Introduction
Papillary thyroid carcinoma (PTC) is a common
endocrine tumor with an incidence that has increased over recent
decades in China. PCT exhibits a high cure rate with 10-year
patient survival rates estimated at 80–90% (1). Currently, surgery remains the
predominant treatment option for PTC; however, even following
curative resection, 20–30% of PTC patients experience recurrence
and/or metastasis, which is associated with increased mortality
(2). Numerous clinical and
pathological factors, including age, gender, tumor size,
histological type, tumor interstitial fibrosis and extrathyroidal
infiltration, have been shown to have limited prognostic value in
PTC (2–7).
In recent years, the study of the molecular
mechanisms of PTC has demonstrated that the BRAF gene mutation is a
significant event in the process of this disease. The V600E
mutation of the BRAF gene was identified in 28–83% of PTC
specimens, however, no mutations were observed in normal thyroid
tissue and tissue from patients with benign thyroid disease
(4,8–11). A
large number of studies have demonstrated that the BRAF gene
mutation is associated with pathological features, including
patient age, extrathyroidal invasion, lymph node metastasis and
tumor stage, which aid in determining patient prognosis (5,6,12–14).
In the present study, multiplex allele-specific PCR technology
combined with denaturing high-performance liquid chromatography
(DHPLC) was established to investigate the correlation between the
BRAF gene mutation and the clinical features, pathological
diagnosis, treatment and prognosis in 187 patients with PTC. The
BRAF V600E mutation was observed in PTC, while no BRAF mutations
were identified in benign-type thyroid diseases. In addition,
significant correlations were observed between the BRAF mutation
and age (>45 years), tumor-lymph node-metastasis stage and tumor
persistence/recurrence.
Patients and methods
Patients
To be eligible for the study, patients were required
to present with pathologically confirmed PTC according to the
tumor-lymph node-metastasis classification system of the
International Union against Cancer and the American Joint Committee
on Cancer. Tumor samples and 20 adjacent normal tissues were
obtained from 187 patients in the West China Hospital, Sichuan
University (Sichuan, China) between May 2009 and May 2011 for use
in this study. The study was reviewed and approved by the West
China Hospital, Sichuan University. Written informed consent was
obtained from the patients. Thyroid cancer K1 cells with the BRAF
V600E mutation and RO82-W-1 cells with wild-type BRAF were obtained
from the Molecular Biology Laboratory of the West China Hospital.
All patients underwent total or near-total thyroidectomy. Patients
were then routinely observed as outpatients, typically every 6–12
months, and were evaluated for cancer persistence/recurrence with
serum thyroglobulin (Tg) testing during the 2-year follow-up. For
all cases, persistent/recurrent PTC was defined as a detectable
basal serum Tg level (>1 ng/ml) (9). A total of 176 patients completed
follow-up.
DNA extraction
Genomic DNA was isolated from formalin-fixed
parafin-embedded tissue sections with a Qiagen TIANamp genomic DNA
kit (Qiagen, Hilden, Germany) and from cells lines with a Tiangen
genomic DNA extraction kit (Tiangen Biotech, Co., Ltd., Beijing,
China), according to the manufacturer’s instructions. DNA was
analyzed by agarose gel electrophoresis and a UV photometer (UVP,
Upland, CA, USA).
PCR primers
The BRAF mutation primers (BRAF mut) were designed
based on the BRAF sequence, with a mismatched nucleotide at the 3′
end, so that wild-type BRAF was not amplified by these primers. The
length of the PCR products was 126 bp. The BRAF sequencing primers
(BRAF seq) were also designed. In addition, TBXAS1 was chosen as a
reference gene. Information concerning these primers is listed in
Table I.
 | Table IAllele-specific PCR primer sequences
for the BRAF gene. |
Table I
Allele-specific PCR primer sequences
for the BRAF gene.
