Introduction
The incidence of adenocarcinomas of the distal
esophagus and esophagogastric junction (AEG) is rising notably in
Western populations with >480,000 new cases diagnosed annually,
accounting for 400,000 mortalities per year (1,2).
Despite adequate preoperative staging and improvements in
perioperative treatment, the overall prognosis remains poor, with a
five-year-survival rate of ~40%. In addition, <30% of patients
exhibit potentially operable tumors and the majority of patients
already have locally advanced tumor stages with involvement of
locoregional lymph nodes on presentation (3). For patients undergoing surgery
following neoadjuvant therapy [chemoradiotherapy (CRT) or
chemotherapy (CT) alone], three-year survival rates vary between 22
and 55% (4–11). To improve long-term survival rates,
multimodal treatment strategies, including preoperative CT and
neoadjuvant CRT, for locally advanced AEGs have been considered and
investigated widely. In an updated meta-analysis, Sjoquist et
al summarized the results of multimodal treatment strategies
indicating a trend in favor of neoadjuvant CRT (12). In addition, a current phase III
trial from the Netherlands has confirmed the feasibility and
superiority of a neoadjuvant CR regimen compared with surgery alone
(10). However, a standard of care
for patients with AEG tumors has not yet been defined. Recently,
the EORTC expert panel voted in favor of preoperative CRT for AEG I
and II tumors and recommended perioperative CT for AEG III tumors
(13).
The current study presents a retrospective analysis
of a single center experience with preoperative CRT or
perioperative CT in addition to surgery in locally advanced but
resectable AEG. The aim of the study was to identify the advantages
and potential disadvantages of the two treatment regimens. Patients
who were treated either with perioperative CT or neoadjuvant CRT
between the years 2006 and 2012 at the Johann Wolfgang Goethe
University Hospital (Frankfurt, Germany) were included in the
analysis. Major surgical and non-surgical complications were
evaluated and a Kaplan-Meier survival analysis was performed to
compare the overall and progression-free survival-estimates between
the two groups.
Patients and methods
Patients and treatment
A retrospective analysis was performed of patients
with advanced but resectable AEG, treated in neoadjuvant intention
with neoadjuvant CRT or perioperative CT between January 2006 and
October 2012. Patients were allocated to the two treatment regimens
almost equally by the consensus decision of a multidisciplinary
tumor board of the Johann Wolfgang Goethe University Hospital. In
total, 29 patients were identified who received identical CRT or CT
schedules. Patients with different treatment regimens or who were
participants in clinical trials were excluded from the analysis to
achieve a homogeneous study population. The study was approved by
the ethics committee of the Johann Wolfgang Goethe University
Hospital. Written informed consent was obtained from the
patients.
Preoperative staging
Initial staging included endoscopy of the upper
gastrointestinal tract with multiple biopsies, computed tomography
scan of the thorax and abdomen and an endoscopic ultrasound. Other
diagnostics, including positron emission tomography/computed
tomography or diagnostic laparotomy, were optional. Physical
examination and laboratory testing were routinely performed in all
patients.
CRT group
The CRT group included 16 patients. Radiation
therapy planning was based on three-dimensional computed tomography
scans of the chest and upper abdomen with a resolution of 3-mm
slice reconstructions. The planning target volume was delineated as
the macroscopic gross tumor volume plus the safety margins of 15 mm
in the circumferential, 30 mm in the oral and 50 mm in the aboral
extension. Patients received a median cumulative dose of 45.0 Gy
(range, 45.0–50.4 Gy) in single fractions of 1.8 Gy/day. In
addition, patients received two cycles of cisplatin and
5-Fluorouracil (5-FU) in the first and fifth week of radiotherapy.
Cisplatin was administered at a dose of 20 mg/m2 from
day one to five of each cycle. 5-FU was administered at a dose of
600 mg/m2 as a continuous infusion from day one to five
of each cycle.
CT group
The CT group included 13 patients who received a
maximum of six three-week cycles of epirubicin, cisplatin and
capecitabine (three cycles preoperatively and postoperatively). On
day one of every three-week cycle, 50 mg/m2 epirubicin
was administered to each patient, followed by the administration of
60 mg/m2 cisplatin. Between days one and 14, 1,000
mg/m2 capecitabine was orally administered twice daily.
