Introduction
As an invasive procedure, surgery for esophageal
carcinoma may lead to serious inflammatory reactions and is
characterized by extreme changes in the serum level of cytokines
and acute phase proteins, particularly interleukin (IL)-6 and
polymorphonuclear neutrophil elastase (1). It is generally well accepted that
excessive inflammation is detrimental to postoperative recovery,
therefore, the use of perioperative corticosteroid therapy to
inhibit inflammatory mediators has been recommended as an approach
to improve prognosis (2,3). However, as reported by Yano et
al(4), the clinical benefits
and risks associated with the administration of preoperative
steroid therapy remain unclear due to controversial study results
and a lack of thorough investigation. A number of surgeons also
express concern with regard to delayed wound healing and the
potential for tumor recurrence following corticosteroid
administration in routine clinical work. Consequently,
perioperative corticosteroid administration has not been widely
accepted or used. The present meta-analysis study aims to
investigate the efficacy and safety of perioperative corticosteroid
administration following esophagectomy.
Materials and methods
Inclusion criteria and outcomes
To avoid potential bias, the present meta-analysis
only included randomized clinical trials. Participants must have
undergone an esophagectomy for a pathologically-confirmed
esophageal carcinoma. Trials must have included an intervention
group, which received perioperative corticosteroid administration,
and a control group, which received a placebo infusion of an inert
substance, such as saline water. Data from eligible studies were
extracted by two independent authors following a protocol accepted
by all authors. Extracted information included population data,
number of participants in each group, number of patients that
preliminarily withdrew or dropped out, completeness of follow-up
data, corticosteroid administration route, dosage and patient
outcomes. The primary outcomes were mortality and morbidity,
including pulmonary disorders, severe infection, anastomotic
leakage, renal and liver failure, cardiovascular disorders, failure
of any organ and additional adverse reactions, such as altered
postoperative plasma levels of IL-6 or −8 and lower postoperative
PaO2/FiO2 ratios.
Literature search sources
A comprehensive search was performed to identify all
relevant studies from the electronic and printed literature. All
included studies were analyzed regardless of the language used. The
key words used for identifying the studies included prednisone,
prednisolone, methylprednisolone, glucocorticoid, hydrocortisone,
corticosteroid, esophagectomy, esophageal cancer and randomized
controlled trial. The following bibliographic databases were
searched: PubMed (up to February 6, 2013), the Cochrane Central
Register of Controlled Trials (CENTRAL; up to February 6, 2013),
MEDLINE (between 1946 and January 31, 2013), EMBASE (between 1974
and February 6, 2013), the Chinese Biomedical Literature Database
(up to February 6, 2013), Chinese National Knowledge Infrastructure
(up to February 6, 2013) and the VIP Database for Chinese Technical
Periodicals (up to February 6, 2013).
Statistical analysis
Two authors selected the relevant studies by
searching publication titles and abstracts. All the extracted data
underwent meta-analysis using Review Manager 5.1 software (The
Cochrane Collaboration, Copenhagen, Denmark). The Mantel-Haenszel
method was used to analyze dichotomous data and the risk ratio (RR)
had 95% confidence intervals (CI). For continuous data, the inverse
variance method was used and mean differences with 95% CIs were
expressed.
The clinical and methodological heterogeneity were
initially assessed. The χ2 test was used to analyze
statistical heterogeneity, and statistical significance was
indicated by a value of P<0.1. The I2 test was also
used to estimate the total variation across all included studies.
The level of heterogeneity, which determined whether a
random-effects model or a fixed-effects model was used for pooled
data analysis, was judged according to the recommendations of
Higgins and Green (5). The risk of
bias was assessed according to criteria described in the Cochrane
Handbook for Systematic Reviews of Interventions (5). The level of evidence quality was
assessed using the Grading of Recommendations Assessment,
Development and Evaluation (GRADE) profiler software (version 3.2
for Windows; developed by Jan Brozek, Andrew Oxman and Holger
Schünemann, 2008).
Results
After analyzing all studies retrieved using the key
word search, only six eligible studies were selected, all of which
were Japanese and used methylprednisolone. In addition, no
treatments were administered orally or following the completion of
surgery. The characteristics of the included studies are listed in
Table I.
 | Table ICharacteristics of the studies
included in the present meta-analysis. |
Table I
Characteristics of the studies
included in the present meta-analysis.