| Genes | Sequence | PCR products, bp |
|---|
| BRAF mut | Forward,
5′-GGTGATTTTGGTCTAGCTACATA-3′ | 126 |
| Reverse,
5′-GGCCAAAAATTTAATCAGTGG-3′ | |
| TBXAS1 | Forward,
5′-GCCCGACATTCTGCAAGTCC-3′ | 100 |
| Reverse,
5′-GGTGTTGCCGGGAAGGGTT-3′ | |
| BRAF seq | Forward,
5′-CTCTTCATAATGCTTGCTCTG-3′ | 269 |
| Reverse,
5′-GAGACCTTCAATGACTTTCTAGTAAC-3′ | |
Multiplex PCR amplification
BRAF and TBXAS1 genes were amplified in the same
25-μl amplification system, which included 30 ng DNA templates, 10
pmol BRAF primers, 5 pmol TBXAS1 primers, 0.1 mol/l dNTPs and 1.5
units Taq enzyme. The thermal cycling protocol for PCR
involved an initial denaturation step at 95°C for 5 min followed by
30 cycles at 94°C for 30 sec, 54°C for 30 sec and 72°C for 30 sec.
The BRAF gene was amplified in a 50 μl amplification system for
direct sequencing.
DHPLC
DHPLC was performed using the Transgenomic Wave
Nucleic Acid Fragment Analysis system with a DNASep column
(Transgenomic, Omaha, NE, USA). The mobile phases comprised 0.05%
acetonitrile in 0.1 M triethylammonium acetate (TEAA; eluent A) and
25% acetonitrile in 0.1 M TEAA (eluent B). The PCR products were
denatured at 95°C for 5 min and then cooled to 50°C to allow the
formation of heterozygote DNA. A 0.9-ml/min flow rate was used, and
the ultraviolet detector was set at 260 nm. The results were
analyzed by Navigator software (Transgenomic, Omaha, NE, USA). The
heterozygous profiles were investigated by visual inspection of the
chromatograms on the basis of the appearance of additional
later-eluting peaks. Corresponding homozygous profiles showed only
one peak. To determine the detection limit of DHPLC, DNA was
extracted from the RO82-W-1 (BRAF wild-type) and K1 (BRAF V600E
mutation) cells. Serial mixtures (BRAF V600E/Total DNA, 50, 25, 10,
3 and 1%) were made for the DHPLC analysis.
Statistical analysis
