Introduction
Lung cancer is a major cause of cancer mortality
worldwide. Resistance to antitumor drugs is a vital obstacle to
overcome for tumor chemotherapy (1). In the past several decades, the
following strategies have been developed to circumvent drug
resistance: i) Inhibition of the functions of the multidrug
resistance (MDR)-associated protein (MRP) or ATP-dependent membrane
drug efflux pump protein, P-glycoprotein (P-gp), by several
generation modulators to decrease the efflux rate of antitumor
drugs; ii) blocking of molecular mechanisms via pathways to defeat
and possibly prevent drug resistance. Villanueva et al
revealed that a combination therapy involving a MAPK/ERK kinase
(MEK) and v-raf murine sarcoma viral oncogene homolog B1 (BRAF)
inhibitor may be an effective strategy to conquer drug resistance
in melanoma (2); acquired
resistance to BRAF inhibitors mediated by a RAF kinase switch in
melanoma may be overcome by co-targeting MEK and IGF-1R/PI3K; and
iii) increasing the uptake and accumulation of antitumor drugs
using therapeutic nanoparticles or liposomes for drug delivery
(3–5). To date, the drug resistance of tumors
remains a big challenge.
As a polymeric nanoparticulate system,
poly(D,L-lactide-co-glycolide) (PLGA) has gained attention
for the preparation of a wide variety of delivery systems
containing several drugs due to its biodegradable and biocompatible
properties and low toxicity (6,7). The
etiology of MDR may be multifactorial, but the classic resistance
to cytotoxic drugs has often been associated with the
overexpression of P-gp, a 170-kd ATP-dependent membrane
transporter, which acts as a drug efflux pump (8–10).
Previously, the inhibition of P-gp as a method of reversing MDR has
been extensively studied (11–13).
Numerous agents that modulate the function of P-gp have been
identified, including calcium channel blockers (14), calmodulin antagonists (15), steroidal agents (16), protein kinase C inhibitors,
immunosuppressive drugs, antibiotics and surfactants. Cyclosporin A
(CsA) has been demonstrated as a broad-spectrum MDR modulator in a
number of previous studies (17,18).
In the current study, to achieve an improved
therapeutic effect of antitumor drug resistant cancer, a more
optimized delivery system using PLGA was adopted, that not only
increases drug [doxorubicin (DOX)] uptake, but also reduces the
efflux rate of DOX by co-delivering the P-gp inhibitor, CsA.
Materials and methods
Cells and agents
The paclitaxel-resistant non-small cell lung cancer
A549 cell line (A549-Taxol), cultured in DMEM medium (10% FBS, 1%
penicillin and 1% streptomycin) and 0.25% trypsin solution, was
purchased from Invitrogen Life Technologies (Carlsbad, CA, USA).
PLGA, DOX and CsA were purchased from Sigma-Aldrich (St. Louis, MO,
USA), phycoerythrin (PE)-labeled mouse anti-human P-gp antibody was
purchased from eBioscience, Inc. (San Diego, CA, USA) and anti-P-gp
antibody (265/F4) for western blot analysis was provided by Wuhan
University (Wuhan, China).
Flow cytometry
The expression levels of human P-gp on the surfaces
of the A549-Taxol cells were examined by fluorescence-activated
cell sorting (FACS) analysis using PE-labeled anti-human P-gp
antibodies. The cells were incubated with or without the antibody
for 45 min at 4°C, followed by washing twice with PBS. Fluorescence
staining levels were measured using FACSCalibur (BD Biosciences,
Franklin Lakes, NJ, USA).
Western blotting
Cell lysates of A549-Taxol cells were separated by
SDS-PAGE and then electrotransferred onto a polyvinylidene
difluoride membrane. The membrane was incubated with 1 mg/ml
anti-P-gp monoclonal antibody (265/F4) purchased from Abcam
(Cambridge, MA, USA), followed by washing 3 times and treatment
with peroxidase-conjugated goat anti-mouse secondary antibody
(Amersham Pharmacia Biotech, Amersham, UK). Subsequently, the
membrane-bound antibody was visualized with the Enhanced
Chemiluminescence Plus Detection kit (Amersham Pharmacia
Biotech).
Preparation of nanovectors
PLGA, CsA-coated PLGA (PLGA-CsA), DOX-loaded PLGA
(PLGA-DOX) and DOX and CsA-loaded PLGA (PLGA-DOX-CsA) were prepared
using a modified procedure of oil in water single emulsion solvent
evaporation. The organic phase consisted of PLGA polymer in a
dichloromethane-acetone mixture (2:1) and the aqueous phase
contained P-gp inhibitor, CsA and DOX (alone or together). The
organic phase was emulsified with the aqueous phase using an
Ultra-Turrax model T25 (IKA®-Werke GmbH & Co. KG,
Staufen, Germany) at 14,000 rpm in an ice bath for 5 min. The
organic mixture was then removed rapidly by evaporation under
nitrogen gas at 37°C. The particles were centrifuged at 100,000 × g
for 30 min, washed 3 times in distilled water and freeze-dried for
use.
