Introduction
Lung cancer is the most common type of malignant
tumor and the leading cause of cancer-related mortality worldwide
(1,2). Non-small cell lung cancer (NSCLC)
accounts for ~80% of primary lung cancer, and approximately
two-thirds of NSCLC patients are diagnosed in advanced stages,
which may contribute to high mortality levels. Chemotherapy and
radiation therapy are useful treatments for patients with NSCLC.
The chemotherapeutic drug, cis-diaminedichloroplatinum
(CDDP), is one of the most frequently used agents in curing or
controlling NSCLC, but NSCLC is insensitive to CDDP-based
chemotherapy in a clinical setting, which is a major clinical
obstacle for the successful treatment of NSCLC. The underlying
mechanism has not been determined (3,4).
MicroRNAs (miRNAs) are a class of small,
single-stranded, non-coding RNAs of 19–25 nt that are involved in
the regulation of cellular development, proliferation,
differentiation, apoptosis and metabolism (5–8).
miRNAs downregulate target mRNAs by interacting with their
3′-untranslated regions through sequence-specific base-pairing
(9). Therefore, miRNAs are pivotal
in regulating gene expression and it is estimated that miRNAs
regulate ~30% of all protein-coding genes (10). In previous years, accumulating
evidence has implicated that dysregulation of miRNAs is associated
with the initiation and progression of cancer (11,12).
miRNA-101 (miR-101) belongs to a family of miRNAs that are involved
in a series of cellular activities, such as cell proliferation,
invasion and angiogenesis (13,14).
Previously, it has been found that miR-101 is expressed in several
types of cancer, including liver, prostate and breast, and emerging
evidence indicates that miRNAs may act as cancer suppressors
(15–17). In addition, it has been reported
that miR-101 induces apoptosis and suppresses tumorigenicity in
vitro and in vivo, and inhibits the migration and
invasion of gastric cancer cells (15). Notably, studies have shown that
miR-101 is evidently downregulated in NSCLC, and it inhibits cell
proliferation and invasion. Moreover, miR-101 enhances
paclitaxel-induced apoptosis in NSCLC cells by directly repressing
the enhancer of zeste homolog 2 expression (18,19).
In addition, the role of miR-101 in chemosensitivity has been
previously identified. Batchu et al reported that miR-101
enhances the chemosensitivity of pancreatic ductal adenocarcinoma
(PDAC) cells by inhibition of mammalian target of rapamycin (mTOR)
signaling via proline-rich Akt substrate 40 (PRAS40) (20). Xu et al reported that miR-101
enhanced apoptosis induced by CDDP in the HepG2 cell line by
inhibiting autophagy (21).
However, limited knowledge is available concerning whether miR-101
expression affects the chemosensitivity of NSCLC, and the
underlying molecular mechanism remains unclear.
In the present study, we provide further evidence
that miR-101 enhances the chemosensitivity of A549 NSCLC cells to
CDDP. Furthermore, miR-101 was shown to significantly promote
CDDP-induced apoptosis and suppress the colony formation by
activating the caspase 3-dependent apoptosis pathway.
Materials and methods
Cell culture and transfection
The A549 cell line was derived from adenocarcinomas
of the lung obtained from the China Center for Type Culture
Collection (Wuhan, China). The lung cancer cells (A549) were
maintained in RPMI-1640 medium (Gibco-BRL, Carlsbad, CA, USA) with
10% fetal bovine serum and antibiotics (100 U/ml penicillin and 100
μg/ml streptomycin) within a humidified atmosphere containing 5%
CO2 at 37°C. Transfection of A549 cells with plasmids,
pre-miR-101 or scrambled pre-miR control (GenePharma, Shanghai,
China), was performed using Lipofectamine 2000 according to the
manufacturer's instructions (Invitrogen Life Technologies,
Carlsbad, CA, USA). Untransfected A549 cells were employed as a
negative control.
Total RNA extraction and real-time
polymerase chain reaction (qPCR)
Total RNA was extracted from cells using a modified
TRIzol one-step extraction method (Invitrogen Life Technologies).
