treatment of
10−9 M E2 with TM was found to arrest endoplasmic
reticulum stress-induced apoptosis by counteracting the inhibitory
effect of TM on Akt, causing an increase in phospho-Ser473-Akt. It
was concluded that low concentrations of E2 are able to counteract
endoplasmic reticulum stress-induced apoptosis by Akt pathway.
Endoplasmic reticulum stress and UPR are highly
induced in various tumor types, such as gastric cancer, whose cells
possess rapid glucose metabolism and fast growth rate, which lead
to poor vascularization of tumor mass, low oxygen supply, nutrient
deprivation, pH changes and express mutant proteins that do not
fold correctly. The primary role of the UPR is to provide survival
signaling pathways required for tumor growth by dissociating GRP78,
which regulates the protein folding process, from three endoplasmic
reticulum stress sensors (including PERK, IRE1α and ATF6) that are
consequently phosphorylated and activated. However, if the attempt
to recover from endoplasmic reticulum stress fails, UPR induces
cell death programs to eliminate the stressed cells (14). It has been previously reported that
the PERK-mediated α-subunit of eukaryotic translation initiation
factor (eIF2α) phosphorylation, which contributes to the
attenuation of translation to alleviate stress damage in the early
stage, may selectively initiate the translation of ATF4 mRNA. This
subsequently activates the expression of genes involved in
endoplasmic reticulum stress-associated apoptosis (9,20).
GADD153/CHOP, downstream of the PERK/eIF2α pathway, mediates
endoplasmic reticulum stress-induced apoptosis (10). In the current study, endoplasmic
reticulum stress induced by TM was shown to result in
caspase-3-mediated apoptosis in SGC7901 cells.
Gastric tumor has been generally considered as a
non-estrogen related tumor. However, a growing number of previous
epidemiological observations have shown that the ratio between the
male and female incidence of gastric cancer is between 2:1 and 3:1.
This difference disappears in females following menopause, and in
addition to estrogen levels, this difference is difficult to
explain with any one of the other known risk factors (21). Oral contraceptives and estrogen
replacement therapy reduce the incidence of gastric cancer
(22). The incidence of gastric
cancer has been found to increase in females receiving oophorectomy
and to decrease in females receiving estrogen replacement therapy
(5). The incidence of gastric
cancer in males with prostate cancer administered E2 treatment is
considerably lower than that in males who have not received
treatment (6). In our previous
study, estrogen at high concentrations inhibited the growth of
gastric cancer cells, while at low concentrations promoted cell
growth (data not published). This highlighted marked explanations
to the epidemiological mystery that the incidence of gastric cancer
in males has been considerably higher than that in females. In
addition, estrogen may induce GRP78, which has been found to
correlate with cell viability and resistance to paclitaxel and
cisplatin in endometrial cancer (11). Administration of a small volume of
E2, prolonged the survival of rats by 3 h by ameliorating
endoplasmic reticulum stress (12).
Isoflavones, which have a structure similar to that of E2, may
protect SH-SY5Y cells from cell death by suppressing endoplasmic
reticulum stress. In the current study, 10−9 M E2 was
found to counteract endoplasmic reticulum stress-induced
apoptosis.
Akt, also known as protein kinase B, is a
serine/threonine protein kinase that has been shown to regulate the
balance between cell survival and apoptosis (23,24).
Misregulation of the Akt signaling pathway has been found to play a
central role in tumorigenesis. Activation of Akt tips the balance
of cells into prosurvival pathways, which is often found to
correlate with tumor progression by directly phosphorylating and
inactivating proteins, including Bad and procaspase 9 (25,26).
However, reduced activity of Akt tips the balance toward apoptosis.
It has been previously reported that endoplasmic reticulum
stress-induced apoptosis is associated with a reduction in
phospho-Akt (27,28). In addition, estrogen activates the
PI3K-Akt pathway through ER α- and ER β-independent mechanisms in
breast cancer (29,30). In the present study, the
dephosphorylation of Akt at Ser473 was found to be involved in
apoptosis induced by endoplasmic reticulum stress. In addition,
simultaneous treatment with E2 at a concentration of
10−9 M may counteract endoplasmic reticulum
stress-induced apoptosis by the Akt pathway.
Acknowledgements
The current study was supported in part by grants
from the National Natural Science Foundation of China (no. 30870981
and 81272754), the Natural Science Foundation of Hubei Province
(no. 2013CFB215) and the Jianghan University Doctor Foundation (no.
2010023).
References
|
1
|
Brenner H, Rothenbacher D and Arndt V:
Epidemiology of stomach cancer. Methods Mol Biol. 472:467–477.
2009. View Article : Google Scholar
|
|
2
|
Lu J, Huang CM, Zheng CH, et al: Analysis
on the clinical and pathological features and prognosis of familial
gastric cancer in South china population: a single-center study of
724 patients. J Oncol. 2012:6412182012.
|
|
3
|
Herszényi L and Tulassay Z: Epidemiology
of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci.
14:249–258. 2010.
|
|
4
|
Sipponen P and Correa P: Delayed rise in
incidence of gastric cancer in females results in unique sex ratio
(M/F) pattern: etiologic hypothesis. Gastric Cancer. 5:213–219.
