Introduction
Tricholemmal carcinoma, a rare malignant tumor, was
first described in 1968 as tricholeptocarcinoma and occurred in
0.05% of the patients who underwent histopathological examinations
(1). Currently, a definitive list
of characteristic clinical signs of tricholemmal carcinoma is not
available. Therefore, diagnosis is usually determined on the basis
of the histological resemblance of the tumor to the outer root
sheath, following the exclusion of other types of skin neoplasms
(2), leading to a high misdiagnosis
rate. Pathologically, tricholemmal carcinoma is characterized by an
abundance of glycogen-rich, clear cells with foci of pillar-type
keratinization, peripheral palisading of cells and subnuclear
vacuolization (3). Additionally,
the high mitotic rate appears to be a constant feature (4).
Despite the seemingly malignant cytological
appearance of these lesions, clinical follow-up in the majority of
cases shows that recurrence or metastasis is rare (2–4).
Therefore, conservative surgical excision with the achievement of
negative margins is considered the treatment of choice for these
neoplasms. Tumor size and thickness are deemed to be the most
important prognostic risk factors (5). Previously, it has also been shown that
adjuvant radiotherapy is required for high-risk cases, when
complete resection is impossible or if recurrence/metastasis has
occurred (6–8).
The current study reports a series of 15 patients
with tricholemmal carcinoma of the head and neck region, including
one case example, with the focus on the clinicopathological
characteristics and management of these lesions.
Patients and methods
Patient diagnosis
All patients provided written informed consent in
accordance with the institutional guidelines of the Ninth People’s
Hospital (Shanghai, China). Between April 1994 and September 2010,
all 15 patients with tumors of the skin, pathologically diagnosed
as tricholemmal carcinoma and treated at the Department of Oral and
Maxillofacial Surgery, Ninth People’s Hospital, were enrolled into
the current study. The pathological material from all the cases had
been checked by two dermatopathologists who were blinded to the
results. A consensus diagnosis of tricholemmal carcinoma was
reached.
Tissue preparation
For all patients, formalin-fixed, paraffin-embedded
tissues were processed for routine microscopy, with histological
sections being stained with hematoxylin and eosin (HE).
Immunohistochemical (IHC) studies were performed on five patients.
Paraffin-embedded tissue sections were incubated with the following
selection of antibodies: Pan-cytokeratin (P-CK), keratin 15
(KRT15), vimentin (Vim), smooth muscle actin (SMA), S-100 protein,
MelanA and human melanoma black (HMB)-45.
Patient characteristics
Clinical information, which included age, gender,
tumor site, size and treatment, was obtained from the medical
records of the patients. The clinical course was followed by
regular return visits and telephone interviews. The cutoff date for
evaluation was June 1, 2012.
Results
Clinical observations
The clinical observations of the 15 patients are
summarized in Table I. In total,
there were 4 males and 11 females (male to female ratio, 1:2.75),
with a mean age of 77.5 years (range, 24–92 years). In the majority
of patients, the typical signs were a slow-growing exophytic or
polypoid mass of recent onset and/or a surface ulcer combined with
a bloody scab and keratotic nodule. Occasionally, patients also
presented with pruritus or pain related to the mass.
 | Table IClinical observations of 15 patients
with tricholemmal carcinoma of the head and neck region. |
Table I
Clinical observations of 15 patients
with tricholemmal carcinoma of the head and neck region.