Surface morphology, particle size and ζ
potential analysis
The surface morphology of PLGA, PLGA-CsA, PLGA-DOX
and PLGA-DOX-CsA was examined by a Hitachi model H-800 transmission
electron microscope (TEM) (Hitachi, Ltd., Tokyo, Japan). Freshly
prepared particles were washed with ddH2O at 100,000 × g
for 1 h and then re-suspended in ddH2O for analysis.
Samples were transferred to a cuvette for dynamic light scattering
analysis to measure the size distribution or subjected to an
electric field for ζ potential determination using Zetasizer Nano
ZS (Malvern Instruments, Malvern, UK).
Accumulation of free DOX, PLGA-DOX and
PLGA-DOX-CsA
The A549-Taxol cells (2×104) were seeded
in 24-well tissue culture plates and incubated with free DOX,
PLGA-DOX and PLGA-DOX-CsA nanoparticles for 6, 12, 24 and 48 h. A
DOX concentration equivalent to 2 μg/ml was maintained in the
solutions. Following incubation, the cells were washed 3 times to
remove the DOX, PLGA-DOX or PLGA-DOX-CsA that had not been
internalized. The cells were subsequently observed and images were
captured using fluorescence microscopy (BX53; Olympus, Corp.,
Tokyo, Japan).
In vitro cytotoxicity assay
The A549-Taxol cells (2×103) were plated
into 96-well plates and incubated with free PLGA (200 mg/ml),
PLGA-CsA (1 mg/ml CsA), free DOX (10 mg/ml), PLGA-DOX (10 mg/ml
DOX) and PLGA-DOX-CsA (10 mg/ml DOX and 1 mg/ml CsA ) for 6, 12,
24, 48 and 72 h. Next, 10 ml WST-1 reagents were added to each well
and incubated at 37°C for 4 h. Finally, the absorbance was measured
using a Perkin-Elmer 2030 VICTOR X Multilabel Plate Reader
(Perkin-Elmer, Waltham, MA, USA) at 450 nm.
Antitumor activity of PLGA-DOX and
PLGA-DOX-CsA
The in vivo antitumor efficacy of PLGA-DOX
and PLGA-DOX-CsA was assessed in female BALB/c background SCID mice
(body weight, 18–20 g). The A549-Taxol cells (2×105)
were subcutaneously injected into the mice. The mice were randomly
divided into six groups (PBS, PLGA, PLGA-CsA, free DOX, PLGA-DOX
and PLGA-DOX-CsA), with six mice in each group. The mice were
treated with a single intravenous (i.v.) injection of a 10 μg/ml
dose, equivalent to DOX, in each group. The control group of mice
received a single i.v. injection of PBS or free PLGA particles. At
predetermined time intervals, the tumor volume was determined by
measuring the tumor dimensions using digital calipers and then
calculated according to the following formula: Tumor volume
(mm3) = width × (length / 2)2. Survival rates
of the mice were observed and calculated for 60 days.
Statistical analysis
A one- and two-way analysis of variance and
Student’s t-test were used to determine statistical significance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
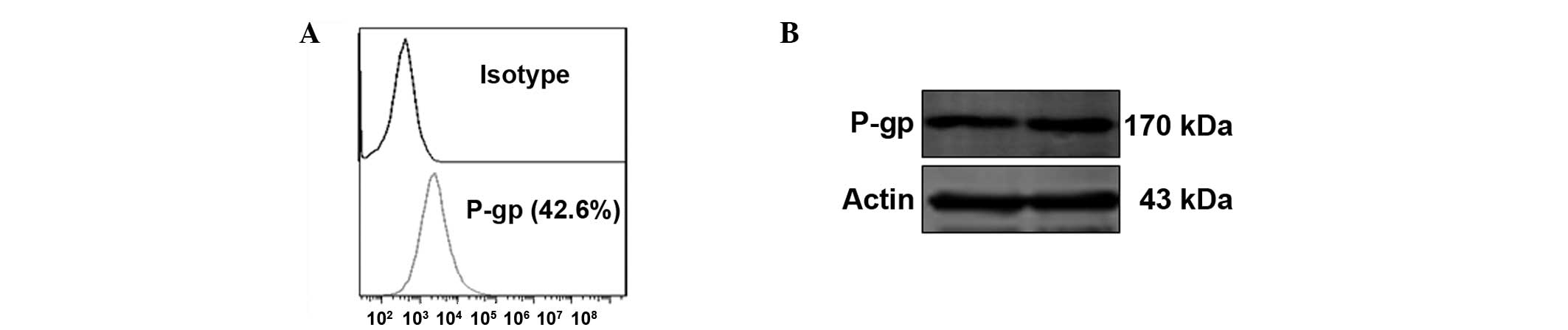
High expression of P-gp in A549-Taxol
cells
FACS and western blot analysis were performed to
confirm the expression of P-gp in the A549-Taxol cells. As
predicted, the cells were positive for P-gp in the FACS (Fig. 1A) and western blot analysis results
(Fig. 1B).