| First author (year)
[ref] | Placebo (dose) | Intervention
(dose) | Administration
time | Intervention group,
n | Placebo group, n |
|---|
| Sato et
al(2002) [11] | Saline (10
mg/kg) | Methylprednisolone
(10 mg/kg) | Within 30 min prior
to surgery | 33 | 33 |
| Yano et
al(2005) [4] | Saline (500
mg/body) | Methylprednisolone
(500 mg/body) | Within 2 h prior to
surgery | 20 | 20 |
| Matsutani et
al(1998) [25] | Saline (10
mg/kg) | Methylprednisolone
(10 mg/kg) | At the time of
induction of anesthesia | 14 | 19 |
| Takeda et
al(2003) [15] | Saline (10
mg/kg) | Methylprednisolone
(10 mg/kg) | Prior to induction of
anesthesia | 7 | 10 |
| Takeda et
al(1997) [26] | Saline (30
mg/kg) | Methylprednisolone
(30 mg/kg) | Prior to induction of
anesthesia | 15 | 15 |
| Sayama et
al(1995) [27] | Saline (250
mg/body) | Methylprednisolone
(250 mg/body) | Within 2–3 h prior to
surgery | 8 | 9 |
The following parameters showed significant
differences between the control and methylprednisolone-treated
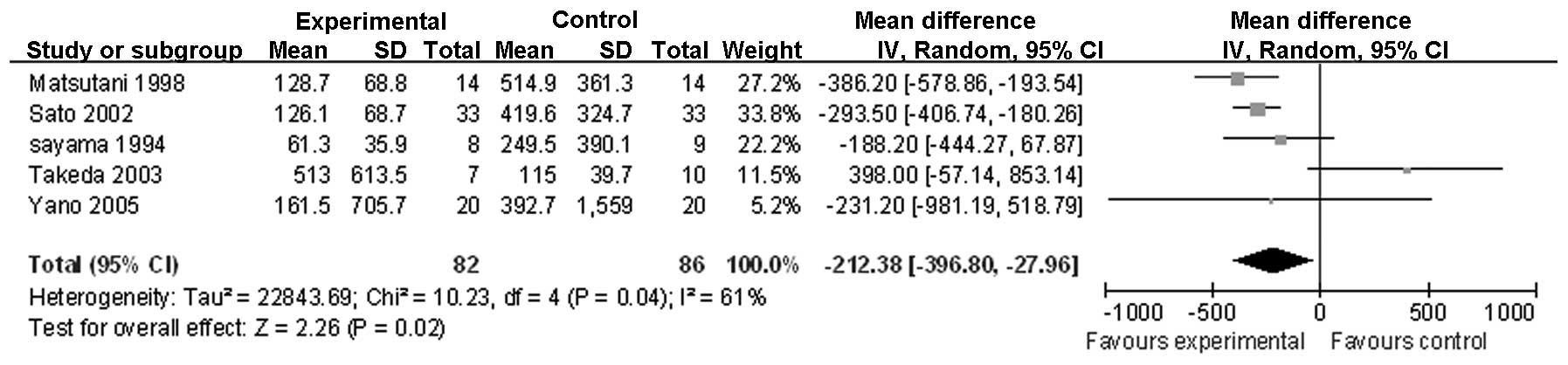
groups, as demonstrated in the following figures: IL-6 following
surgery (Fig. 1); IL-6 on
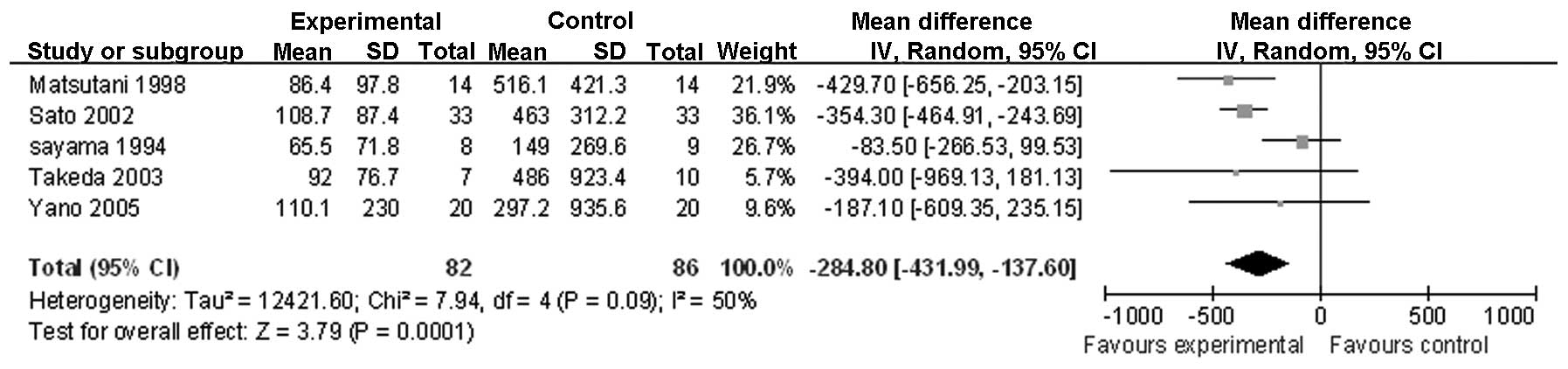
postoperative day (POD) 1 (Fig. 2);
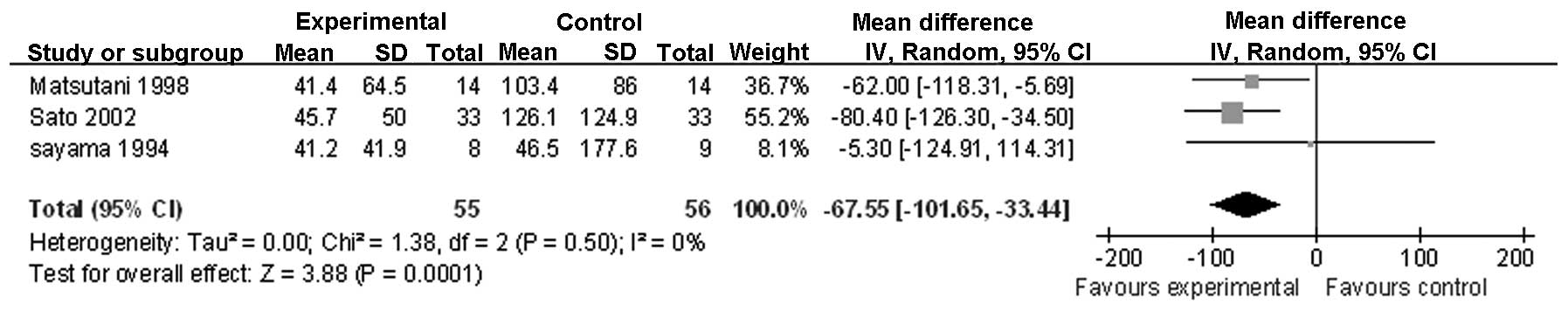
IL-6 on POD 3 (Fig. 3); IL-8
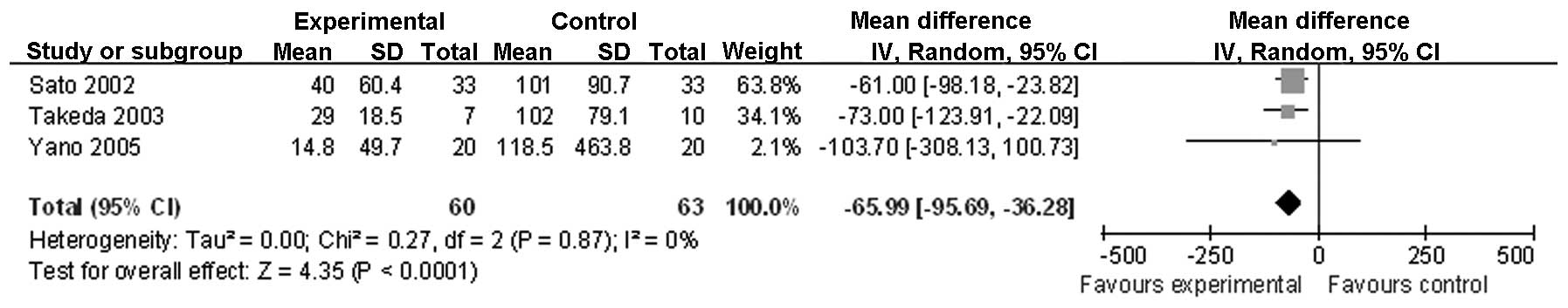
following surgery (Fig. 4);