| Case no. | Gender/age,
years | Site | Longest diameter,
cm | Recurrence | LN metastasis | Prognosis |
|---|
| 1 | F/78 | Nose | 1.5 | No | Yes | Mortality |
| 2 | F/88 | Temple | 5.0 | No | No | Mortalityb |
| 3 | F/24 | Face | 3.0 | No | No | Survival |
| 4 | F/83 | Bilateral face | 3.5 | Yes | No | Mortality |
| 5 | F/91 | Face | 6.0 | No | Yesa | Survival |
| 6 | M/87 | Face | 1.0 | No | No | Survival |
| 7 | M/69 | Bilateral temple | 13.0 | Yes | No | Mortality |
| 8 | F/83 | Temple | 2.0 | No | No | Survival |
| 9 | M/81 | Face | 5.0 | No | Yesa | Survival |
| 10 | F/90 | Face | 3.0 | Yes | No | Mortality |
| 11 | F/92 | Face | 2.5 | No | No | Survival |
| 12 | M/66 | Face | 3.0 | Missing | Missing | Lost to
follow-up |
| 13 | F/81 | Face | 3.0 | No | No | Survival |
| 14 | F/57 | Face (multiple
lesions) | 2.0 | Missing | Missing | Lost to
follow-up |
| 15 | F/92 | Face | 2.0 | No | No | Survival |
Lesions were distributed on the face (n=11), temple
(n=3) and nose (n=1). Multiple lesions were identified on the face
of 1 of the 15 patients, and two other patients exhibited bilateral
lesions (on the face and temples, respectively). The size of the
tumors ranged between 1–13 cm in largest diameter. The lesions were
most frequently misdiagnosed clinically as basal cell carcinoma. In
total, 7 of the 15 cases underwent pre-operative biopsy or
intraoperative frozen pathological testing. However, none of the
primary cases were diagnosed as tricholemmal carcinoma prior to the
results of the paraffin sections, reported following staining with
HE and/or IHC.
All patients were treated by radical surgical
excision, and neck dissection was performed for any patients with
suspicion of lymph node (LN) metastasis (n=5). Ultimately, the neck
LNs of two patients were positive, but all tumor margins were
negative. The repair or reconstruction method for the defect, which
was determined by the surgeons according to their clinical
practice, included adjacent flap (n=11), pectoralis major
myocutaneous flap (PMMF; n=2), free skin graft (n=2) and forearm
flap (n=1). Post-operative radiotherapy was performed on two
patients.
The follow-up results were available for 13
patients, since two patients were lost to follow-up. The median OS
was 21 months (range, 7–99 months). Of the 13 patients, five
(38.5%) succumbed to causes that included recurrence (n=3) and neck
LN metastasis (n=1). One patient succumbed to a cause unrelated to
the cancer (Parkinson’s disease). None of the patients developed
distant metastases following treatment. The DFS and OS were
31.1±7.8 and 32.9±7.4 months (mean ± SE), respectively, and the DFS
and OS rates were 69.2 and 61.5%, respectively.
Pathological observations
All 15 cases showed an exophytic appearance and a
whitish cut surface. Microscopically, the majority of cases showed
an intraepithelial lesion that featured large cells with abundant
clear cytoplasm. Specific cells showed intracytoplasmic
keratohyalin granules and abrupt keratinization without a granular
layer. The basal layer showed a tendency toward palisading and was
associated with a prominent basement membrane. Nuclei were large
and atypical; the degree of nuclear atypia and the high mitotic
rate being sufficient to indicate malignancy, although no signs of
dermal involvement were identified.
Although the majority of cases showed sharp borders,
three cases showed an infiltrative neoplasm in which the epidermis
and a large section of the dermis were replaced by large, cellular
lobules. In addition, perineural invasion was observed in one
case.