Following surgical resection, patients underwent adjuvant treatment
with the same CT regimen.
Surgery and follow-up
Surgery in the two groups was performed between four
and six weeks following preoperative treatment. Extended
gastrectomy and distal esophagectomy, with Roux-en-Y
esophagojejunostomy and two-field D2-lymph node dissection, was
performed in patients with AEG II/III. Transthoracic esophagectomy
and proximal gastrectomy with en bloc removal of the
esophagus and adjacent lymph nodes were performed in patients with
AEG I. Patients were first seen for physical examination six to
eight weeks following the termination of therapy. Technical
examination (esophagogastroduodenoscopy or computed tomography) was
performed at the discretion of the attending physician. For
follow-up, patients were observed every three months in the first
year and every six months in the following years. Medical reports
and information from the attending physicians were also taken into
account for analysis.
Statistical analysis
Data were analyzed and compiled using BiAS software
for Windows (version 9.11; Epsilon-Verlag, Darmstadt, Germany),
SPSS version 20 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5
for Windows (version 5; GraphPad Software Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference. Follow-up time was defined as the time between
initiation of preoperative therapy and mortality or final contact.
The primary endpoint of the study was overall survival (OS),
calculated between initiation of preoperative therapy and
mortality. Progression-free survival (PFS) was calculated between
the initiation of neoadjuvant treatment and reported initial
reaction (defined as locoregional relapse or distant metastases) or
mortality.
Acute hematological side effects were recorded
according to Common Toxicity Criteria, version 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm).
For TNM staging, the current TNM classification at diagnosis was
used respecting the 2009 revision of TNM classification for
esophageal cancer (www.uicc.org).
Results
Patient follow-up
Median follow-up was 25.5 months (range, 6.0–73.3
months) in the CRT group versus 22.0 months in the CT group (range,
5.3–64.7 months). Patients and tumor characteristics are shown in
Table I.
 | Table IPatient and tumor characteristics. |
Table I
Patient and tumor characteristics.
| Characteristics |
Chemoradiotherapy | Chemotherapy |
|---|
| Patients, n | 16 (100.0) | 13 (100.0) |
| Gender |
| Male | 13 (81.0) | 12 (92.0) |
| Female | 3 (19.0) | 1 (8.0) |
| Age, years |
| Median (range) | 63.8 (36.4–77.6) | 58.8 (29.1–79.1) |
| <50 | 2 (13.0) | 4 (31.0) |
| 51–60 | 4 (25.0) | 3 (23.0) |
| 61–70 | 5 (31.0) | 2 (15.0) |
| >70 | 5 (31.0) | 4 (31.0) |
| Tumor sitea |
| AEG I | 11 (69.0) | 8 (62.0) |
| AEG II | 3 (19.0) | 3 (23.0) |
| AEG III | 2 (12.0) | 2 (15.0) |
| Preoperative T
stage |
| 2 | 2 (12.5) | 4 (31.0) |
| 3 | 12 (75.0) | 9 (69.0) |
| 4 | 2 (12.5) | 0 (0.0) |
| Preoperative N
stage |
| 0 | 3 (19.0) | 4 (31.0) |
| + | 13 (81.0) | 9 (69.0) |
Acute side effects and feasibility of
perioperative and preoperative therapy
In the two groups, no acute non-hematological
adverse events ≥grade 3, leading to treatment modifications, were
recorded. Acute hematological toxicity grade 3 or 4 was reported
more frequently in the CRT group [eight of the 16 patients (50%)]
compared with the CT group [two of the 13 patients (15%)] (P=0.02).