Statistical analysis was performed using SPSS 13.0
statistical software (SPSS, Inc., Chicago, IL, USA) and a
χ2 test was used for the comparisons. The category data
were estimated by odds ratio (OR) and 95% confidence intervals (CI)
in a meta-analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical data
A total of 187 cases of PTC, consisting of 159
females and 28 males (age, 8–80 years; mean ± SD, 42.57±12.88),
were selected for systematic analysis. Among them, 59 patients
presented with bilateral leaf papillary carcinoma, 47 with PTC of
the left lobe, 78 with PTC of the right lobe and three with
papillary carcinoma of the thyroid isthmus.
Multiplex allele-specific PCR sensitivity
detection
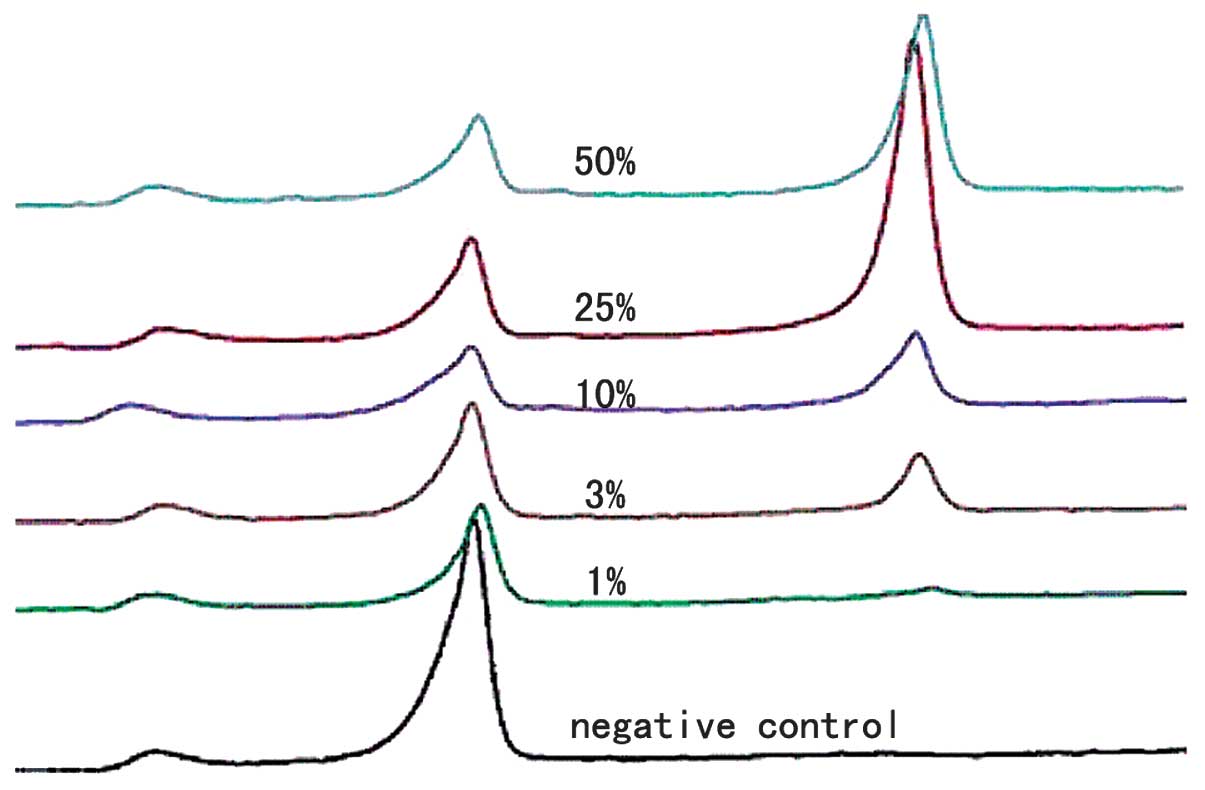
In order to investigate the sensitivity of multiplex
allele-specific PCR combined with DHPLC, different concentrations
of V600E mutation PCR products (1, 3, 10, 25 and 50%) were detected
by this method. According to the DHPLC results, two elution peaks
were observed in the presence of a V600E mutation (Fig. 1); the right elution peak was due to
the BRAF mutation and the left was due to the internal reference
TBXAS1 gene. It was demonstrated that multiplex allele-specific PCR
detection sensitivity may be up to 1% (Fig. 1).
Prevalence of BRAF V600E mutation in PTC
samples
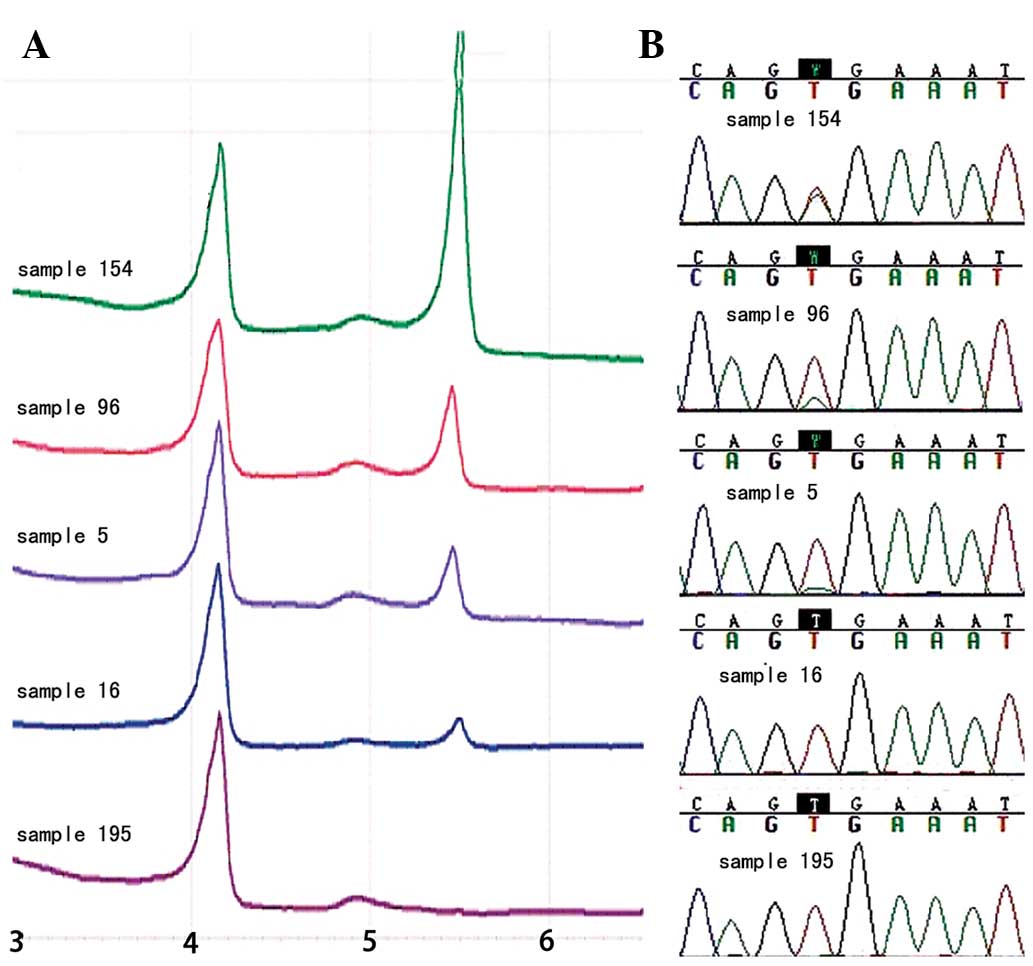
The BRAF V600E mutation was detected in 63.6%
(119/187) of PTCs (Fig. 2A). No
mutation was identified in the 20 benign thyroid lesions (data not
shown). In addition, these results were confirmed by direct
sequencing (Fig. 2B).
Association of BRAF V600E mutation status
with PTC pathological features
In patients with conventional PTC, the BRAF V600E
mutation was associated with age, tumor stage and prognosis
(P<0.05). However, the frequency of the BRAF V600E mutation was