Characteristics of PLGA, PLGA-DOX,
PLGA-CsA and PLGA-DOX-CsA
To synthesize PLGA-DOX, PLGA-CsA and PLGA-DOX-CsA,
DOX and CsA (alone or together) were encapsulated into PLGA
nanoparticles. TEM was first performed to observe the prepared
nanoparticles. As shown in Fig. 2A,
all nanoparticles were dispersed as individual particles with a
well-defined spherical shape and homogeneously distributed
diameters of ~120 nm. Size distribution (Fig. 2B) and ζ potential (Fig. 2C) analyses of the nanoparticles
revealed that the average size of free PLGA was 98.57±3.6 nm,
PLGA-CsA was 107.9±6.9 nm, PLGA-DOX was 123.5±12.3 nm and
PLGA-DOX-CsA was 172.9±14.6 nm, and all nanoparticles were
negatively charged (PLGA, −39.4±2.6; PLGA-CsA, −32.3±4.7; PLGA-DOX,
−34.6±7.1; and PLGA-DOX-CsA, −20±2.9 mV).
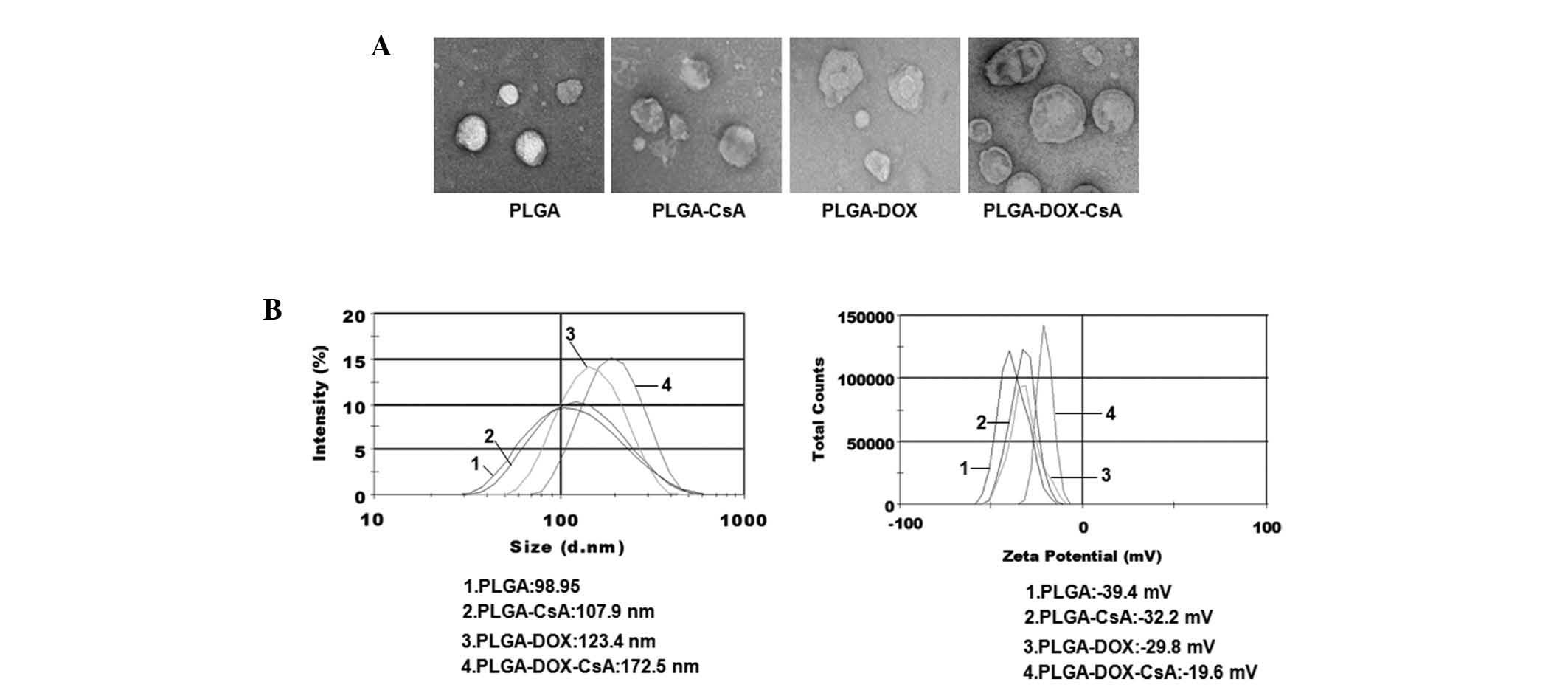 | Figure 2Characteristics of nanoparticles PLGA,
PLGA-DOX, PLGA-CsA and PLGA-DOX-CsA prepared as described in the
Materials and methods section. (A) the morphology of the