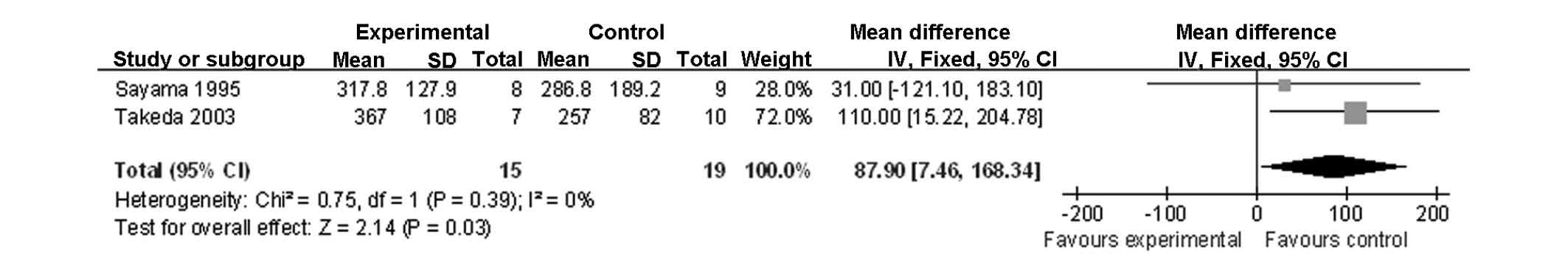
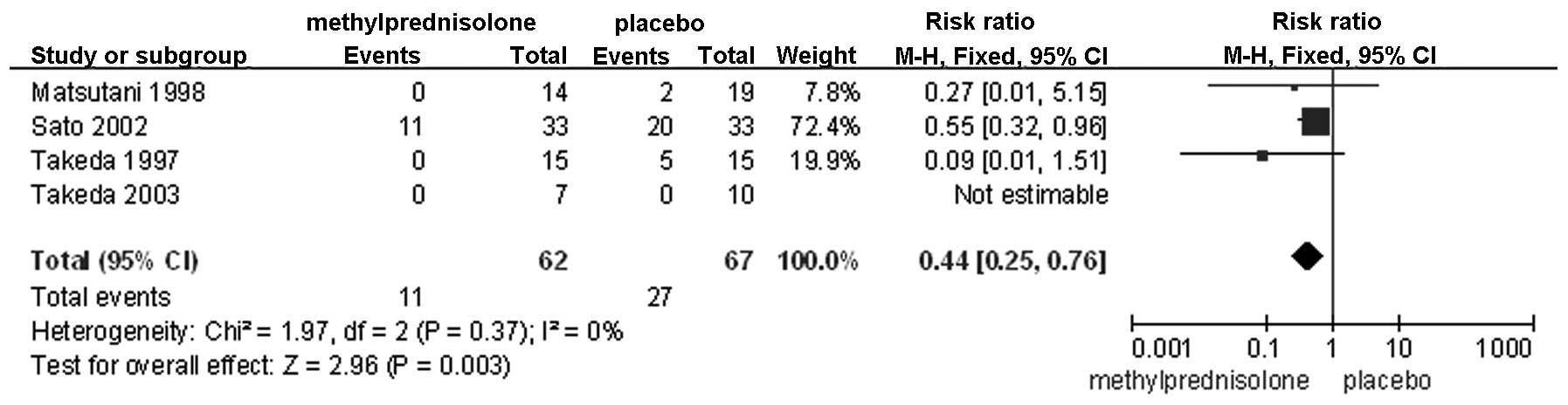
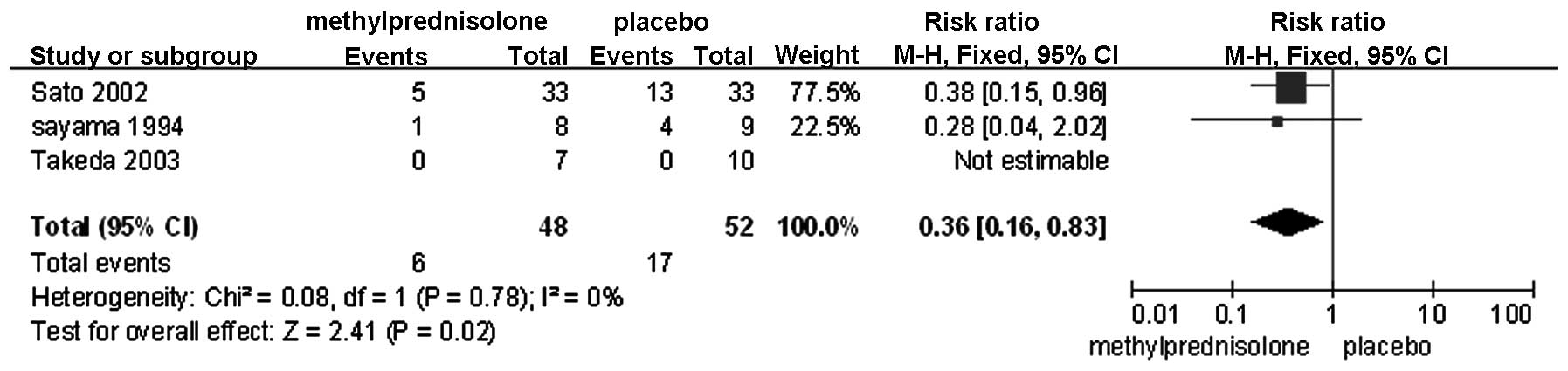
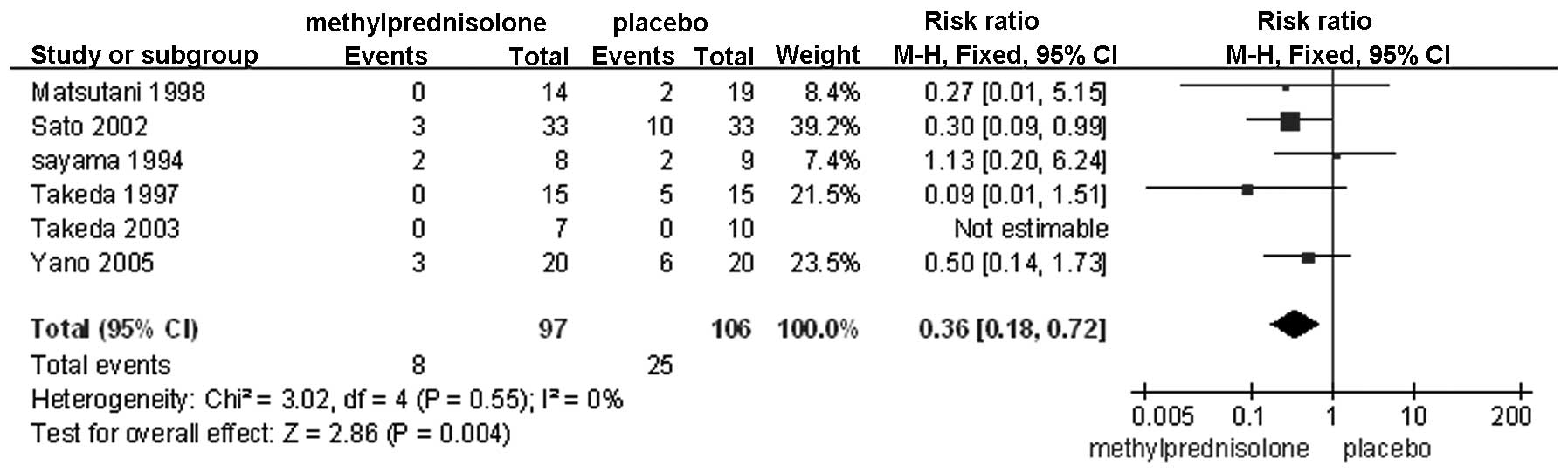
PaO2/FiO2 on POD 3 (Fig. 5); failure of any organ (Fig. 6); cardiovascular disorders (Fig. 7); and pulmonary disorders (Fig. 8). The remaining factors showed no
significant differences, notably, IL-8 on POD 1, IL-6 prior to
surgery, IL-6 on POD 5, PaO2/FiO2 following
surgery, mortality, anastomotic leakage, severe infection and renal
and hepatic failure (Table
II).
 | Table IINon-significant outcomes associated
with methylprednisolone treatment. |
Table II
Non-significant outcomes associated
with methylprednisolone treatment.