Therefore, a dose reduction of CT was necessary in 50% of CRT
patients and 15% of CT patients. In 15 of the 16 patients (94%) in
the CRT group and 12 of the 13 patients (92%) in the CT group, all
scheduled preoperative CT cycles were able to be administered. In
the CT group, eight of the 13 patients (62%) were unable to receive
adjuvant CT due to prolonged hematological toxicity or
deterioration of general condition (Table II).
 | Table IIAcute toxicity, treatment
characteristics and comorbidity. |
Table II
Acute toxicity, treatment
characteristics and comorbidity.
| Treatment |
Chemoradiotherapy | Chemotherapy |
|---|
| Patients | 16 (100) | 13 (100) |
| Acute toxicity |
|
Non-hematological | 0 (0) | 0 (0) |
| Hematological (CTC
grade 3/4) | 8 (50)a | 2 (15)a |
| Cumulative dose of
irradiation, Gy (range) | 45.0
(45.0–66.6)b | NA |
| Preoperative
Chemotherapy |
| All scheduled cycles
of chemotherapy | 15 (94) | 12 (92) |
| Dose reduction of
chemotherapy during preoperative treatment | 8 (50) | 2 (15) |
| Postoperative
Chemotherapy |
| Receiving
postoperative chemotherapy | NA | 5 (38) |
Major surgical and non-surgical
postoperative complications and associated mortality
Postoperative pulmonary and pleural complications,
including pneumonia, pneumothorax, relevant pleural-effusion and
pleural empyema, occurred more frequently in the CRT group than in
the CT group (44 vs. 8%, respectively; P=0.04). One patient in each
group succumbed to their condition during hospitalization due to
septic complications following anastomotic leakage (Table III). The two groups did not differ
significantly in the number of major surgical complications (CRT
group, 69% vs. CT group, 77%; P=0.63; Table IV).
 | Table IIISurgical complications and
mortality. |
Table III
Surgical complications and
mortality.