nanoparticles was image captured by TEM. (B) Size distribution and
(C) ζ potential of nanoparticles were measured using dynamic light
scattering analysis. PLGA, poly(D,L-lactide-co-glycolide);
DOX, doxorubicin; CsA, cyclosporin A; PLGA-CsA, CsA-coated PLGA;
PLGA-DOX, DOX-loaded PLGA; PLGA-DOX-CsA, DOX and CsA-loaded PLGA;
TEM, transmission electron microscopy. |
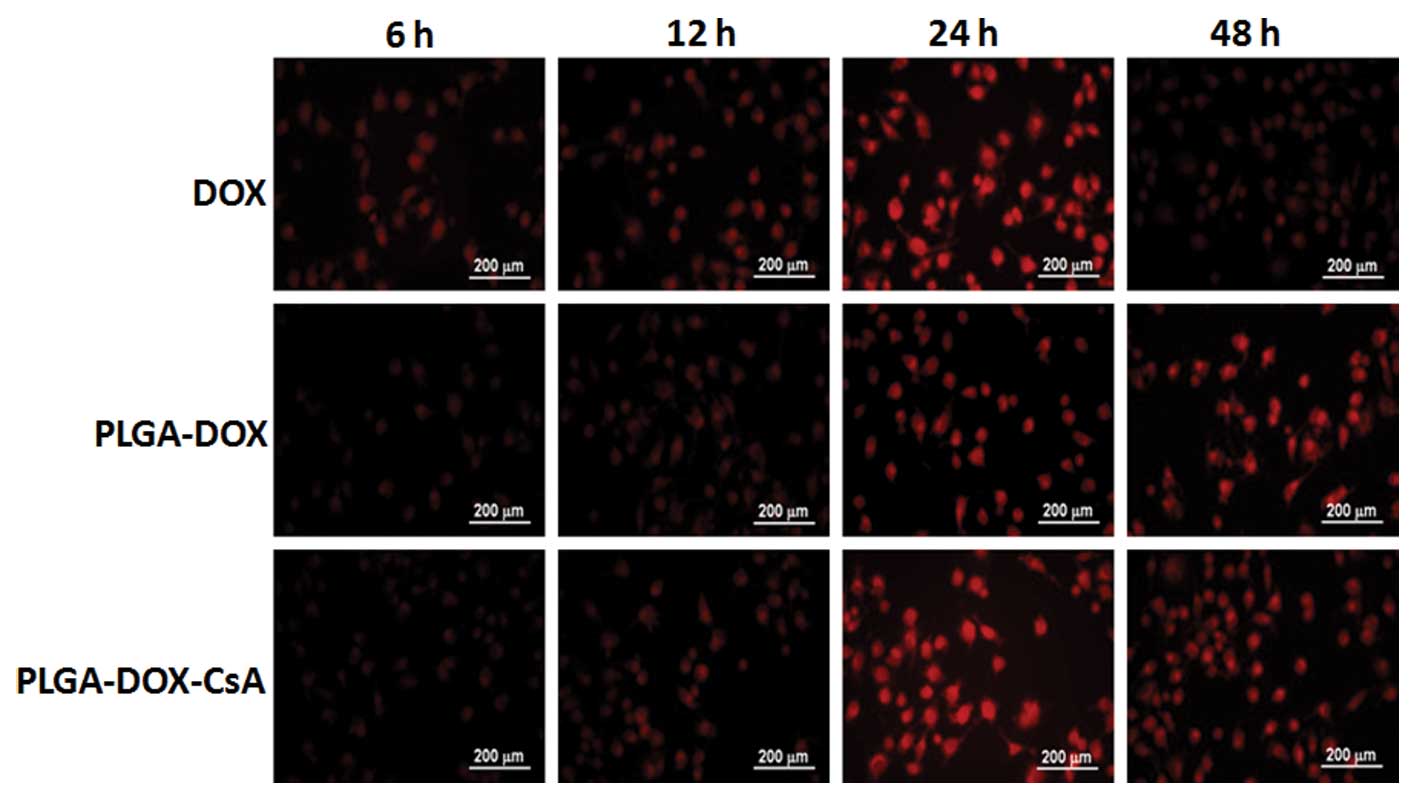
PLGA or PLGA-CsA nanoparticles reduce the
efflux of DOX in A549-Taxol cells
To investigate whether PLGA or PLGA-CsA increased
the accumulation of DOX in the A549-Taxol cells, fluorescence
microscopy was used to examine the intensity of DOX. A549-Taxol
cells were plated in 12-well plates and incubated with free DOX,
PLGA-DOX and PLGA-DOX-CsA, respectively. At various times (6, 12,
24 and 48 h), the cells were washed and images were captured. As
indicated in Fig. 3, free DOX was
notably reduced at 48 h, while the accumulation of PLGA-DOX, and
particularly PLGA-DOX-CsA, remained at a high level.