Stem-loop reverse transcription for mature miR-101 and U6 primers
was performed as previously described. U6 RNA was used as an miRNA
internal control. The primers used for stem-loop
reverse-transcription PCR for miR-101 were purchased from Guangzhou
RiboBio Co., Ltd. (Guangzhou, China). Each sample was conducted in
triplicate and the results were calculated using the
2−ΔΔCt method.
3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl
tetrazolium bromide (MTT) assay
The effect of miR-101 on cell growth was measured by
MTT assay. A549 cells that had been mock-transfected or transfected
with pre-miR-101 were seeded respectively into 96-well plates at a
density of 5×103 cells/well and allowed to grow
overnight. Cells were then treated with various concentrations of
CDDP (QiLu Pharmaceutical Co., Ltd., Jinan, China). Following 24 h
of CDDP treatment, 20 μl MTT (5 mg/ml; Sigma-Aldrich, St Louis, MO,
USA) was added to the cells, which were incubated in the dark for 4
h. Following the removal of the cell supernatants, formazan
crystals were dissolved in 150 μl dimethylsulfoxide. The viability
of treated cells was calculated from the average OD570 values
compared with those of the untreated cells with an enzyme-linked
immunosorbent assay reader (Infinite M200; Tecan Group Ltd.,
Männedorf, Switzerland). Each group was run in triplicate
wells.
Colony formation assay
A549 cells were transfected and treated with CDDP.
Following 24 h of treatment, cells were reseeded into six-well
plates with a density of 500 cells per well for 2–3 weeks. The
medium was discarded and each well was carefully washed twice with
phosphate-buffered saline (PBS). The colonies were fixed in
methanol for 20 min and then stained with Giemsa staining solution.
The number of colonies with ≥50 cells were counted and colony
forming efficiency was calculated using the following formula:
Percentage of colonies (%)= number of colonies formed/number of
cells inoculated ×100. Experiments were repeated three times.
Cell death analysis
Cell death was assessed by flow cytometry using
propidium iodide (PI). A549 cells were transfected and treated with
CDDP as described above. The cells from various treated groups were
collected and washed twice with cold PBS. The cells were then
resuspended in binding buffer at a concentration of
1×106 cells/ml. In total, 100 μl cell suspension
(1×105 cells) was transferred to a 5-ml culture tube.
Following this, 10 μl PI was added to the cell suspension and
incubated for 15 min at room temperature in the dark. The stained
cells were then diluted to 500 μl with binding buffer and analyzed
with a FACSCalibur flow cytometer (Beckman Coulter, Miami, FL,
USA). Triplicate assays were performed.
Apoptosis detection by terminal
deoxynucleotidyl transferase-mediated dUTP nick end labeling
(TUNEL) staining
To evaluate the degree of CDDP-induced apoptosis,
TUNEL assays were performed. A549 cells were incubated in six-well
plates and transfected as previously described. Following 24 h of
transfection and then 24 h with or without CDDP stimuli exposure,
the cells in each well were washed twice with cold PBS and fixed
with 4% (v/v) formaldehyde in PBS for 10 min at room temperature.
TUNEL was performed using the In Situ Cell Death Detection Kit,
Fluorescein (Roche Diagnostics, Mannheim, Germany) according to the
manufacturer's instructions. Randomly selected microscopic fields
(n=8) were counted under a fluorescent microscope (Leica, Wetzlar,
Germany) and the ratio of TUNEL-positive cells to the total number
of cells was calculated in three independent experiments using
ImageJ software (National Institutes of Health, Bethesda, MD,
USA).
Western blot analysis
A549 cells from various treated groups were washed
twice in cold PBS and lysed for 20 min using RIPA buffer (Thermo
Fisher Scientific, Waltham, MA, USA), with freshly added protease
inhibitor and phosphatase inhibitor (Roche Diagnostics,
Indianapolis, IN, USA). Protein concentration in the cell lysate
was determined using the BCA assay (Pierce Biotechnology, Inc.,
Rockford, IL, USA). In total, 50 μg of protein was separated on
SDS-PAGE and then transferred onto PVDF membranes (Millipore,
Billerica, MA, USA). Next, the membranes were blocked with 2% BSA
in TBST containing 0.1% Tween-20 for 1 h at room temperature.