| Complication | Chemoradiotherapy, n
(%) | Chemotherapy, n
(%) | P-valuea |
|---|
| Patients | 11 (69) | 10 (77) | NS |
| Type of major
surgical complication |
| Anastomotic
leakage | 4 (25) | 4 (31) | NS |
|
Mediastinitis/sepsis | 1 (6) | 1 (8) | NS |
| Implantation of
esophageal stent | 2 (13) | 2 (15) | NS |
| Pulmonary
complications | 7 (44) | 1 (8) | 0.04 |
| Secondary
surgery | 3 (19) | 1 (8) | NS |
| Necrosis of
intrathoracic gastric tube/neoesophagus | 1 (6) | 0 (0) | NS |
| Lymphatic
fistula | 1 (6) | 0 (0) | NS |
| Other | 5 (31) | 3 (23) | NS |
|
Complication-associated mortality | 1 (6) | 1 (8) | NS |
 | Table IVSurgical outcome and survival
data. |
Table IV
Surgical outcome and survival
data.
| Outcome |
Chemoradiotherapy | Chemotherapy | P-valuea |
|---|
| R0
resectionb | 16 (100) | 10 (77) | 0.05 |
| pCRc | 3 (19) | 0 (0) | 0.23 |
| Median OS,
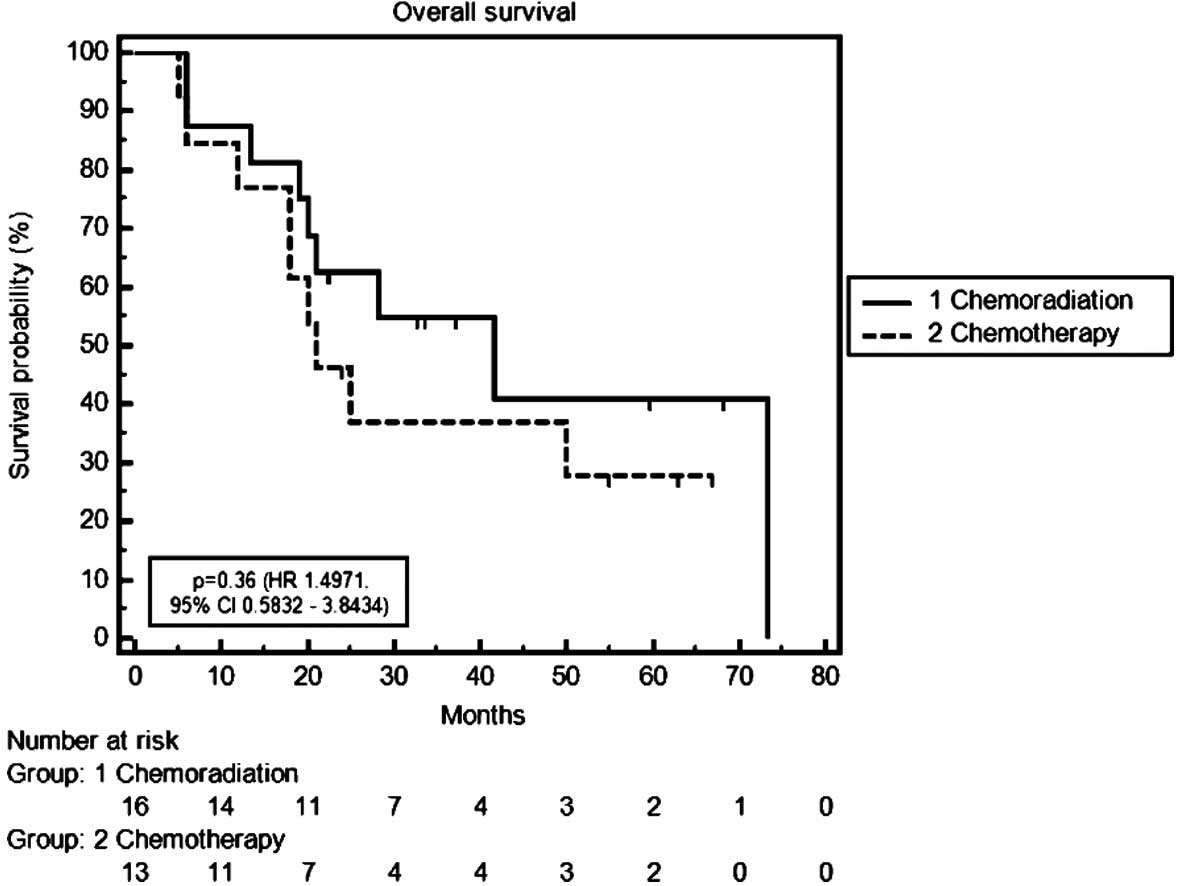
months | 41.7 | 21.0 | 0.36 |
| 3-year OS rate,
% | 55.0 | 38.0 | NS |
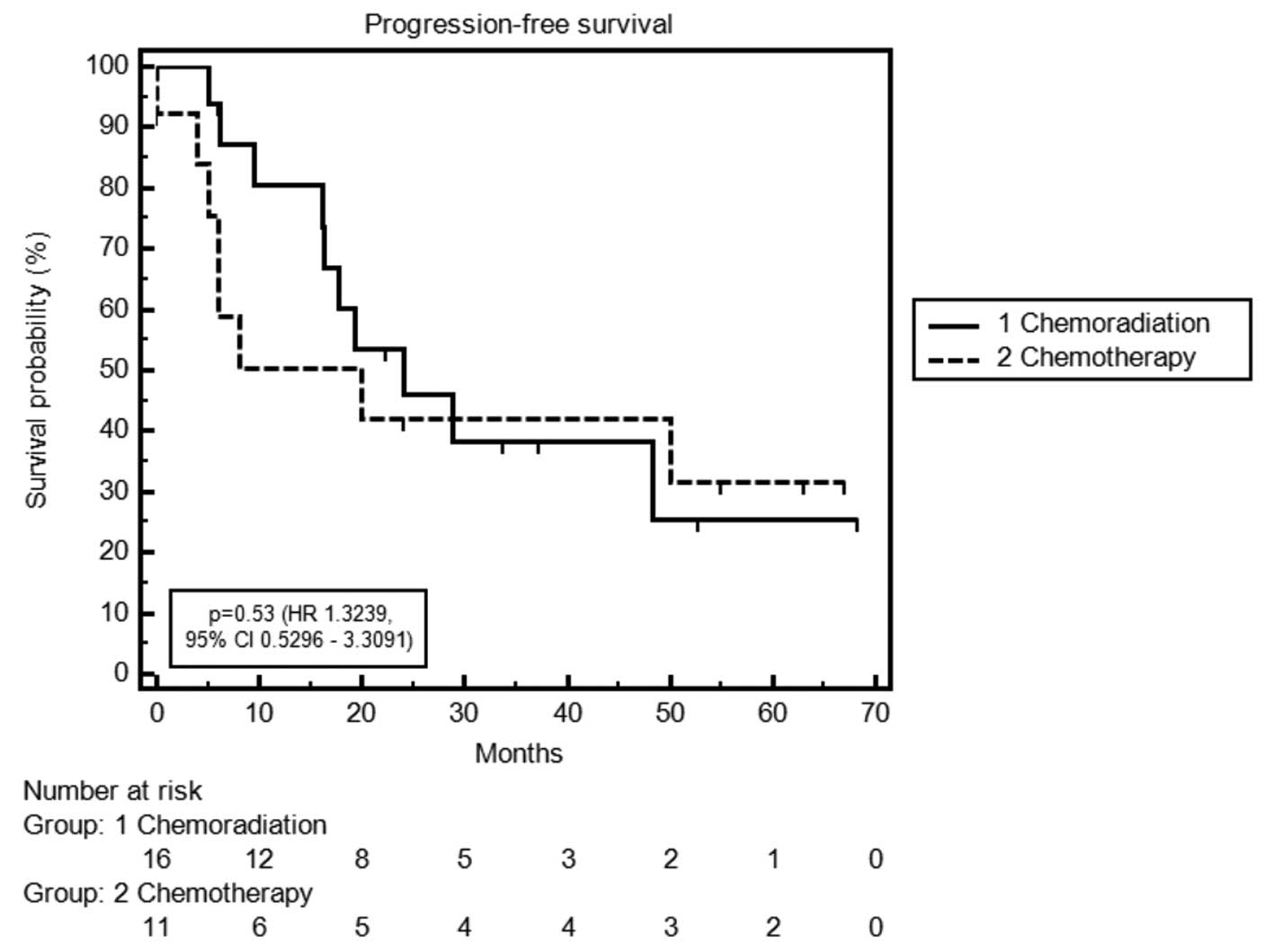
| Median PFS,
months | 24.1 | 20.0 | 0.71 |
| Pattern of
recurrence |
| Locoregional | 2 (13) | 4 (31) | NS |
| Distant | 7 (44) | 4 (31) | NS |
| Locoregional and
distant | 1 (6) | 1 (8) | NS |
Pathological complete remission and rate
of R0 resection
Pathological complete remission (pCR) of the tumors
was observed in three patients in the CT group (CT group, 19% vs.
CRT group, 0%; P=0.11). In addition, the R0 resection rate
(complete tumor-free resection margins) was significantly higher in
the CRT group compared with the CT group (100 vs. 77%,
respectively; P=0.05; Table
IV).
OS and PFS
Median OS time was 21.0 months in the CT group
versus 41.7 months in the CRT group [P=0.36; hazard ratio (HR),
1.50; 95% confidence interval (CI), 0.58–3.84]. Three-year survival