PLGA-DOX and PLGA-DOX-CsA effectively
inhibit cell viability in vitro
Nanoparticles without DOX (PLGA) were found to have
no toxic effects on the cells, as shown in Fig. 4. The free DOX group exhibited
proliferation of the A549-Taxol cells compared with the control,
PLGA and PLGA-CsA groups (P<0.01). PLGA-DOX and PLGA-DOX-CsA
further enhanced the inhibitory function of DOX in vitro
(P<0.05 and P<0.01, respectively). Furthermore, the
CsA-loaded PLGA group reduced the proliferation of A549-Taxol cells
compared with the control and PLGA groups (P<0.05).
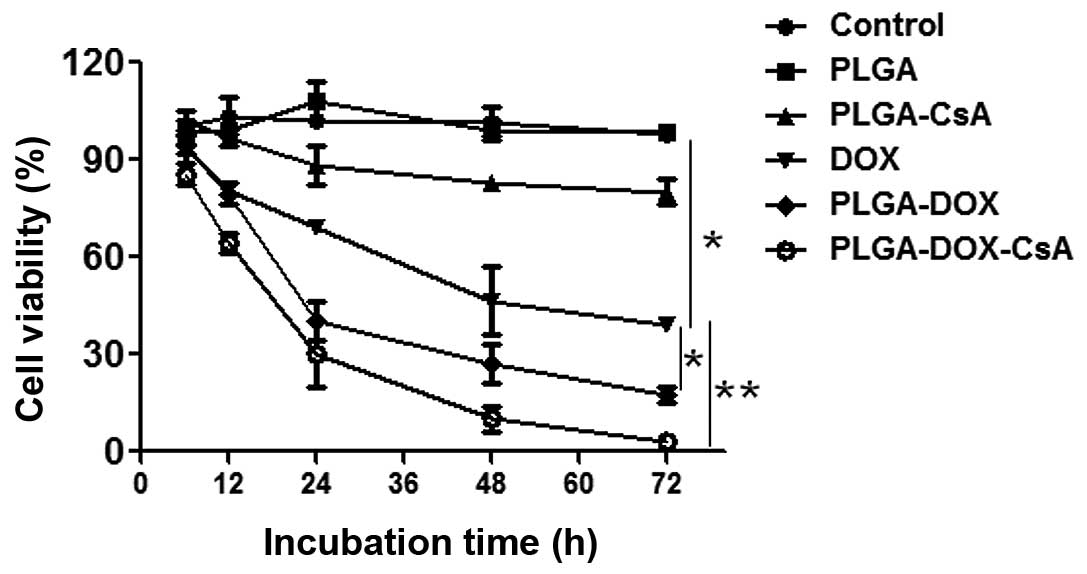 | Figure 4Cell viability following exposure to
PBS, PLGA, PLGA-CsA, free DOX, PLGA-DOX and PLGA-DOX-CsA at various
culture times at 37 °C. Compared with the free DOX, PLGA-DOX and
PLGA-DOX-CsA significantly inhibited cell viability.
*P<0.05, vs. PLGA-DOX; **P<0.01, vs.
PLGA-DOX-CsA. DOX, doxorubicin; PLGA,
poly(D,L-lactide-co-glycolide); CsA, cyclosporin A; PLGA-CsA,
CsA-coated PLGA; PLGA-DOX, DOX-loaded PLGA; PLGA-DOX-CsA,
CsA-loaded PLGA. |
PLGA-DOX and PLGA-DOX-CsA enhance
antitumor activity
The in vivo antitumor activity of free DOX,
PLGA-DOX, PLGA-CsA and PLGA-DOX-CsA was evaluated with A549-Taxol
tumor-bearing mice (6 per group). Treatments were performed as i.v.
injections of PBS, free PLGA, free DOX, PLGA-DOX, PLGA-CsA or
PLGA-DOX-CsA in tumor-bearing mice with average tumor volumes of
100 mm3, and this day was designated as day 1. Fig. 5A shows the variations in tumor
volume compared with the number of days after treatment in the
A549-Taxol tumor-bearing mice. Mice treated with free DOX exhibited
effectively inhibited tumor growth compared with the PBS, PLGA and
PLGA-CsA groups (P<0.05). As predicted, the PLGA-DOX and
PLGA-DOX-CsA groups exhibited improved tumor inhibitory effects
compared with the free DOX group (P<0.05 and P<0.01,
respectively). The survival rates in Fig. 5B indicated that the co-delivery of
DOX and CsA further increased the survival rate of
A549-Taxol-bearing mice (P<0.01, vs. PBS, PLGA and PLGA-CsA
groups).