Membranes were then incubated overnight at 4°C with antibodies
against caspase 3, (1:1,000; Cell Signaling Technology, Inc.,
Beverly, MA, USA) and β-actin (1:2,000; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA). After washing, the membranes were
incubated with horseradish peroxidase-conjugated secondary antibody
(1:2,000) followed by ECL detection (Amersham Pharmacia Biotech,
Amersham, UK).
Statistical analysis
All statistical analyses were conducted using SPSS
11.5 software (SPSS, Inc., Chicago, IL, USA). All numerical data
were generated from three independent experiments and are presented
as means ± standard error of the mean. Differences between groups
were examined using one-way analysis of variance or Student's
t-test, as appropriate. P<0.05 was considered to indicate a
statistically significant difference.
Results
Overexpression of miR-101 correlates with
cytotoxic activity of CDDP in A549 cells
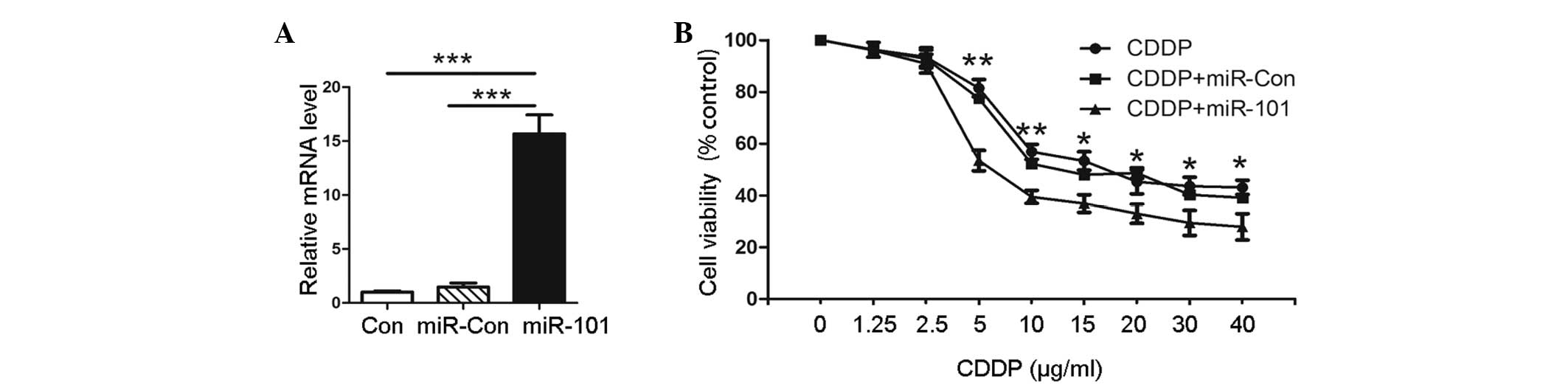
In order to explore the role of miR-101 in A549
cells, transfection with plasmids, pre-miR-101 or scrambled pre-miR
control, was performed. The expression levels of miR-101 were
confirmed by qPCR, as shown in Fig.
1A. Pre-miR-101 (miR-101)-transfected cells showed a higher
miR-101 expression than the untransfected negative control (Con)
and empty vector-transfected (miR-Con) groups. To evaluate the
effect of miR-101 on the cytotoxic activity of CDDP in A549 cells,
MTT assay was performed on the pre-miR-101 transfected cells and
the Con and miR-Con groups combined with various concentrations of
CDDP. The results showed that the viability of A549 cells with
miR-101 overexpression was significantly decreased compared with
that of the miR-Con or Con groups at the same concentration of CDDP
(Fig. 1B). This indicated that
miR-101 sensitizes A549 cells to CDDP compared with the Con group.
This observation suggested that the overexpression of miR-101
facilitates the cytotoxic activity of CDDP in A549 cells.