rates were 38% in the CT group and 55% in the CRT group. Median PFS
time was 20.0 months in the CT group compared with 24.1 months in
the CRT group (P=0.71; HR, 1.19; 95% CI, 0.46–3.05) (Table IV; Figs. 1 and 2).
Patterns of recurrence and secondary
malignancies detected during the follow-up period
The rate of local and/or distant recurrence during
the follow-up period was 56% in the CRT and 69% in the CT group
(Table IV). In the CRT group,
three patients were diagnosed with secondary malignancies,
including non-small cell lung cancer, colon carcinoma and
cholangiocarcinoma. At least one patient in the CRT group succumbed
to a secondary malignancy and not to the AEG tumor. In the other
two cases, the cause of mortality was not clearly identifiable.
Discussion
Although modern perioperative treatment regimens
have shown improved outcomes for patients with advanced AEG tumors,
there remains a lack of profound data to define the most effective
treatment approach. The current study compared the outcome of a
homogeneous patient population treated with preoperative CRT or
perioperative CT at a single center. An updated meta-analysis in
2011 analyzing the administration of neoadjuvant CT or CRT in
addition to surgery was unable to determine a clear advantage
between the treatment regimens, although, a proportionately larger
survival benefit was observed for CRT versus CT (12). However, concern remains that this
benefit is achieved at the expense of an increase in morbidity and
mortality. The present study found no significant differences in
overall morbidity and mortality between the two treatment arms.
This is consistent with the results of a number of randomized
trials that found similar overall morbidity and mortality rates for
perioperative CT and preoperative CRT when compared with surgery
alone (Table V) (4–11,15–18).