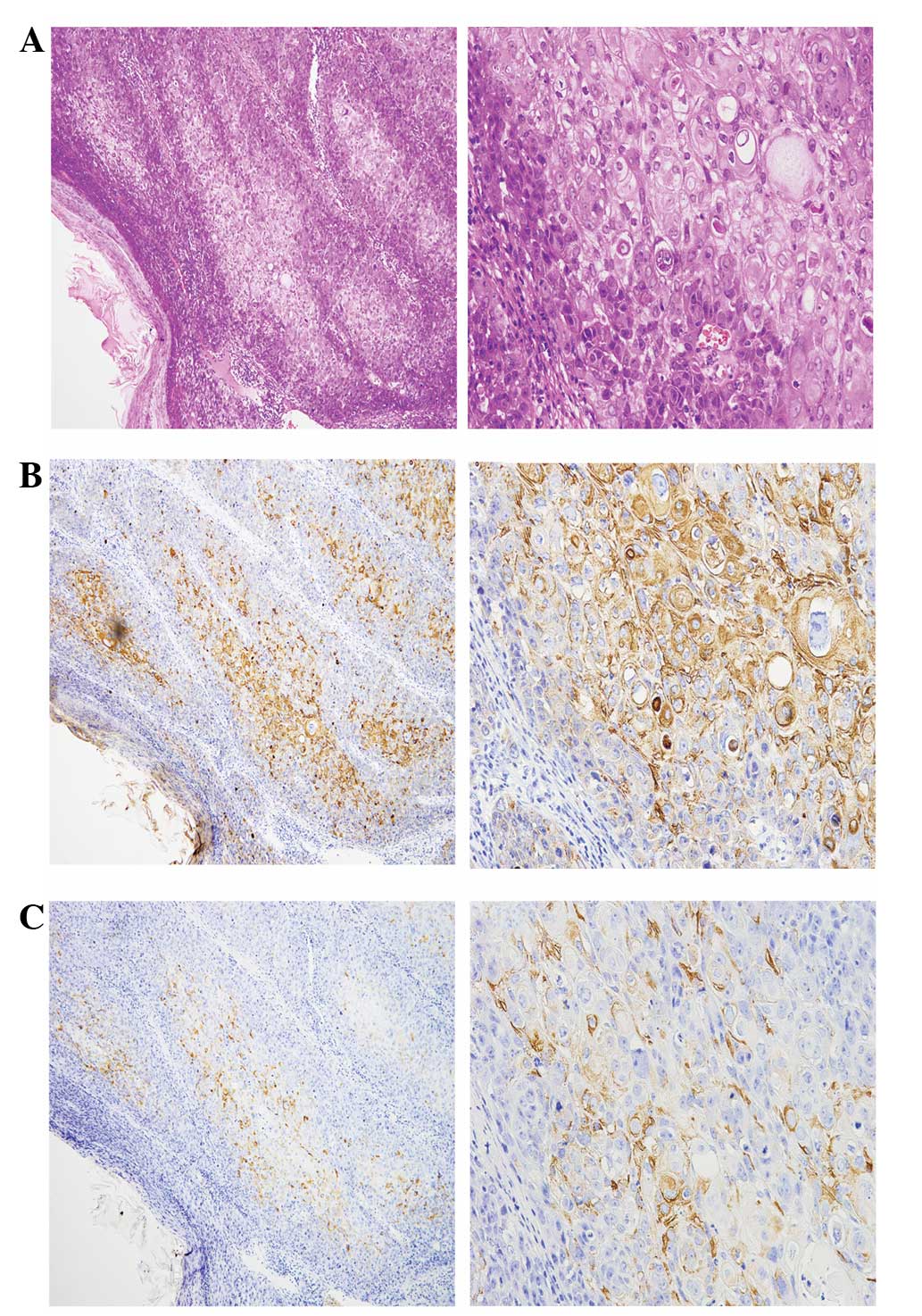
The typical results of IHC staining were as follows:
P-CK and KRT15 (a marker of outer root sheath originated tumors)
(9) were positive, and Vim, S-100,
SMA, MelanA and HMB-45 were negative. The HE and positive
pathological features (P-CK and KRT15) are shown in Fig. 1.