| Outcome | Studies, n | Participants, n | Effect estimate, mean
(range) | P-value |
|---|
| IL-8 on POD
1a | 3 | 123 | −15.73
(−34.64–3.18) | 0.10 |
| IL-6 prior to
surgerya | 3 | 111 | 1.73
(−16.22–19.68) | 0.85 |
| IL-6 on POD
5a | 2 | 94 | −54.95
(−140.70–30.79) | 0.21 |
|
PaO2/FiO2 following
surgerya | 2 | 34 | −3.77
(−85.94–78.40) | 0.93 |
|
PaO2/FiO2 on POD
1a | 2 | 34 | −44.88
(−115.77–26.01) | 0.21 |
|
Mortalityb | 2 | 96 | 0.13
(0.01–2.12) | 0.15 |
| Anastomotic
leakagec | 5 | 186 | 0.73
(0.26–2.07) | 0.56 |
| Severe
infectionc | 5 | 186 | 0.57
(0.23–1.38) | 0.21 |
| Renal
failurec | 3 | 113 | 0.79
(0.34–1.85) | 0.59 |
| Hepatic
failurec | 3 | 113 | 0.38
(0.09–1.56) | 0.18 |
Following evaluation of the GRADE profile, the
quality of evidence was acceptable for the description of
postoperative complications, including anastomotic leakage, organ
failure, severe infection and pulmonary disorders. By contrast, for
mortality, cardiovascular disorders, renal and hepatic failure,
inflammatory cytokines and the PaO2/FiO2
ratio, the evidence was significantly weaker (Table III).
 | Table IIIQuality of evidence assessed by GRADE
profile. |
Table III
Quality of evidence assessed by GRADE
profile.
| | Summary of
observations | |
|---|
| |
| |
|---|
| Quality
assessment | Patients, n
(%) | Effect | | |
|---|
|
|
|
| | |
|---|
| Outcome | Studies, n | Design | Limitations | Inconsistency | Indirections | Imprecision | Other
considerations | Study | Control | RR (95% CI) | Absolute
(range) | Quality | Importance |
|---|
| IL-6 prior to
surgery | 3 | Randomized
trial | Seriousa,b | Not serious | Not serious | Seriousc | Reporting
biasd | 55 | 56 | - | MD 1.73 (−16.22 to
19.68) | Extremely low | Important |
| IL-6 following
surgery | 5 | Randomized
trial | Seriousa,b | Seriouse | Not serious | Not serious | None | 82 | 86 | - | MD 212.38 (−396.8
to 27.96) | Low | Important |
| IL-6 on POD 1 | 5 | Randomized
trial | Seriousa,b | Seriousf | Not serious | Not serious | None | 82 | 86 | - | MD −284.8 (−431.99
to −137.61) | Low | Important |
| IL-6 on POD 3 | 3 | Randomized
trial | Seriousa,b | Not serious | Not serious | Not serious | Reporting
biasg | 55 | 56 | - | MD −67.55 (−101.65
to −33.44) | Low | Important |
| IL-6 on POD 5 | 2 | Randomized
trial | Seriousa,b | Serioush | Not serious | Seriousc,i | Reporting
biasj | 47 | 47 | - | MD −54.95 (−140.7
to 30.79) | Extremely low | Important |
| IL-8 following
surgery | 3 | Randomized
trial | Seriousa,b | Not serious | Not serious | Not serious | Reporting
biasd | 60 | 63 | - | MD −65.99 (−95.69
to −36.28) | Low | Important |
| IL-8 on POD 1 | 3 | Randomized
trial | Seriousa,b | Not serious | Not serious | Seriousc | Reporting
biasd | 60 | 63 | - | MD −15.73 (−34.64
to 3.18) | Extremely low | Important |
|
PaO2/FiO2 following
surgery | 2 | Randomized
trial | Seriousa,k | Not serious | Not serious | Seriousc,i | Reporting
biasj | 15 | 19 | - | MD −3.77 (−85.94 to
78.4) | Extremely low | Critical |
|
PaO2/FiO2 on POD
1 | 2 | Randomized
trial | Seriousa,k | Not serious | Not serious | Seriousc,i | Reporting
biasj | 15 | 19 | - | MD −44.88 (−115.77
to 26.01) | Extremely low | Critical |
|
PaO2/FiO2 on POD
3 | 2 | Randomized
trial | Seriousa,b | Not serious | Not serious | Seriousi | Reporting
biasj | 15 | 19 | - | MD 87.9 (7.46 to
168.34) | Extremely low | Critical |
| Anastomotic
leakage | 5 | Randomized
trial | Seriousa,b | Not serious | Not serious | Not seriousc | None | 5/89 (5.6) | 7/97 (6.1) | 0.73
(0.26–2.07) | 16 fewer/1,000 | Moderate | Critical |
| Mortality | 2 | Randomized
trial | Seriousa,b | Not serious | Not serious | Seriousc | Reporting
biasj | 0/48 (0) | 2/48 (6.7) | 0.13
(0.01–2.12) | 57 fewer/1,000 | Extremely low | Critical |
| Any organ
failure | 4 | Randomized
trial | Seriousa,b | Not serious | Not serious | Seriousl | Strong
associationm | 11/62 (17.7) | 27/67 (21.9) | 0.44
(0.25–0.76) | 122
fewer/1,000 | Moderate | Critical |
| Severe
infection | 5 | Randomized
trial | Seriousa,b | Not serious | Not serious | Not serious | None | 6/89 (6.7) | 11/97 (6.1) | 0.57
(0.23–1.38) | 26 fewer/1,000 | Moderate | Critical |
| Pulmonary
disorder | 6 | Randomized
trial | Seriousa,b | Not serious | Not serious | Not serious | None | 8/97 (8.2) | 25/106 (26.1) | 0.36
(0.18–0.72) | 167
fewer/1,000 | Moderate | Critical |
| Cardiovascular
failure | 3 | Randomized
trial | Seriousa,b | Not serious | Not serious | Seriousd | Reporting
biasn | 6/48 (12.5) | 17/52 (39.4) | 0.36
(0.16–0.83) | 252
fewer/1,000 | Extremely low | Critical |
| Renal failure | 3a,b | Randomized
trial | Seriousa,b | Not serious | Not serious | Seriousc,d | Reporting
biasn | 7/55 (12.7) | 9/58 (6.7) | 0.79
(0.34–1.85) | 14 fewer/1,000 | Extremely low | Critical |