However, in the current study, a significantly higher rate of
pulmonary complications (44%) was observed in the CRT compared with
the CT group. Similar rates ranging between 20 and 50% have been
reported by a number of authors investigating neoadjuvant CRT
followed by surgery versus surgery alone. Notably, in these
studies, the two groups (neoadjuvant CRT plus surgery vs. surgery
alone) showed similar pulmonary morbidity rates. This indicates
that pulmonary complications are not likely to be predominantly
caused by the addition of radiotherapy or CT alone (6,8–10,19).
However, the differences demonstrated in the current study are not
well explained and highlight issues of target volume definition,
radiotherapy dose/fractionation and lung sparing techniques, which
must be accounted for in future studies. An additional difference
between the treatment groups was observed in the frequency of
hematological side effects, with 50% of the patients in the CRT
group developing grade 3/4 hematotoxicity compared with only 15% in
the CT group. The extremely low hematotoxicity in the CT group is
contradictory to an additional single center phase II study
investigating a similar preoperative CT regimen
(epirubicin/cisplatin/capecitabine) that only differed by the dose
of capecitabine. In this trial, the reported grade 3/4 neutropenia
was 62% (20). By contrast, a
recent phase III trial comparing preoperative CRT in addition to
surgery with surgery alone reported considerably low rates of grade
3 and 4 hematotoxicity (<10%) with a treatment compliance of
>90%, using a new chemotherapeutic regimen consisting of
carboplatin and paclitaxel, with a radiation dose of 41.1 Gy in 1.8
Gy fractions. No differences in morbidity or mortality were
observed in the two groups, however, patients in the CRT group
showed significantly improved survival outcomes (10). Previously, the long-term results of
the MRC OEO2 trial, which compared the additional effect of
preoperative CT with surgery alone, showed that patients with
microscopically complete resection (R0 resection) exhibited an OS
rate of 42.4% compared with 18% of patients with microscopically
incomplete resection (R1 resection) and 8.6% of patients with a
remaining macroscopic tumor (R2 resection) (4). The present series identified a
significantly higher R0 resection rate for patients who received
CRT (100%) compared with those who received CT (77%). Furthermore,
only patients in the CRT group (n=3) achieved pCR. Consecutively, a
non-significant trend to a higher PFS and OS and an improved local
control rate was identified in the CRT group. By contrast,
preoperative CT was noted to decrease the incidence of distant
metastasis by 31 versus 44% in the CRT group. Overall, these
results reflect the results of the only two randomized trials that
have directly compared preoperative CT with preoperative CRT.
However, the two trials closed prematurely due to poor accrual.
Stahl et al reported three-year OS rates of 47.4% in the CRT
group and 27.7% in the CT group, with an increased number of
patients in the CRT group experiencing pathological downstaging and
pCR compared with the CT group (15.6 vs. 2%, respectively).
Notably, all patients with pCR survived (11). Similarly, Burmeister et al
was unable to demonstrate a significant survival benefit with the
addition of radiation therapy to preoperative CT. The authors
reported a prolonged time to progression, a significantly higher
pCR rate and a trend to an improved R0 resection rate in the CRT
group. The reported OS rates at three years were 49 (CT) versus 52%
(CRT) and are consistent with the current results (6).
 | Table VStudies on multimodal treatment
strategies in AEG. |
Table V
Studies on multimodal treatment
strategies in AEG.