Typical case
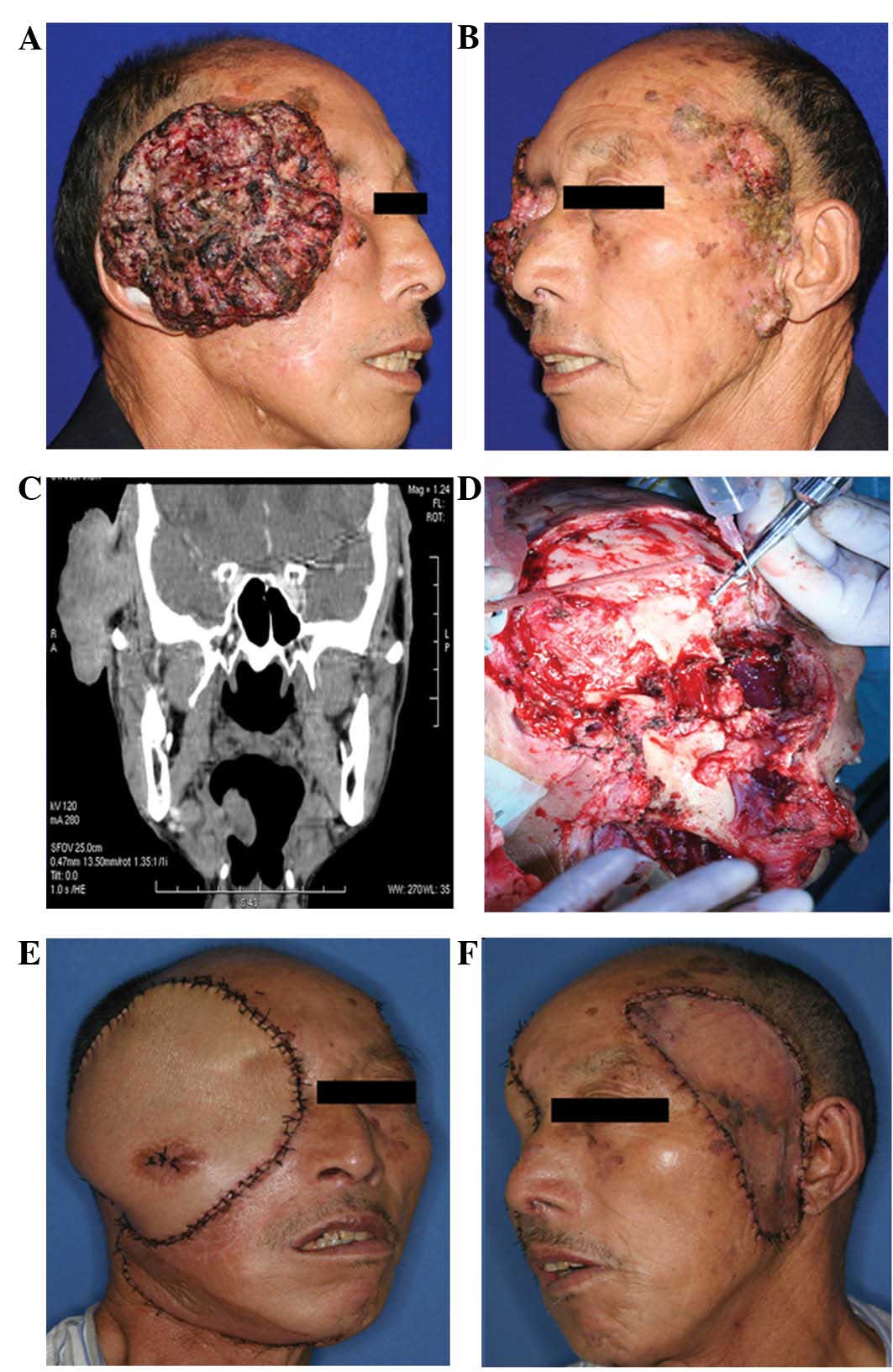
The clinical signs and treatment of a patient with a
giant lesion with recurrence/multiple lesions are shown in Fig. 2 (case no. 7). This 69-year-old male
presented with a 12×13-cm cutaneous tumor on the right temple and
multiple cutaneous tumors on the left temple and left lower
anterior tragus. Two years prior to the current admittance to the
Department of Oral and Maxillofacial Surgery, Ninth People’s
Hospital, the patient had undergone two surgeries at an alternative
hospital in order to excise tumors from his right temple and orbit;
the histological diagnosis being tricholemmal carcinoma. Only one
year later, the tumor recurred again on the right temple. The size
of the lesion on the right temple rapidly increased, whilst lesions
on the left temple and maxillofacial region occurred in succession
(Fig. 2A and 2B). The patient was
transferred to the Department of Oral and Maxillofacial Surgery,
Ninth People’s Hospital for further treatment.
A computed tomography scan of the cranial and
maxillofacial region showed that the tumor was extensively
infiltrating the soft tissues beneath the skin of the right temple.