| Hepatic
failure | 3 | Randomized
trial | Seriousa,b | Not serious | Not serious | Seriousc,d | Reporting
biasn | 2/55 (3.6) | 6/58 (6.7) | 0.38
(0.09–1.56) | 41 fewer/1,000 | Extremely low | Critical |
Discussion
As one of the more radical therapies for esophageal
cancer, esophagectomy is associated with a high incidence of
postoperative complications (6). In
addition, esophagectomy is stressful and induces an aggressive
inflammatory response (7). There
appears to be a plausible correlation between high levels of
inflammation and the incidence of postoperative complications
(8). It is well known that
postoperative immunological function, particularly cell-mediated
immunity, is profoundly repressed by an excessive inflammatory
response (9). Nekhaev et
al(10) reported that
prophylactic administration of granulocytic colony-stimulating
factor reduced the incidence of specific postoperative
complications, as well as the length of hospitalization. In this
respect, maintaining a sufficient inflammatory stress reaction may
modulate the patient's levels of immunity in a way that it is
beneficial for recovery. Consistent with this concept, Sato et
al(11) and Shimada et
al(2) reported that the
perioperative administration of methylprednisolone restricted
inflammatory cytokines to a moderate level and improved the
postoperative clinical course. The present study was designed to
highlight a comprehensive meta-analysis of the efficacy and safety
of perioperative corticosteroid administration, associated with
recovery from esophagectomy.
A predominant observation of the current study was
that corticosteroid treatment decreased the levels of postoperative
inflammatory molecules. For example, while the preoperative levels
of IL-6 were not different between the control and
methylprednisolone-treated groups, the postoperative IL-6 levels in
patients treated with methylprednisolone were significantly lower
on PODs 1 and 3. A similar change was observed for the
postoperative levels of IL-8. This is likely to be attributed to
the evidence that glucocorticoids are potent anti-inflammatory
agents that inhibit the activity of a number of immunoregulatory
genes (12), including nuclear κB
(12,13). An additional mechanism hypothesized
by Munck et al(14) states
that glucocorticoids stabilize the lysosome membrane and contain
these molecules.
Takeda et al(15) reported a negative correlation
between the levels of IL-8 and the PaO2/FiO2
ratio in bronchoalveolar lavage fluid. A previous study also
reported that IL-8 may be important for increasing the permeability
of the pulmonary endothelium through the activation of neutrophils
that generate toxic agents, including hyperoxide and protease
(16). Therefore, in theory, once
IL-8 levels are suppressed by methylprednisolone, pulmonary
function must improve. The results of the current meta-analysis
showed significant differences in the
PaO2/FiO2 ratio between the control and
methylprednisolone-treated groups on POD 3, with a higher ratio in
the treated group. By contrast, the postoperative
PaO2/FiO2 ratio was not significantly
different between the groups on POD 1. The decrease in the
oxygenation index following surgery is likely to be associated with
a systemic inflammatory response, lung injury and/or ischemic
reperfusion injury of the pulmonary vasculature. This, in turn, may
activate neutrophils to generate toxic substances and result in
further lung injury, thickening of the respiratory membrane and
increased pulmonary endothelium permeability. Although a
preoperative single dose of methylprednisolone does not completely
buffer the stress resulting from all these injurious factors, the
repression of inflammatory cytokines by the steroid is clear. This
effect may ultimately lead to a decrease in the incidence of
postoperative pulmonary disorders, a hypothesis that is consistent
with the present meta-analysis.
In addition, it must be noted that the IL-6 levels
on POD 5 and the IL-8 levels on POD 1 showed no significant
differences between the control and methylprednisolone-treated
groups. This was likely to be due to the administration of only a
single dose of methylprednisolone in all the trials and as
methylprednisolone exhibits a relatively short half-life of ~2.8 h
in blood. Thus, with decreasing drug concentration, the
anti-inflammatory effect is likely to decrease within a few hours
to days following surgery. Yeager et al(17) also proposed that the dose-dependent
effects of anti-inflammatory agents are likely to be more
prominent. These conclusions indicate that preoperative
administration of methylprednisolone alone is not sufficient to
attain the highest degree of anti-inflammatory effects and that
perioperative administration must be considered.