not correlated with gender, tumor size, lymph node metastasis or
location of the lesion (Table II).
In addition, the BRAF V600E mutation was significantly different in
the central lymph nodes and lateral neck lymph nodes (75.3 vs.
49.3%; P=0.002) of patients with lymph node metastasis.
 | Table IICorrelation analysis between BRAF
V600E mutation and clinical features. |
Table II
Correlation analysis between BRAF
V600E mutation and clinical features.
| | BRAF V600E mutation,
% | |
|---|
| |
| |
|---|
| Clinical
features | Cases, n | Positive | Negative | P-value |
|---|
| Gender | | | | 0.401 |
| Male | 28 | 20 | 8 | |
| Female | 159 | 99 | 60 | |
| Age, years | | | | 0.002 |
| >45 | 75 | 58 | 17 | |
| <45 | 112 | 61 | 51 | |
| Tumor size, cm | | | | 0.279 |
| >2 | 144 | 95 | 49 | |
| ≤2 | 43 | 24 | 19 | |
| Lymph node
metastasis | | | | 0.582 |
| N1 | 146 | 91 | 55 | |
| N0 | 41 | 28 | 13 | |
| Metastasis
region | | | | 0.002 |
| Central node | 73 | 55 | 18 | |
| Lateral node | 73 | 36 | 37 | |
| Tumor stage | | | | |
| I and II | 119 | 66 | 53 | 0.003 |
| III and IV | 68 | 53 | 15 | |
| Unilateral or
bilateral lesions | | | | 0.138 |
| Bilateral | 59 | 33 | 26 | |
| Unilateral | 125 | 85 | 40 | |
| Lesion location | | | | 0.698 |
| Left lobe | 47 | 31 | 16 | |
| Right lobe | 78 | 54 | 24 | |
| Prognosis | | | | 0.03 |
| Normal | 117 | 69 | 48 | |
|
Relapse/metastasis | 59 | 45 | 14 | |
BRAF V600E mutation status as a
prognostic factor in PTC
A correlation analysis of PTC diagnosis and clinical
features was conducted. Based on the univariate analysis, tumor
recurrence/metastasis was associated with tumor size and lymph
metastasis, but not with gender, age and tumor stage. Furthermore,
the multivariate logistic regression analysis showed that lymph
node metastasis and BRAF V600E mutation were independent factors
that predicted tumor prognosis (Table
III).