| A, Studies on
perioperative CT followed by OP versus OP alone. |
|---|
|
|---|
| | n/OS rate, % | |
|---|
| |
| |
|---|
| Author (year)
[ref] | Years of
accrual | CT and OP | OP | Postoperative
toxicity |
|---|
| Kelsen et
al(1998) [7] | 1990–1995 | 124/23
(3-year) | 120/26
(3-year) | No difference in
morbidity/mortality |
| Allum et
al(2009) [4] and MRC OEO2
(2009) [17] | 1992–1998 | 265/22.6
(3-year) | 268/17.6
(3-year) | No difference in
morbidity/mortality |
| Cunningham et
al(2006) [14] | 1994–2002 | 65/38 absolute | 66/31 absolute | No difference in
morbidity/mortality |
| Ychou et
al(2011) [15] | 1995–2003 | 109/38
(5-year) | 110/24
(5-year) | No difference in
morbidity/mortality |
|
| B, Studies on
preoperative CRT followed by OP versus OP alone. |
|
| | n/OS rate, % | |
| |
| |
| Author (year)
[ref] | Years of
accrual | CRT and OP | OP | Postoperative
toxicity |
|
| Urba et
al(2001) [8] | 1989–1994 | 37/30 (3-year) | 38/16 (3-year) | No difference in
morbidity/mortality |
| Walsh et
al(1996) [9] | 1990–1995 | 58/32 (3-year) | 55/6 (3-year) | No difference in
morbidity/higher mortality for CRT |
| Burmeister et
al(2005) [5] | 1994–2000 | 78/28 (3-year) | 83/30 (3-year) | No difference in
morbidity/mortality |
| Tepper et
al(2008) [16] | 1997–2000 | 23/39 (5-year) | 19/16 (5-year) | No difference in
morbidity/mortality |
| van Hagen et
al(2012) [10] | 2004–2008 | 134/~55a (3-year) | 141/~45a (3-year) | No difference in
morbidity/mortality |
|
| C, Studies on
preoperative CRT followed by surgery versus preoperative CT
followed by OP. |
|
| | n/OS rate, % | |
| |
| |
| Author (year)
[ref] | Years of
accrual | CRT and OP | OP | Postoperative
toxicity |
|
| Stahl et
al(2009) [11] | 2000–2005 | 60/47.4
(3-year) | 59/27.7
(3-year) | Postoperative
mortality was not significantly increased for CRT group |
| Burmeister et
al(2011) [6] | 2000–2006 | 39/52 (3-year) | 36/49 (3-year) | No difference in
morbidity/mortality |
| | 39/45 (5-year) | 39/36 (5-year) | |
To determine the best multimodal treatment regimen,
further studies are required. At present, an ongoing study, the
international phase III TOPGEAR trial (launched in 2012), is
investigating whether preoperative CRT (two cycles of ECF followed
by 45 Gy of radiation with concurrent 5-FU) or preoperative CT
(three cycles of ECF) alone is more effective in patients with
resectable gastric and esophagogastric cancer. Following surgery,
the two groups were scheduled to receive three additional cycles of
ECF (unpublished data).
Despite the major limitations of the present small
and retrospective analysis, the results confirmed the results of
recent randomized trials addressing the issue of whether
preoperative CT or CRT is superior for the treatment of AEG tumors.
The current study demonstrated significantly higher R0 resection
rates and an increased number of pCR in the CRT group. These
results appear to indicate a trend for improved PFS and OS for the
CRT group. As postoperative morbidity and mortality rates were
similar in the two groups, the results of the current study support
the use of CRT for patients with advanced AEG. Nevertheless, large
trials integrating the best available treatment schedules are
required to define a standard treatment approach for this
increasingly common tumor entity.
References
|
1
|
Pera M: Epidemiology of esophageal cancer,
especially adenocarcinoma of the esophagus and esophagogastric
junction. Recent Results Cancer Res. 155:1–14. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Trivers KF, Sabatino SA and Stewart SL:
Trends in esophageal cancer incidence by histology, United States,
1998–2003. Int J Cancer. 123:1422–1428. 2008.
|
|
3
|
Breaux JR, Bringaze W, Chappuis C and Cohn
I Jr: Adenocarcinoma of the stomach: a review of 35 years and 1,710
cases. World J Surg. 14:580–586. 1990.PubMed/NCBI
|
|
4
|
Allum WH, Stenning SP, Bancewicz J, Clark
PI and Langley RE: Long-term results of a randomized trial of
surgery with or without preoperative chemotherapy in esophageal
cancer. J Clin Oncol. 27:5062–5067. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burmeister BH, Smithers BM, Gebski V, et
al; Trans-Tasman Radiation Oncology Group; Australasian
Gastro-Intestinal Trials Group. Surgery alone versus
chemoradiotherapy followed by surgery for resectable cancer of the
oesophagus: a randomised controlled phase III trial. Lancet Oncol.