Of the included studies in the present
meta-analysis, the patients with postoperative cardiovascular
disorders all exhibited underlying conditions, including abnormal
changes in the preoperative electrocardiogram, and an elderly age
(18). Surgical manipulation
directly irritates the heart, particularly in a procedure such as
an esophagectomy (19). In
addition, postoperative hypoxemia caused by conditions, including
low oxygenation index or pulmonary complications, is a crucial
factor in the pathogenesis of cardiovascular disorders (20). As discussed, the administration of
methylprednisolone has been hypothesized to alleviate excessive
systemic inflammation and improve the oxygenation index and
pulmonary function. Thus, it is likely that is also decreases the
rate of postoperative cardiovascular disorders. The results of the
present meta-analysis showed a significant difference in the
incidence of these disorders between the control and
methylprednisolone-treated groups, with a lower incidence in the
treated group.
Postoperative organ failure is attributable to
multiple etiological factors, notably severe infection, serious
trauma and sepsis, which are all factors that activate the
inflammatory cascade (21). If this
response is not repressed, organ failure is likely to occur in any
organ, thus illustrating the usefulness of perioperative
administration of an anti-inflammatory agent, such as
methylprednisolone. The current meta-analysis showed that morbidity
associated with organ failure was lower in the
methylprednisolone-treated group compared with the control group,
with the exception of renal and hepatic failure, which showed no
significant difference. This clear discrepancy is hypothesized to
be due to the data associated with organ failure, as it was
assessed in the present meta-analysis by combining data from all
organs, which is likely to magnify the effect. Furthermore, the
anastomotic leakage and mortality rates were similar in the groups,
indicating that the use of methylprednisolone is likely to be well
tolerated. However, since a few of the trials that were included
had small quantities of participants, specific clinical differences
may not have been detected.
As aforementioned, there are multiple predisposing
causes of postoperative complications, among which hypernomic
inflammation is significant. Nevertheless, a moderate inflammatory
response is indispensable for postoperative recovery, particularly
when severe infection occurs (22).
Thus, it is crucial to maintain a delicate balance between pro- and
anti-inflammation. The present meta-analysis showed that the rate
of severe infection between the control and
methylprednisolone-treated groups was similar. The dose of
methylprednisolone that was used in the included trials varied
between 250 mg/body and 30 mg/kg. Calandra et al reported
that low concentrations of glucocorticoids may activate the
secretion of macrophage migration inhibitory factor (MIF) by
macrophages (23), and the
proinflammatory effects of MIF are then able to overcome the
anti-inflammatory effects of the steroids (24). Furthermore, Gao et al and
Donnelly et al found that a high concentration of MIF in
alveoli contributes to acute respiratory distress syndrome
(25,26). From these previous studies, it
appears that certain physiological mechanisms of methylprednisolone
remain to be elucidated (27), with
a clear requirement for future investigation of the administration
time and optimal dosage.
In conclusion, the present meta-analysis indicates
that methylprednisolone treatment may be associated with reduced
levels of the IL-6 and −8 inflammatory cytokines and higher
PaO2/FiO2 ratios by POD 3. However, this
association requires confirmation due to the smaller size and a
lack of rigorous randomized controlled design in a number of the
included studies. A marked association was demonstrated in the
administration of methylprednisolone with a lower incidence of
organ failure and pulmonary disorder. One significant cause of
heterogeneity is the variation in dosage and time of
administration, which weakened the evidence quality. Thus, future
rigorous randomized controlled trials with a greater number of
participants are likely to be useful for clarifying the conclusions
of the current meta-analysis and for determining the optimal
administration time and dosage of methylprednisolone.
References
|
1
|
Kikuchi K, Kurokawa H, Matsumoto F,
Iwashita S, Miyagi F, Nagai K and Nara N: Responses of cytokines,
acute phase proteins, and polymorphonuclear cell elastase to
surgical stress in the patients with esophageal cancer. Rinsho
Byori. 44:579–584. 1996.(In Japanese).
|
|
2
|
Shimada H, Ochiai T, Okazumi S, Matsubara
H, Nabeya Y, Miyazawa Y, et al: Clinical benefits of steroid
therapy on surgical stress in patients with esophageal cancer.
Surgery. 128:791–798. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kikuchi T and Kugimiya T: Clinical effects
of steroid in the perioperative management of patients undergoing
extensive esophagectomy. Masui. 51:722–727. 2002.(In Japanese).
|
|
4
|
Yano M, Taniguchi M, Tsujinaka T, Fujiwara
Y, Yasuda T, Shiozaki H and Monden M: Is preoperative
methylprednisolone beneficial for patients undergoing
esophagectomy? Hepatogastroenterology. 52:481–485. 2005.
|
|
5
|
Higgins JPT and Green S: Assessing risk of
bias in included studies. Cochrane Handbook for Systematic Reviews
of Interventions Version 5.0.2. John Wiley & Sons, Inc; New
York, NY: pp. 183–242. 2009
|
|
6
|
Lagarde SM, de Boer JD, ten Kate FJ, Busch
OR, Obertop H and van Lanschot JJ: Postoperative complications
after esophagectomy for adenocarcinoma of the esophagus are related
to timing of death due to recurrence. Ann Surg. 247:71–76. 2008.
View Article : Google Scholar
|
|
7
|
Ni Choileain N and Redmond HP: Cell
response to surgery. Arch Surg. 141:1132–1140. 2006.
|
|
8
|
Lin E, Calvano SE and Lowry SF:
Inflammatory cytokines and cell response in surgery. Surgery.