Overexpression of miR-101 in A549 cells
increases CDDP-induced cell death
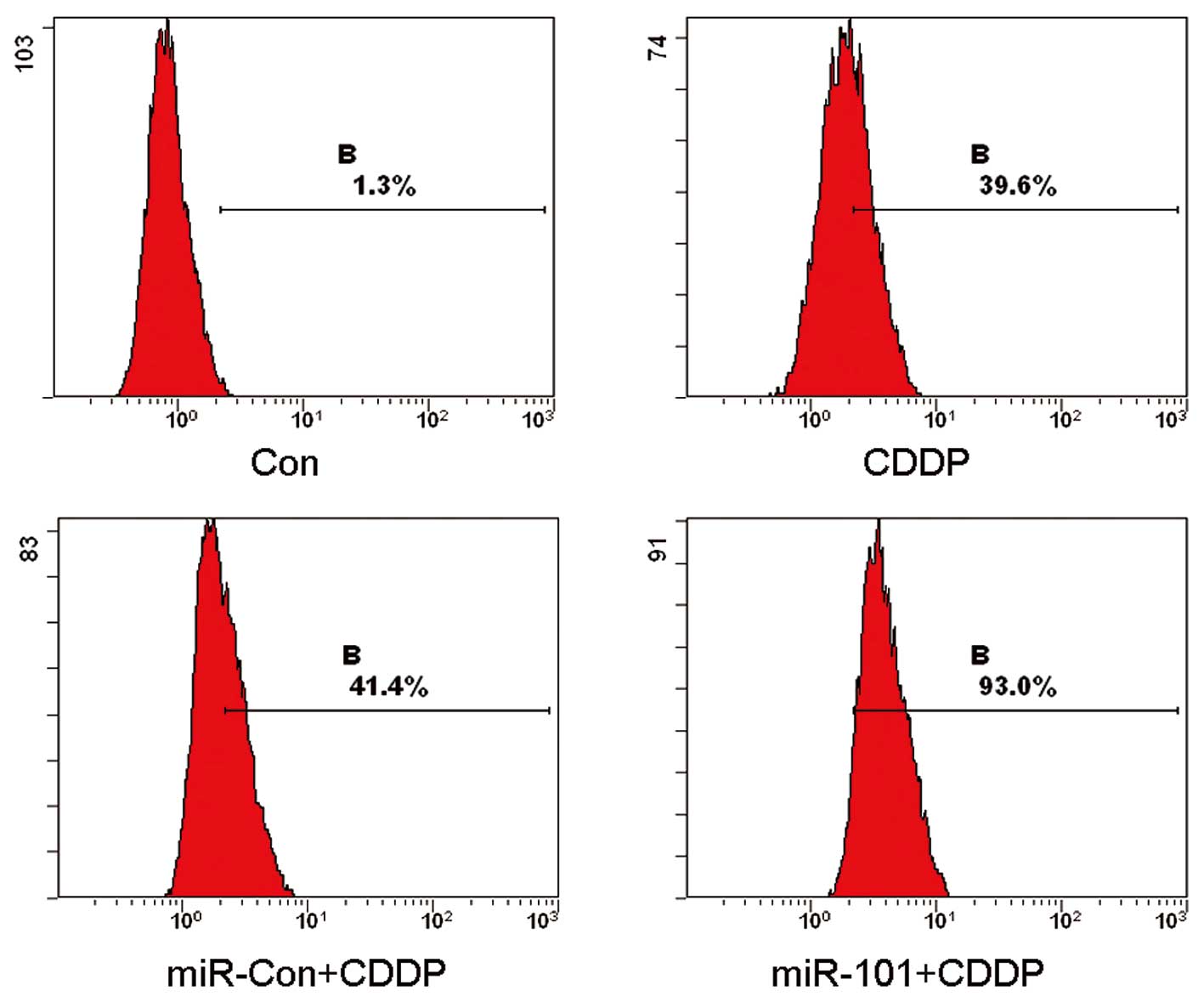
In order to explore the manner in which the
modulation of miR-101 in the A549 cell line affected CDDP-induced
cell death, pre-miR-101 transfected cells and the Con and miR-Con
groups were treated with CDDP and analyzed by flow cytometry. The
results of the flow cytometry revealed that the mean rates of cell
death were 1.3, 39.6, 41.4 and 93.0% for the Con, CDDP, CDDP plus
miR-Con and CDDP plus miR-101 groups, respectively (Fig. 2). These results clearly indicated
that the overexpression of miR-101 enhances CDDP-induced cell death
in A549 cells.