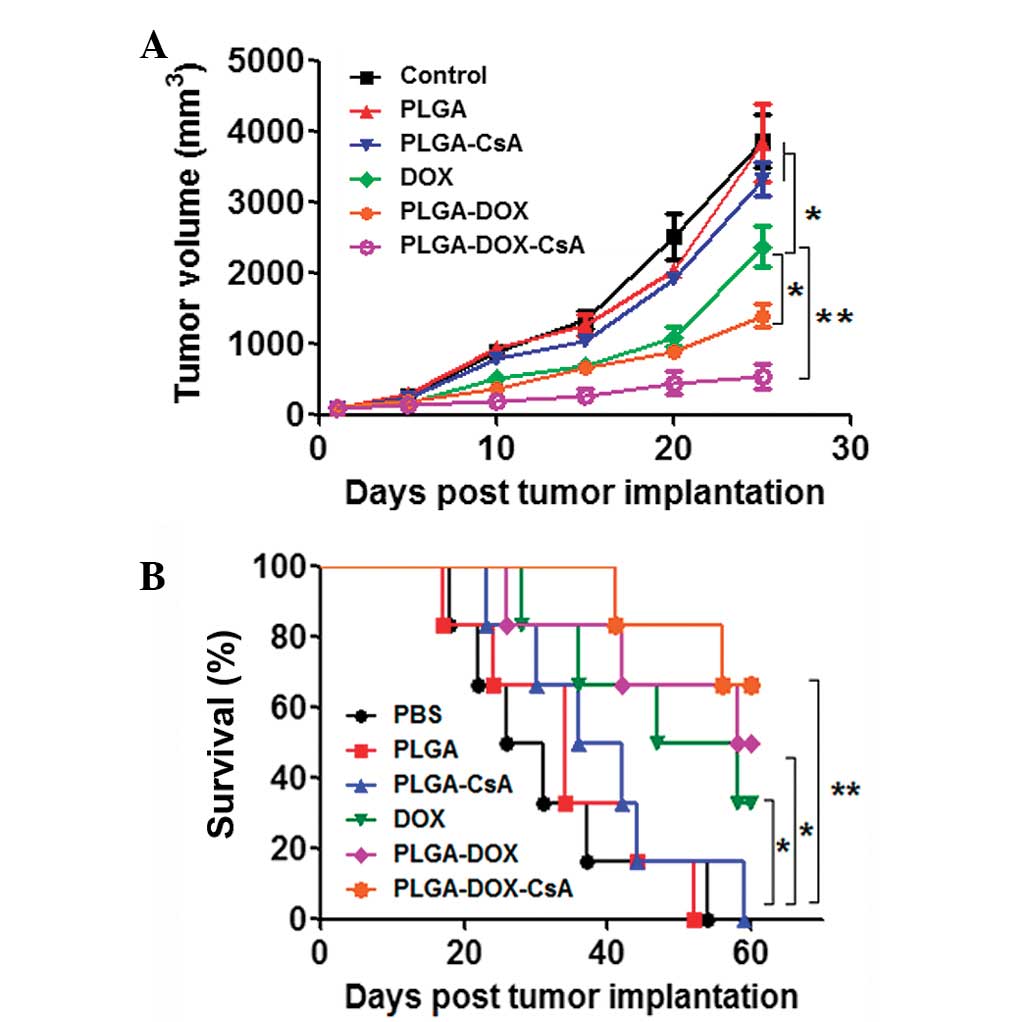 | Figure 5In vivo tumor growth and
survival rate of tumor-bearing mice. (A) The tumor inhibitory
effect of PLGA-DOX and PLGA-DOX-CsA was compared with free DOX,
PLGA vector and PBS control in the A549-Taxol tumor model (n=6).
DOX and PLGA-DOX-CsA demonstrated significant tumor inhibition. (B)
Compared with the PBS, PLGA and PLGA-CsA groups the survival rate
of A549-Taxol-bearing mice was significantly improved in the
PLGA-DOX and PLGA-DOX-CsA groups. *P<0.05, vs.