127:117–126. 2000. View Article : Google Scholar
|
|
9
|
Kimura F, Shimizu H, Yoshidome H, Ohtsuka
M and Miyazaki M: Immunosuppression following surgical and
traumatic injury. Surg Today. 40:793–808. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nekhaev IV, Sviridova SP and Kiselevskiĭ
MV: Possibilities of immune prevention of pyo-septic complications
in cancer patients by granulocytic colony stimulating factors.
Anesteziol Reanimatol. Sep–Oct;64–67. 2001.(In Russian).
|
|
11
|
Sato N, Koeda K, Ikeda K, Kimura Y, Aoki
K, Iwaya T, et al: Randomized study of the benefits of preoperative
corticosteroid administration on the postoperative morbidity and
cytokine response in patients undergoing surgery for esophageal
cancer. Ann Surg. 236:184–190. 2002. View Article : Google Scholar
|
|
12
|
LeVan TD, Behr FD, Adkins KK, Miesfeld RL
and Bloom JW: Glucocorticoid receptor signaling in a bronchial
epithelial cell line. Am J Physiol. 272:L838–L843. 1997.
|
|
13
|
Auphan N, DiDonato JA, Rosette C, Helmberg
A and Karin M: Immunosuppression by glucocorticoids: inhibition of
NF-kappa B activity through induction of I kappa B synthesis.
Science. 270:286–290. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Munck A, Guyre PM and Holbrook NJ:
Physiological functions of glucocorticoids in stress and their
relation to pharmacological actions. Endocr Rev. 5:25–44. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takeda S, Takeda S, Kim C, Ikezaki H,
Nakanishi K, Sakamoto A, et al: Preoperative administration of
methylprednisolone attenuates cytokine-induced respiratory failure
after esophageal resection. J Nippon Med Sch. 70:16–20. 2003.
View Article : Google Scholar
|
|
16
|
Hashimoto S, Gon Y, Matsumoto K, Maruoka
S, Takeshita I, Hayashi S, Asai Y, Jibiki I, Machino T and Horie T:
Selective inhibitor of p38 mitogen-activated protein kinase
inhibits lipopolysaccharide-induced interleukin-8 expression in
human pulmonary vascular endothelial cells. J Pharmacol Exp Ther.
293:370–375. 2000.
|
|
17
|
Yeager MP, Rassias AJ, Pioli PA, Beach ML,
Wardwell K, Collins JE, et al: Pretreatment with stress cortisol
enhances the human systemic inflammatory response to bacterial
endotoxin. Crit Care Med. 37:2727–2732. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xue L, Pan T, Xu Z, Zhao X, Zhong L, Wu L,
et al: Multi-factor investigation of early postoperative cardiac
arrhythmia for elderly patients with esophageal or cardiac
carcinoma. World J Surg. 33:2615–2619. 2009. View Article : Google Scholar
|
|
19
|
Shimizu H, Inoue T, Fujimura M, et al:
Cerebral blood flow after surgery for unruptured cerebral
aneurysms: effects of surgical manipulation and irrigation fluid.
Neurosurgery. 69:677–688. 2011. View Article : Google Scholar
|
|
20
|
Jensen LA, Onyskiw JE and Prasad NG:
Meta-analysis of arterial oxygen saturation monitoring by pulse
oximetry in adults. Heart Lung. 27:387–408. 1998. View Article : Google Scholar
|
|
21
|
Werner J and Büchler MW: Pancreatic
necrosis: pro surgical therapy. Chirurg. 82:507–513. 2011.(In
German).
|
|
22
|
Nathan C: Points of control in
inflammation. Nature. 420:846–852. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Calandra T, Bernhagen J, Metz CN, Spiegel
LA, Bacher M, Donnelly T, et al: MIF as a glucocorticoid-induced
modulator of cytokine production. Nature. 377:68–71. 1995.
View Article : Google Scholar
|
|
24
|
Javeed A and Zhao Y and Zhao Y:
Macrophage-migration inhibitory factor: role in inflammatory
diseases and graft rejection. Inflamm Res. 57:45–50. 2008.
View Article : Google Scholar
|
|
25
|
Gao L, Flores C, Fan-Ma S, Miller EJ,
Moitra J, Moreno L, et al: Macrophage migration inhibitory factor
in acute lung injury: expression, biomarker, and associations.
Transl Res. 150:18–29. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Donnelly SC and Bucala R: Macrophage
migration inhibitory factor: a regulator of glucocorticoid activity
with a critical role in inflammatory disease. Mol Med Today.
3:502–507. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sorrells SF and Sapolsky RM: An
inflammatory review of glucocorticoid actions in the CNS. Brain
Behav Immun. 21:259–272. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsutani T, Onda M, Sasajima K and
Miyashita M: Glucocorticoid attenuates a decrease of antithrombin
III following major surgery. J Surg Res. 79:158–163. 1998.
View Article : Google Scholar
|
|
29
|
Takeda S, Ogawa R, Nakanishi K, Kim C,
Miyashita M, Sasajima K, et al: The effect of preoperative high
dose methylprednisolone in attenuating the metabolic response after
oesophageal resection. Eur J Surg. 163:511–517. 1997.PubMed/NCBI
|
|
30
|
Sayama J, Shineha R, Yokota K, Ueda H,
Hirayama K and Ooe H: The effectiveness of preoperative steroid
therapy in preventing postoperative circulatory system
complications in surgery of esophageal cancer. Nihon Kyobu Geka
Gakkai Zasshi. 43:652–655. 1995.(In Japanese).
|