Overexpression of miR-101 promotes
CDDP-induced caspase 3-dependent apoptosis
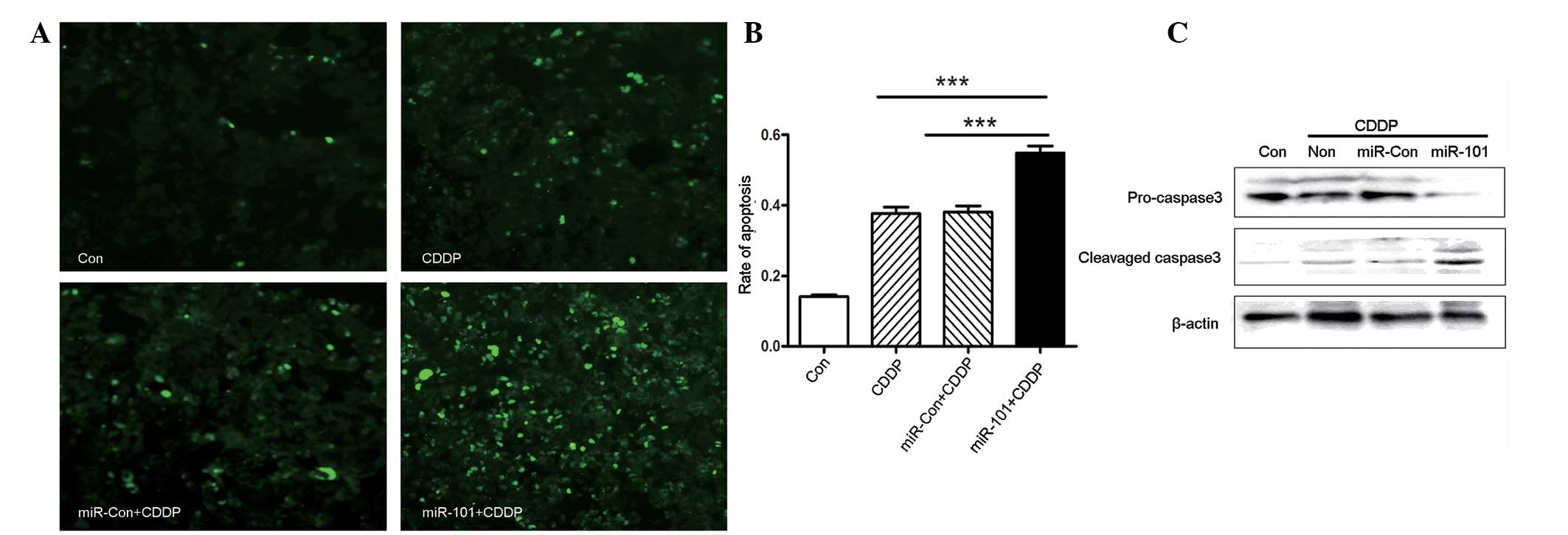
To explore whether miR-101 expression may alter
CDDP-induced apoptosis, apoptosis was measured by TUNEL staining.
As shown in Fig. 3A and B, miR-101
overexpression increased the number of apoptotic cells following
treatment with CDDP. In addition, western blot analysis
demonstrated that miR-101 was involved in caspase 3-dependent
apoptosis, as shown in Fig. 3C.
CDDP led to an increased level of cleaved caspase 3 in A549-miR-101
cells, to a greater extent than that in A549 or A549-miR-Con cells.