PLGA-DOX; **P<0.01, vs. PLGA-DOX-CsA. DOX,
doxorubicin; PLGA, poly(D,L-lactide-co-glycolide); CsA,
cyclosporin A; PLGA-CsA, CsA-coated PLGA; PLGA-DOX, DOX-loaded
PLGA; PLGA-DOX-CsA, CsA-loaded PLGA. |
Discussion
Cancer is one of the most significant causes of
mortality in humans, and the incidence and mortality rates of
cancer are continuously rising (19). A thorough ‘cure for cancer’ remains
elusive for a number of reasons. One of the critical reasons is the
strong toxic side-effects of free drugs or traditional drug
delivery vectors, mainly due to drug leakage prior to reaching the
cancer site. In addition, the intrinsic or acquired MDR of cancer
is primarily responsible for the final failure of cancer
chemotherapy, with >90% of patients with malignant tumors
succumbing due to a certain degree of MDR. Therefore, MDR in cancer
has become a major obstacle in the chemotherapeutic treatment of
numerous types of human cancer (20). Overcoming the currently untreatable
MDR in cancer remains important in antitumor research.
Current strategies to overcome tumor MDR generally
resort to multi-drug combined chemosensitization, reconstruction of
primary drugs and bio-/nanotechnologies. The combined use of two or
several strategies is being recognized as a realistic route to
successful chemotherapeutic treatment. By integrating multi-drug
chemosensitization with nanotechnology, specific nano drug delivery
systems based on organic or inorganic nanocarriers have been
designed. These systems overcome MDR and also enhance drug efficacy
against drug-sensitive and -resistant cancer cells, mainly by
improving drug bioaccessibility and chemosensitivity. Varying types
of combinations against MDR in cancer have been identified,
including the combination of proapoptotic compounds with
chemotherapeutics (21),
MDR-targeted siRNA with chemotherapeutics (22), nanoparticles co-encapsulating
hydrophobic and hydrophilic drugs (23), nanoparticles with precise
ratiometric drug loading (24) and
efflux pump inhibitors with chemotherapeutics (25).
According to previous studies on drug resistant
tumor therapy, PLGA, which contains a solid, polymer-filled core
that is more suited for water-insoluble drug payloads as a delivery
tool, and CsA (26), which
functions as a drug efflux pump inhibitor to further enhance the
accumulation and reduce the efflux of DOX, were selected for use in
the present study. Following the synthesis and characterization of
PLGA-DOX, PLGA-CsA and PLGA-DOX-CsA, the accumulation of free DOX,
PLGA-DOX and PLGA-DOX-CsA were initially investigated in A549-Taxol
cells and the results demonstrated that the PLGA-DOX and
PLGA-DOX-CsA groups exhibited increased accumulation of DOX
compared with the naked DOX group. Furthermore, >80% of the A549
cells at 48 h and 90% at 72 h were killed by PLGA-DOX-CsA and ~70%
of the cells at 48 h and 80% at 72 h were extirpated by PLGA-DOX at
a DOX concentration of 10 mg/ml, while only 40% of the cells were
eliminated by free DOX. The in vivo antitumor model also
indicated that PLGA-DOX and PLGA-DOX-CsA not only inhibited the
tumor growth, but also increased the survival rate of
A549-Taxol-bearing mice.
Collectively, PLGA is considered to be a safe
delivery system tool due to its biodegradability and
biocompatibility, since a number of PLGA-based drug systems have
been approved by the FDA. This modified delivery vector is further
empowered by co-loading with tumor-targeted molecules,
tumor-sensitive drugs, inhibitors associated with tumor progression
and modulators of drug resistance. In addition, this type of
multi-targeted therapeutic method should be a more effective tumor
therapeutic method.
Acknowledgements
This study was supported by a grant from the
Scientific and Technological Projects of Wuhan (no. 200507017).
References
|
1
|
Schimrosczyk K, Song YH, Vykoukal J, et
al: Liposome-mediated transfection with extract from neonatal rat
cardiomyocytes induces transdifferentiation of human
adipose-derived stem cells into cardiomyocytes. Scand J Clin Lab
Invest. 68:464–472. 2008. View Article : Google Scholar
|
|
2
|
Villanueva J, Infante JR, Krepler C, et
al: Concurrent MEK2 mutation and BRAF amplification confer
resistance to BRAF and MEK inhibitors in melanoma. Cell Rep.