6:659–668. 2005. View Article : Google Scholar
|
|
6
|
Burmeister BH, Thomas JM, Burmeister EA,
et al: Is concurrent radiation therapy required in patients
receiving preoperative chemotherapy for adenocarcinoma of the
oesophagus? A randomised phase II trial. Eur J Cancer. 47:354–360.
2011. View Article : Google Scholar
|
|
7
|
Kelsen DP, Ginsberg R, Pajak TF, et al:
Chemotherapy followed by surgery compared with surgery alone for
localized esophageal cancer. N Engl J Med. 339:1979–1984. 1998.
View Article : Google Scholar
|
|
8
|
Urba SG, Orringer MB, Turrisi A,
Iannettoni M, Forastiere A and Strawderman M: Randomized trial of
preoperative chemoradiation versus surgery alone in patients with
locoregional esophageal carcinoma. J Clin Oncol. 19:305–313.
2001.
|
|
9
|
Walsh TN, Noonan N, Hollywood D, Kelly A,
Keeling N and Hennessy TP: A comparison of multimodal therapy and
surgery for esophageal adenocarcinoma. N Engl J Med. 335:462–467.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Hagen P, Hulshof MC, van Lanschot JJ,
et al; CROSS Group. Preoperative chemoradiotherapy for esophageal
or junctional cancer. N Engl J Med. 366:2074–2084. 2012.
|
|
11
|
Stahl M, Walz MK, Stuschke M, et al: Phase
III comparison of preoperative chemotherapy compared with
chemoradiotherapy in patients with locally advanced adenocarcinoma
of the esophagogastric junction. J Clin Oncol. 27:851–856. 2009.
View Article : Google Scholar
|
|
12
|
Sjoquist KM, Burmeister BH, Smithers BM,
et al; Australasian Gastro-Intestinal Trials Group. Survival after
neoadjuvant chemotherapy or chemoradiotherapy for resectable
oesophageal carcinoma: an updated meta-analysis. Lancet Oncol.
12:681–692. 2011. View Article : Google Scholar
|
|
13
|
Lutz MP, Zalcberg JR, Ducreux M, et al;
First St Gallen EORTC Gastrointestinal Cancer Conference 2012
Expert Panel. Highlights of the EORTC St. Gallen International
Expert Consensus on the primary therapy of gastric,
gastroesophageal and oesophageal cancer - differential treatment
strategies for subtypes of early gastroesophageal cancer. Eur J
Cancer. 48:2941–2953. 2012. View Article : Google Scholar
|
|
14
|
Siewert JR, Hölscher AH, Becker K and
Gössner W: Cardia cancer: attempt at a therapeutically relevant
classification. Chirurg. 58:25–32. 1987.(In German).
|
|
15
|
Cunningham D, Allum WH, Stenning SP, et
al; MAGIC Trials Participants. Perioperative chemotherapy versus
surgery alone for resectable gastroesophageal cancer. N Engl J Med.
355:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ychou M, Boige V, Pignon JP, et al:
Perioperative chemotherapy compared with surgery alone for
resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD
multicenter phase III trial. J Clin Oncol. 29:1715–1721. 2011.
View Article : Google Scholar
|
|
17
|
Tepper J, Krasna MJ, Niedzwiecki D, et al:
Phase III trial of trimodality therapy with cisplatin,
fluorouracil, radiotherapy, and surgery compared with surgery alone
for esophageal cancer: CALGB 9781. J Clin Oncol. 26:1086–1092.
2008. View Article : Google Scholar
|
|
18
|
Medical Research Council Oesophageal
Cancer Working Group. Surgical resection with or without
preoperative chemotherapy in oesophageal cancer: a randomised
controlled trial. Lancet. 359:1727–1733. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leibl BJ, Vitz S, Schäfer W, Alfrink M,
Gschwendtner A and Grabenbauer GG: Adenocarcinoma of the
esophagogastric junction: neoadjuvant radiochemotherapy and radical
surgery: early results and toxicity. Strahlenther Onkol.
187:231–237. 2011. View Article : Google Scholar
|
|
20
|
Starling N, Okines A, Cunningham D, et al:
A phase II trial of preoperative chemotherapy with epirubicin,
cisplatin and capecitabine for patients with localised
gastro-oesophageal junctional adenocarcinoma. Br J Cancer.
100:1725–1730. 2009. View Article : Google Scholar
|