Overall, miR-101 overexpression enhanced chemosensitivity to CDDP
in the A549 NSCLC cancer cell line via caspase 3-dependent
apoptosis.
Overexpression of miR-101 inhibits A549
cell colony formation
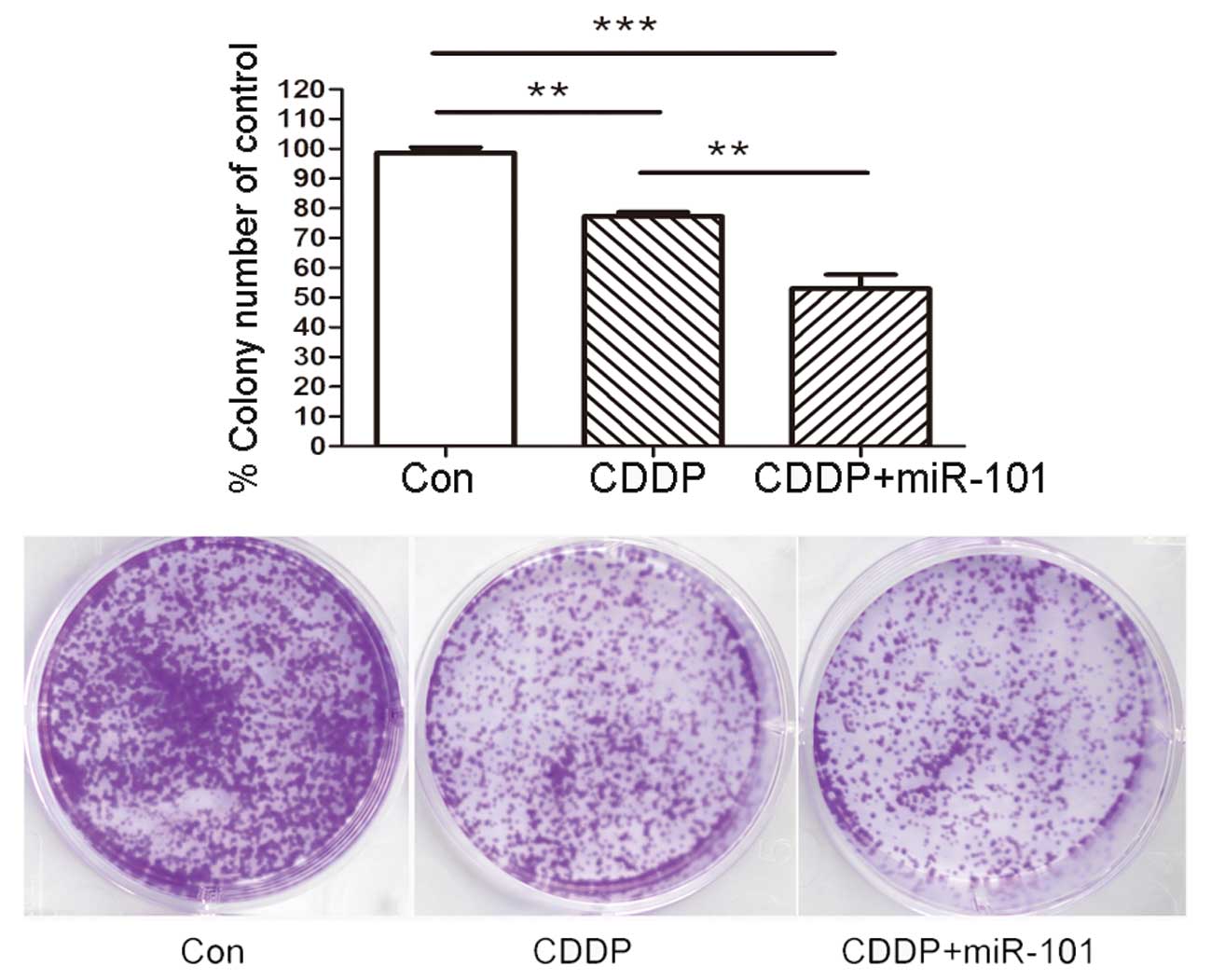
To confirm the long-term survival rate of cells, a
colony assay was performed in the A549 cell line with various
treatments as described previously. As shown in Fig. 4, overexpression of miR-101 in A549
cells caused a significant reduction in the number and diameter of
the colonies at day 14 compared with those of the Con and miR-Con
groups.