 | Table IIIAssociations between the prognosis of
PTC and clinical features. |
Table III
Associations between the prognosis of
PTC and clinical features.
| Clinical
features | Univariate analysis
P-value | Multivariate
regression analysis |
|---|
|
|---|
| P-value | HR | 95% CI |
|---|
| Gender | 0.119 | 0.134 | | |
| Age, years | 0.871 | 0.999 | | |
| Tumor size, cm | 0.004 | 0.074 | 2.545 | 0.915–7.092 |
| Lymph metastasis | 0.003 | 0.044 | 3.003 | 1.027–8.771 |
| Tumor stage | 0.406 | 0.999 | | |
| BRAF mutation | 0.030 | 0.021 | 2.471 | 1.149–5.312 |
Discussion
BRAF V600E mutation detection in PTC demonstrated
that the BRAF V600E mutation was present in ~63.6% of the tumor
tissue samples, predominantly in those of PTC. In addition, no BRAF
mutations were observed in other benign-type thyroid diseases,
indicating that this genetic event was likely to be a key
determinant of the papillary cancer phenotype.
The occurrence and development of PTC involves
multiple genetic abnormalities, in which the BRAF V600E gene
mutation is the most common variant and contributes to the
destabilization of the kinase encoded by the gene. The majority of
studies have demonstrated that the BRAF V600E mutation is common in
PTC and that the frequency varies from 18 to 87%. Although certain
studies have indicated that geographical and histological subtype
classification factors may account for these differences, the
reliability of the detection method must also be taken into
consideration (7,15). In the present study, multiplex
allele-specific PCR combined with DHPLC was used to detect the
mutation. It was demonstrated that detection sensitivity may be up
to 1% by this method, showing that this would be a reliable method
of detection in clinical samples.
Although the prognostic value of the BRAF V600E
mutation in PTC is controversial, several studies have observed an
association between the mutation and a poor prognosis (5,13,16).
To analyze this association in Chinese patients, 187 PTC samples
were analyzed for the mutation and its association with clinical
features. It was observed that the BRAF V600E mutation was
associated with age, tumor stage and prognosis. In addition, the
frequency of the BRAF V600E mutation was significantly different in
the central and lateral neck lymph nodes of patients with lymph
node metastasis, which is consistent with previous studies
(14,17,18).
However, the BRAF V600E mutation was not correlated with gender,
tumor size, lymph node metastasis and location of the lesion.
Furthermore, the correlation of a PTC diagnosis and
the clinical features were analyzed by univariate and multivariate
logistic regression analyses. The results indicated that tumor
persistence/metastasis was significantly associated with tumor
size, lymph node metastasis and BRAF mutation in the univariate
analysis. Concurrent with other studies, lymph node metastasis and
BRAF mutation were independent factors in the prediction of tumor
prognosis in the multivariate logistic regression analysis
(Table III) (5,12,16,19,20).
In conclusion, the BRAF V600E mutations in PTC and
the lymph nodes are independent factors in the prediction of tumor
prognosis. Moreover, the BRAF V600E mutation is significantly
different in central lymph nodes and lateral neck lymph nodes of
patients with lymph node metastasis. Testing for this mutation may
be useful for selecting initial therapy mode and for follow-up
monitoring in PTC patients.
Acknowledgements
This study was supported by grants from the
Department of Sichuan Province, Science and Technology Support
Program (grant no. 2011SZ0147).
References
|
1
|
Ries LAG, Melbert D, Krapcho M, et al:
SEER Cancer Statistics Review, 1975–2005. National Cancer
Institute; Bethesda, MD: 2007
|
|
2
|
Schlumberger M and Pacini F: Prognostic
factors. Thyroid Tumors. Nucleon Editions; Paris: pp. 111–125.