The right temporalis muscle and soft tissue of the lateral orbital
margin were invaded (Fig. 2C).
Therefore, a wide excision of the right-sided tumor was performed
(Fig. 2D). A right radical neck
dissection was also performed, and a PMMF was selected for
reconstruction of the defect (Fig.
2E). The left-sided lesions were widely excised and the defect
was repaired simultaneously with a free skin graft from the abdomen
(Fig. 2F). The pathological
diagnosis was recurrent tricholemmal carcinoma. All resection
margins were negative, as were all neck LNs.
In view of the huge tumor size and thickness,
post-operative radiotherapy (4–6 weeks after surgery) was strongly
recommended for this patient. However, following an uneventful
post-operative course, the patient declined to accept radiotherapy
on schedule. Only 2 months later, the tumor recurred on the right
temple and the patient succumbed to recurrence 7 months after
surgery.
Discussion
Tricholemmal carcinoma occurs predominantly on the
scalps of elderly females, only occasionally arising outside the
scalp. Lesion size is usually <2 cm, but sizes of ≤25 cm have
been reported and 90% of the patients are Caucasian (10,11).
The current study focused on the clinicopathological features and
management of head and neck tricholemmal carcinoma in China. As
previously described, tricholemmal carcinoma of the head and neck
region has been most frequently misdiagnosed clinically as basal
cell carcinoma (3).
In the 15 patients presented in the current study,
the proportion of females was markedly higher compared with males
(73.3 vs. 26.7%), which is consistent with the majority of previous
reports (10), although the cause
of this higher incidence rate among females is not clear. Recent
preclinical and clinical observations have indicated that estrogen
and its receptors may be involved in the development of skin cancer
(12,13). Therefore, the hormonal changes of
menopausal women may be an additional pathogenic factor in
tricholemmal carcinoma of the head and neck region, in addition to
sun exposure.
In the majority of previous studies, tricholemmal
carcinoma was described as a solitary lesion (3,4,6), with
multiple lesions being extremely rare. However, among the 15
patients of the present study, three patients (20.0%) had bilateral
or multiple lesions. Frequent physical stimulation, which includes
insolation and mechanical stimulation, is considered a key cause of
carcinogenesis in the skin of the head and neck. These stimulatory
factors cause various high-risk regions of the skin to transform
into cancer, simultaneously or in succession. The results of the
current study indicate that besides the main lesions in the head
and neck, other plaques and abnormal skin must be closely followed
up. Biopsies and preventative resections may be effective measures
to decrease the occurrence of second cancers and improve cure
rates.
The biological behavior of tricholemmal carcinoma
remains controversial. A number of studies consider that these
tumors have a favorable prognosis (3,4). Even
for invasive cases and giant tumors, the reported rates of
post-operative recurrence and metastasis have been extremely low
following conservative, but thorough, excision (2,6).
However, other studies consider the biological behavior of
tricholemmal malignancy to be difficult to judge, reporting that
although numerous patients have undergone local excision and
post-operative radiotherapy, recurrence or LN metastasis is
frequently found (14–16). An analysis of the prognosis in the
present study showed three patients (20.0%) who exhibited neck LN
metastasis and three patients who developed recurrence, which
included one patient with repeated recurrences. As a result of
recurrence or metastasis, four patients (30.8%) succumbed. In view
of the evidence that none of the patients developed distant
metastasis following treatment, we hypothesize that the biological
behavior of head and neck tricholemmal carcinoma is more inclined
to be locoregionally aggressive.
Further analysis showed that in three-quarters of
the patients with recurrence or metastasis, the original tumor
diameter was >3 cm, a result that is consistent with previous
conclusions that tumor size and thickness are key prognostic
factors for cutaneous carcinoma (5). Therefore, it is recommended that, for
the management of tricholemmal carcinoma of large tumor size or
thickness, wide excision and neck dissection must be performed.
Post-operative radiotherapy for recurrent/LN metastasis patients
may improve the locoregional control rate. For patients with small
tumors, radical resection may lead to an improved prognosis, but
close follow-up for possible neck LN metastasis is required.