Discussion
Although chemotherapeutic agents, including CDDP,
are widely used in the treatment of lung cancer, their efficacy is
often limited by the existence or development of chemoresistance.
Factors that enhance the sensitivity of NSCLC cells to
chemotherapeutic drugs may highlight predictive biomarkers or
targets for therapy. miR-101 is an miRNA, which has been previously
demonstrated to regulate a variety of biological processes by
modulating the expression of several target genes (22,23). A
growing number of studies have implicated that miR-101 may function
as a tumor suppressor gene to inhibit the expression of
tumor-promoting genes. In addition, miR-101 has been demonstrated
to be downregulated in several types of human cancer, including
lung cancer. Emerging evidence suggests that miR-101 induces
apoptosis, suppresses tumorigenicity, inhibits migration and
invasion, and is crucial in promoting chemosensitivity (15,17,24–26).
The present study has demonstrated for the first time that miR-101
sensitizes the A549 NSCLC cell line to CDDP.
To date, the mechanism by which miR-101 enhances
chemosensitivity remains unclear. Previously, Batchu et al
reported that miR-101 enhances the chemosensitivity of PDAC cells
by inhibition of mTOR signaling via PRAS40 (20). Xu et al reported that miR-101
enhanced apoptosis induced by CDDP in the HepG2 cell line by
inhibiting autophagy (21). In
order to explore whether miR-101 expression affects the
chemosensitivity of lung tumors, the human A549 cell line was used
for transfection with the miR-101 overexpression vector. The
cytotoxic activity, proliferation and apoptosis of CDDP were then
examined in A549-miR-101 and A549-mock cells. MTT assays
demonstrated that A549-miR-101 and A549-mock cells were
differentially sensitive to CDDP. It was also found that miR-101
overexpression led to cell death, caspase 3-dependent apoptosis to
CDDP and inhibited cell colony formation. The evidence that high
miR-101 levels result in drug sensitivity indicated that miR-101
may regulate the sensitivity of chemotherapeutic drugs in NSCLC.
Overall, the results of the current study show that miR-101
overexpression effectively promotes CDDP-induced apoptosis and
inhibits colony formation in the A549 NSCLC cell line.
In conclusion, the present study demonstrated for
the first time that miR-101 functions as an inducer of
chemotherapeutic sensitivity in NSCLC by activating the caspase
3-dependent apoptosis pathway. These results established that
miR-101 transfer in combination with CDDP therapy may be a target
to reverse chemotherapeutic resistance. Further investigations with
regard to the miR-101 regulation of chemosensitivity are likely to
provide insights into the mechanistic details of this regulatory
network.
Abbreviations:
|
miR-101
|
microRNA-101
|
|
NSCLC
|
non-small cell lung cancer
|
|
CDDP
|
cis-diaminedichloroplatinum
|
|
PDAC
|
pancreatic ductal adenocarcinoma
|
|
mTOR
|
mammalian target of rapamycin
|
|
PRAS40
|
proline-rich Akt substrate 40
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kelly K, Crowley J, Bunn PA Jr, et al:
Randomized phase III trial of paclitaxel plus carboplatin versus
vinorelbine plus cisplatin in the treatment of patients with
advanced non-small-cell lung cancer: a Southwest Oncology Group
trial. J Clin Oncol. 19:3210–3218. 2001.PubMed/NCBI
|
|
4
|
Lee MW, Kim DS, Min NY and Kim HT: Akt1
inhibition by RNA interference sensitizes human non-small cell lung
cancer cells to cisplatin. Int J Cancer. 122:2380–2384. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng AM, Byrom MW, Shelton J and Ford LP:
Antisense inhibition of human miRNAs and indications for an
involvement of miRNA in cell growth and apoptosis. Nucleic Acids
Res. 33:1290–1297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu P, Guo M and Hay BA: MicroRNAs and the
regulation of cell death. Trends Genet. 20:617–624. 2004.