2003
|
|
3
|
Witt RL: Initial surgical management of
thyroid cancer. Surg Oncol Clin N Am. 17:71–91. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim TH, Park YJ, Lim JA, et al: The
association of the BRAF(V600E) mutation with prognostic factors and
poor clinical outcome in papillary thyroid cancer: a meta-analysis.
Cancer. 118:1764–1773. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xing M, Clark D, Guan H, et al: BRAF
mutation testing of thyroid fine-needle aspiration biopsy specimens
for preoperative risk stratification in papillary thyroid cancer. J
Clin Oncol. 27:2977–2982. 2009. View Article : Google Scholar
|
|
6
|
Gong RX, Zhou Y, Luo SH, et al: An
investigation of BRAF mutation in papillary thyroid carcinoma and
its clinical value. Zhonghua Yi Xue Yi Chuan Xue Za Zhi.
26:310–313. 2009.(In Chinese).
|
|
7
|
Kebebew E, Weng J, Bauer J, et al: The
prevalence and prognostic value of BRAF mutation in thyroid cancer.
Ann Surg. 246:466–471. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Davies H, Bignell GR, Cox C, et al:
Mutations of the BRAF gene in human cancer. Nature. 417:949–954.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
DeLuca AM, Srinivas A and Alani RM: BRAF
kinase in melanoma development and progression. Expert Rev Mol Med.
10:e62008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xing M: BRAF mutation in thyroid cancer.
Endocr Relat Cancer. 12:245–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ball DW: Selectively targeting mutant BRAF
in thyroid cancer. J Clin Endocrinol Metab. 95:60–61. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Costa AM, Herrero A, Fresno MF, et al:
BRAF mutation associated with other genetic events identifies a
subset of aggressive papillary thyroid carcinoma. Clin Endocrinol
(Oxf). 68:618–634. 2008. View Article : Google Scholar
|
|
13
|
Howell GM, Carty SE, Armstrong MJ, et al:
Both BRAF V600E mutation and older age (≥ 65 years) are associated
with recurrent papillary thyroid cancer. Ann Surg Oncol.
18:3566–3571. 2011.
|
|
14
|
Bollag G, Tsai J, Zhang J, et al:
Vemurafenib: the first drug approved for BRAF-mutant cancer. Nat
Rev Drug Discov. 11:873–886. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tufano RP, Teixeira GV, Bishop J, et al:
BRAF mutation in papillary thyroid cancer and its value in
tailoring initial treatment: a systematic review and meta-analysis.
Medicine (Baltimore). 91:274–286. 2012. View Article : Google Scholar
|
|
16
|
Henderson YC, Shellenberger TD, Williams
MD, et al: High rate of BRAF and RET/PTC dual mutations associated
with recurrent papillary thyroid carcinoma. Clin Cancer Res.
15:485–491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alzahrani AS and Xing M: Impact of lymph
node metastases identified on central neck dissection (CND) on the
recurrence of papillary thyroid cancer: potential role of BRAFV600E
mutation in defining CND. Endocr Relat Cancer. 29:13–22. 2013.
View Article : Google Scholar
|
|
18
|
Bozec A, Dassonville O, Chamorey E, et al:
Clinical impact of cervical lymph node involvement and central neck
dissection in patients with papillary thyroid carcinoma: a
retrospective analysis of 368 cases. Eur Arch Otorhinolaryngol.
268:1205–1212. 2011. View Article : Google Scholar
|
|
19
|
Elisei R, Viola D, Torregrossa L, et al:
The BRAF(V600E) mutation is an independent, poor prognostic factor
for the outcome of patients with low-risk intrathyroid papillary
thyroid carcinoma: single-institution results from a large cohort
study. J Clin Endocrinol Metab. 97:4390–4398. 2012. View Article : Google Scholar
|
|
20
|
O’Neill CJ, Bullock M, Chou A, et al:
BRAF(V600E) mutation is associated with an increased risk of nodal
recurrence requiring reoperative surgery in patients with papillary
thyroid cancer. Surgery. 148:1139–1145. 2010.
|