4:1090–1099. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Niu C, Sun Q, Zhou J, et al:
Folate-functionalized polymeric micelles based on biodegradable
PEG-PDLLA as a hepatic carcinoma-targeting delivery system. Asian
Pac J Cancer Prev. 12:1995–1999. 2011.PubMed/NCBI
|
|
4
|
Dreaden EC, Austin LA, Mackey MA and
El-Sayed MA: Size matters: gold nanoparticles in targeted cancer
drug delivery. Ther Deliv. 3:457–478. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parhi P, Mohanty C and Sahoo SK:
Nanotechnology-based combinational drug delivery: an emerging
approach for cancer therapy. Drug Discov Today. 17:1044–1052. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shive MS and Anderson JM: Biodegradation
and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv
Rev. 28:5–24. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Makadia HK and Siegel SJ: Poly
Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug
Delivery Carrier. Polymers (Basel). 3:1377–1397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ullah MF: Cancer multidrug resistance
(MDR): a major impediment to effective chemotherapy. Asian Pac J
Cancer Prev. 9:1–6. 2008.PubMed/NCBI
|
|
9
|
Pluchino KM, Hall MD, Goldsborough AS, et
al: Collateral sensitivity as a strategy against cancer multidrug
resistance. Drug Resist Updat. 15:98–105. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L, Xiao R, Xiong J, et al: Activated
ERM protein plays a critical role in drug resistance of MOLT4 cells
induced by CCL25. PLoS One. 8:e523842013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Larsen UL, Hyldahl Olesen L, Guldborg
Nyvold C, et al: Human intestinal P-glycoprotein activity estimated
by the model substrate digoxin. Scand J Clin Lab Invest.
67:123–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han M, Lv Q, Tang XJ, et al: Overcoming
drug resistance of MCF-7/ADR cells by altering intracellular
distribution of doxorubicin via MVP knockdown with a novel siRNA
polyamidoamine-hyaluronic acid complex. J Control Release.
163:136–144. 2012. View Article : Google Scholar
|
|
13
|
Nieto Montesinos R, Béduneau A, Pellequer
Y and Lamprecht A: Delivery of P-glycoprotein substrates using
chemosensitizers and nanotechnology for selective and efficient
therapeutic outcomes. J Control Release. 161:50–61. 2012.
|
|
14
|
Shen F, Chu S, Bence AK, et al:
Quantitation of doxorubicin uptake, efflux, and modulation of
multidrug resistance (MDR) in MDR human cancer cells. J Pharmacol
Exp Ther. 324:95–102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu R, Zhang Y, Chen Y, et al: A novel
calmodulin antagonist O-(4-ethoxyl-butyl)-berbamine overcomes
multidrug resistance in drug-resistant MCF-7/ADR breast carcinoma
cells. J Pharm Sci. 99:3266–3275. 2010.
|
|
16
|
Harmsen S, Meijerman I, Febus CL, et al:
PXR-mediated induction of P-glycoprotein by anticancer drugs in a
human colon adenocarcinoma-derived cell line. Cancer Chemother
Pharmacol. 66:765–771. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liow JS, Lu S, McCarron JA, et al: Effect
of a P-glycoprotein inhibitor, Cyclosporin A, on the disposition in
rodent brain and blood of the 5-HT1A receptor radioligand,
[11C](R)-(-)-RWAY. Synapse. 61:96–105. 2007.PubMed/NCBI
|
|
18
|
Warren KE, Patel MC, McCully CM, et al:
Effect of P-glycoprotein modulation with cyclosporine A on
cerebrospinal fluid penetration of doxorubicin in non-human
primates. Cancer Chemother Pharmacol. 45:207–212. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He Q, Gao Y, Zhang L, et al: A
pH-responsive mesoporous silica nanoparticles-based multi-drug
delivery system for overcoming multi-drug resistance. Biomaterials.
32:7711–7720. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen YT, Feng B and Chen LB: Update of
research on drug resistance in small cell lung cancer chemotherapy.
Asian Pac J Cancer Prev. 13:3577–3581. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van Vlerken LE, Duan Z, Little SR, et al:
Augmentation of therapeutic efficacy in drug-resistant tumor models
using ceramide coadministration in temporal-controlled
polymer-blend nanoparticle delivery systems. AAPS J. 12:171–180.
2010.
|
|
22
|
Shin HC, Alani AW, Cho H, et al: A 3-in-1
polymeric micelle nanocontainer for poorly water-soluble drugs. Mol
Pharm. 8:1257–1265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakamura K, Abu Lila AS, Matsunaga M, et
al: A double-modulation strategy in cancer treatment with a
chemotherapeutic agent and siRNA. Mol Ther. 19:2040–2047. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Batist G, Gelmon KA, Chi KN, et al:
Safety, pharmacokinetics, and efficacy of CPX-1 liposome injection
in patients with advanced solid tumors. Clin Cancer Res.
15:692–700. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu J, Lu Y, Lee A, et al: Reversal of
multidrug resistance by transferrin-conjugated liposomes
co-encapsulating doxorubicin and verapamil. J Pharm Pharm Sci.
10:350–357. 2007.PubMed/NCBI
|
|
26
|
Qadir M, O’Loughlin KL, Fricke SM, et al:
Cyclosporin A is a broad-spectrum multidrug resistance modulator.
Clin Cancer Res. 11:2320–2326. 2005. View Article : Google Scholar : PubMed/NCBI
|