2002. View Article : Google Scholar
|
|
5
|
Frise S, Kreiger N, Gallinger S, Tomlinson
G and Cotterchio M: Menstrual and reproductive risk factors and
risk for gastric adenocarcinoma in women: findings from the
canadian national enhanced cancer surveillance system. Ann
Epidemiol. 16:908–916. 2006. View Article : Google Scholar
|
|
6
|
Lindblad M, Ye W, Rubio C and Lagergren J:
Estrogen and risk of gastric cancer: a protective effect in a
nationwide cohort study of patients with prostate cancer in Sweden.
Cancer Epidemiol Biomarkers Prev. 13:2203–2207. 2004.PubMed/NCBI
|
|
7
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gardner BM, Pincus D, Gotthardt K,
Gallagher CM and Walter P: Endoplasmic reticulum stress sensing in
the unfolded protein response. Cold Spring Harb Perspect Biol.
5:a0131692013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Scheuner D, Song B, McEwen E, et al:
Translational control is required for the unfolded protein response
and in vivo glucose homeostasis. Mol Cell. 7:1165–1176. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang K and Kaufman RJ: Identification and
characterization of endoplasmic reticulum stress-induced apoptosis
in vivo. Methods Enzymol. 442:395–419. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luvsandagva B, Nakamura K, Kitahara Y, et
al: GRP78 induced by estrogen plays a role in the chemosensitivity
of endometrial cancer. Gynecol Oncol. 126:132–139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kozlov AV, Duvigneau JC, Hyatt TC, et al:
Effect of estrogen on mitochondrial function and intracellular
stress markers in rat liver and kidney following trauma-hemorrhagic
shock and prolonged hypotension. Mol Med. 16:254–261. 2010.
View Article : Google Scholar
|
|
13
|
Park YJ, Jang YM and Kwon YH: Isoflavones
prevent endoplasmic reticulum stress-mediated neuronal degeneration
by inhibiting tau hyperphosphorylation in SH-SY5Y cells. J Med
Food. 12:528–535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zinszner H, Kuroda M, Wang X, et al: CHOP
is implicated in programmed cell death in response to impaired
function of the endoplasmic reticulum. Genes Dev. 12:982–995. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu ZC, Fu ZQ, Song J, et al: Bip enhanced
the association of GSK-3beta with tau during ER stress both in vivo
and in vitro. J Alzheimers Dis. 29:727–740. 2012.PubMed/NCBI
|
|
16
|
Delhanty PJ, van Koetsveld PM, Gauna C, et
al: Ghrelin and its unacylated isoform stimulate the growth of
adrenocortical tumor cells via an anti-apoptotic pathway. Am J
Physiol Endocrinol Metab. 293:E302–E309. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu ZQ, Yang Y, Song J, et al: LiCl
attenuates thapsigargin-induced tau hyperphosphorylation by
inhibiting GSK-3beta in vivo and in vitro. J Alzheimers Dis.
21:1107–1117. 2010.PubMed/NCBI
|
|
18
|
Deng H, Zhen H, Fu Z, Huang X, Zhou H and
Liu L: The antagonistic effect between STAT1 and Survivin and its
clinical significance in gastric cancer. Oncol Lett. 3:193–199.
2012.PubMed/NCBI
|
|
19
|
Parodi AJ: Role of N-oligosaccharide
endoplasmic reticulum processing reactions in glycoprotein folding
and degradation. Biochem J 348 Pt. 1:1–13. 2000. View Article : Google Scholar
|
|
20
|
Harding HP, Zhang Y, Zeng H, et al: An
integrated stress response regulates amino acid metabolism and
resistance to oxidative stress. Mol Cell. 11:619–633. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chandanos E and Lagergren J: Oestrogen and
the enigmatic male predominance of gastric cancer. Eur J Cancer.
44:2397–2403. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fernandez E, Gallus S, Bosetti C,
Franceschi S, Negri E and La Vecchia C: Hormone replacement therapy
and cancer risk: a systematic analysis from a network of
case-control studies. Int J Cancer. 105:408–412. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheung M and Testa JR: Diverse Mechanisms
of AKT Pathway Activation in Human Malignancy. Curr Cancer Drug
Targets. 13:234–244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang X, Tang N, Hadden TJ and Rishi AK:
Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta.
1813:1978–1986. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cardone MH, Roy N, Stennicke HR, et al:
Regulation of cell death protease caspase-9 by phosphorylation.
Science. 282:1318–1321. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Datta SR, Dudek H, Tao X, et al: Akt
phosphorylation of BAD couples survival signals to the
cell-intrinsic death machinery. Cell. 91:231–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu L, Pang Y, He DW and Liu XW: Effects
of propofol on PI3K/Akt signaling pathway and endoplasmic reticulum
stress pathway of apoptosis induced by ischemia-reperfusion in
isolated rat hearts. Zhonghua Yi Xue Za Zhi. 92:2611–2614. 2012.(In
Chinese).
|
|
28
|
Song L, De Sarno P and Jope RS: Central
role of glycogen synthase kinase-3beta in endoplasmic reticulum
stress-induced caspase-3 activation. J Biol Chem. 277:44701–44708.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Yi L, Zhu Y, Zou J, Hong Y and
Zheng W: AKT signaling pathway in invasive ductal carcinoma of the
breast: correlation with ERa, ERbeta and HER-2 expression. Tumori.
97:185–190. 2011.PubMed/NCBI
|
|
30
|
Tsai EM, Wang SC, Lee JN and Hung MC: Akt
activation by estrogen in estrogen receptor-negative breast cancer
cells. Cancer Res. 61:8390–8392. 2001.PubMed/NCBI
|