View Article : Google Scholar
|
|
8
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010.PubMed/NCBI
|
|
9
|
Doench JG and Sharp PA: Specificity of
microRNA target selection in translational repression. Genes Dev.
18:504–511. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bentwich I, Avniel A, Karov Y, et al:
Identification of hundreds of conserved and nonconserved human
microRNAs. Nat Genet. 37:766–770. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar
|
|
12
|
O'Day E and Lal A: MicroRNAs and their
target gene networks in breast cancer. Breast Cancer Res.
12:2012010. View
Article : Google Scholar
|
|
13
|
Smits M, Nilsson J, Mir SE, et al: miR-101
is down-regulated in glioblastoma resulting in EZH2-induced
proliferation, migration, and angiogenesis. Oncotarget. 1:710–720.
2010.PubMed/NCBI
|
|
14
|
Semaan A, Qazi AM, Seward S, et al:
MicroRNA-101 inhibits growth of epithelial ovarian cancer by
relieving chromatin-mediated transcriptional repression of
p21(waf(1)/cip(1)). Pharm Res. 28:3079–3090. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang HJ, Ruan HJ, He XJ, et al:
MicroRNA-101 is down-regulated in gastric cancer and involved in
cell migration and invasion. Eur J Cancer. 46:2295–2303. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pang Y, Young CY and Yuan H: MicroRNAs and
prostate cancer. Acta Biochim Biophys Sin (Shanghai). 42:363–369.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su H, Yang JR, Xu T, et al: MicroRNA-101,
down-regulated in hepatocellular carcinoma, promotes apoptosis and
suppresses tumorigenicity. Cancer Res. 69:1135–1142. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang JG, Guo JF, Liu DL, Liu Q and Wang
JJ: MicroRNA-101 exerts tumor-suppressive functions in non-small
cell lung cancer through directly targeting enhancer of zeste
homolog 2. J Thorac Oncol. 6:671–678. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen S, Wang H, Ng WL, Curran WJ and Wang
Y: Radiosensitizing effects of ectopic miR-101 on non-small-cell
lung cancer cells depend on the endogenous miR-101 level. Int J
Radiat Oncol Biol Phys. 81:1524–1529. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Batchu RB, Gruzdyn O, Qazi AM, Bouwman D,
Gruber SA and Weaver DW: MicroRNA-101 (miR-101) enhances
chemosensitivity of pancreatic ductal adenocarcinoma (PDAC) cells
by inhibition of MTOR signaling via PRAS40. J Surg Res.
172:2332012. View Article : Google Scholar
|
|
21
|
Xu Y, An Y, Wang Y, et al: miR-101
inhibits autophagy and enhances cisplatin-induced apoptosis in
hepatocellular carcinoma cells. Oncol Rep. 29:2019–2024.
2013.PubMed/NCBI
|
|
22
|
Sachdeva M, Wu H, Ru P, Hwang L, Trieu V
and Mo YY: MicroRNA-101-mediated Akt activation and
estrogen-independent growth. Oncogene. 30:822–831. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gui T and Shen K: miRNA-101: a potential
target for tumor therapy. Cancer Epidemiol. 36:537–540. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chiang CW, Huang Y, Leong KW, et al:
PKCalpha mediated induction of miR-101 in human hepatoma HepG2
cells. J Biomed Sci. 17:352010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Buechner J, Tomte E, Haug BH, et al:
Tumor-suppressor microRNAs let-7 and mir-101 target the
proto-oncogene MYCN and inhibit cell proliferation in
MYCN-amplified neuroblastoma. Br J Cancer. 105:296–303. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Varambally S, Cao Q, Mani RS, et al:
Genomic loss of microRNA-101 leads to overexpression of histone
methyltransferase EZH2 in cancer. Science. 322:1695–1699. 2008.
View Article : Google Scholar : PubMed/NCBI
|