The present study reported the clinicopathological
features of head and neck tricholemmal carcinoma, which included
multiple lesions, recurrence/metastasis and invasive growth,
consistent with the overall biological behavior of this tumor being
more aggressive. The recommended management for head and neck
tricholemmal carcinoma must, therefore, be radical resection, with
post-operative radiotherapy also performed for recurrent/metastatic
lesions.
Acknowledgements
The current study was supported by the Shanghai
Leading Academic Discipline Project (project no. S30206) and the
Foundation of Medical School, Shanghai Jiao Tong University
(YZ1023) and the China Postdoctoral Science Foundation Project Fund
(no. 2013M530495)
References
|
1
|
Holmes EJ: Tumors of lower hair sheath.
Common histogenesis of certain so-called ‘sebaceous cysts,’
acanthomas and ‘sebaceous carcinomas’. Cancer. 21:234–248.
1968.PubMed/NCBI
|
|
2
|
Knoeller SM, Haag M, Adler CP and Reichelt
A: Skeletal metastasis in tricholemmal carcinoma. Clinic Orthop
Relat Res. 423:213–216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wong TY and Suster S: Tricholemmal
carcinoma. A clinicopathologic study of 13 cases. Am J
Dermatopathol. 16:463–473. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boscaino A, Terracciano LM, Donofrio V,
Ferrara G and De Rosa G: Tricholemmal carcinoma: a study of seven
cases. J Cutan Pathol. 19:94–99. 1992. View Article : Google Scholar
|
|
5
|
Brantsch KD, Meisner C, Schönfisch B, et
al: Analysis of risk factors determining prognosis of cutaneous
squamous-cell carcinoma: a prospective study. Lancet Oncol.
9:713–720. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wollina U, Bayyoud Y, Kittner T and Dürig
E: Giant tricholemmal squamous cell carcinoma with cranial
infiltration. J Clin Aesthet Dermatol. 4:34–37. 2011.PubMed/NCBI
|
|
7
|
Garcia-Serra A, Hinerman RW, Mendenhall
WM, et al: Carcinoma of the skin with perineural invasion. Head
Neck. 25:1027–1033. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gluck I, Ibrahim M, Popovtzer A, et al:
Skin cancer of the head and neck with perineural invasion: defining
the clinical target volumes based on the pattern of failure. Int J
Radiat Oncol Biol Phys. 74:38–46. 2009.
|
|
9
|
Kanitakis J, Bourchany D, Faure M and
Claudy A: Expression of the hair stem cell-specific keratin 15 in
pilar tumors of the skin. Eur J Dermatol. 9:363–365.
1999.PubMed/NCBI
|
|
10
|
Sau P, Graham JH and Helwig EB:
Proliferating epithelial cysts. Clinicopathological analysis of 96
cases. J Cutan Pathol. 22:394–406. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Casas JG and Woscoff A: Giant pilar tumor
of the scalp. Arch Dermatol. 116:13951980. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Verdier-Sevrain S, Yaar M, Cantatore J,
Traish A and Gilchrest BA: Estradiol induces proliferation of
keratinocytes via a receptor mediated mechanism. FASEB J.
18:1252–1254. 2004.PubMed/NCBI
|
|
13
|
Asgari MM, Efird JT, Warton EM and
Friedman GD: Potential risk factors for cutaneous squamous cell
carcinoma include oral contraceptives: results of a nested
case-control study. Int J Environ Res Public Health. 7:427–442.
2010. View Article : Google Scholar
|
|
14
|
Mathis ED, Honningford JB, Rodriguez HE,
Wind KP, Connolly MM and Podbielski FJ: Malignant proliferating
trichilemmal tumor. Am J Clin Oncol. 24:351–353. 2001. View Article : Google Scholar
|
|
15
|
Batman PA and Evans HJ: Metastasising
pilar tumour of scalp. J Clin Pathol. 39:757–760. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noto G, Pravatà G and Aricò M: Malignant
proliferating trichilemmal tumor. Am J Dermatopathol. 19:202–204.
1997. View Article : Google Scholar
|