Introduction
Gastric cancer is a significant health problem
worldwide, particularly in developing countries, and it accounts
for approximately one million new cancer cases per year. In 2008,
up to 72% of new cases occurred in developing countries, resulting
in 738,000 cancer-related mortalities (1). Furthermore, China alone accounts for
42% of the worldwide gastric cancer cases (2). To date, a number of improvements have
been made for early detection and surgical approaches in the
treatment of early gastric cancer. The overall five-year survival
rate is as high as 95–100% for early cancer patients. However, the
majority of patients are diagnosed at an advanced stage of disease,
which makes a cure by surgery impossible, leading to a poor overall
five-year survival rate. If patients are able to undergo a complete
radical surgery, the overall five-year survival rate may reach
30–40%. Therefore, an early diagnosis of gastric cancer and surgery
are essential for patients to achieve an improved prognosis. Thus,
the development and identification of biomarkers for early
detection and prognosis prediction are urgently required.
The Notch1 signaling pathway, an evolutionarily
conserved cell interaction mechanism, is involved in embryo
development and normal cell proliferation, differentiation,
survival and apoptosis, including the induction of radial glia and
astrocyte differentiation. However, alterations of this gene
pathway contribute to the development of various human cancers and
their progression (3,4). Normally, Notch1, a transmembrane
protein, is activated by ligand-induced proteolysis, leading to the
release of the Notch1 intracellular domain (NICD) from the
cytolemma and in turn translocation into the nuclei of cells for
controlling the expression of certain genes, including Hes-1 and
Hes-5. These downstream target genes are typically regulated
through an interaction between NICD and the DNA-binding
transcription factor protein, CSL, which maintains normal
homeostasis in the human body (5).
However, dysregulation of Notch1 or the expression of its
functional domain, NICD, may be involved in tumorigenesis. A
previous study has shown that abnormal Notch1 signaling contributes
to the development and occurrence of gastric cancer (6).
Furthermore, p21/WAF1 protein, also known as
cyclin-dependent kinase (CKD) inhibitor 1, is able to bind to and
inhibit the activity of cyclin-CDK2 complexes, thus regulating the
G1 phase progression of the cell cycle. Normally, p21 expression is
tightly controlled by the tumor suppressor protein, p53, to mediate
p53-dependent G1 arrest of the cell cycle in response to a variety
of stress stimuli. A number of studies have shown that the
activation of Notch1 signaling promotes p21 expression in certain
types of tumor cells, but inhibits p21 expression in other types
(7,15). Specifically, a previous study has
shown that Notch signaling induced cell cycle arrest in small cell
lung cancer cells (7). Another
study has revealed that activated Notch1 interacted with p53 to
inhibit its phosphorylation and transactivation (12). In addition, Notch1 has been shown to
regulate the Akt signaling pathway and the expression of the cell
cycle regulatory proteins cyclin D1, CDK2 and p21 in T-cell acute
lymphoblastic leukemia cell lines (15). Therefore, the association between
NICD and p21 proteins and the expression of these proteins in
gastric cancer development and progression requires further
investigation. Thus, in the present study, an immunohistochemical
analysis of the two proteins in gastric tissues with varying
degrees of histological development was performed to assess their
association with gastric cancer.
Patients and methods
Tissue specimens
In the present study, 109 surgically resected tissue
specimens were retrospectively retrieved from gastric cancer
patients who underwent surgery between 2007 and 2009 at The First
Affiliated Hospital of Wenzhou Medical College, Wenzhou, China. The
patient group comprised 83 males and 26 females, with a mean age of
60.5 years old [standard deviation (SD), ±11.3]. All patients were
histopathologically diagnosed with well-differentiated
adenocarcinoma (n=5), moderately differentiated adenocarcinoma
(n=42) or poorly differentiated adenocarcinoma (n=62) of the
stomach. The patients were diagnosed according to the
tumor-node-metastasis staging system by the 1997 International
Union Against Cancer, with stage I (n=15, 13.8%), stage II (n=18,
16.5%), stage III (n=46, 42.2%) and stage IV (n=30, 27.5%) tumors.
No patients were administered any neoadjuvant therapy prior to
surgery. In addition, biopsy specimens were obtained from 17
subjects with normal gastric mucosa (who were healthy persons or
presented with some symptoms but had histologically normal gastric
mucosae), 50 patients with chronic superficial gastritis and 57
patients with precancerous gastric lesions (four cases with a
gastric ulcer, two cases with a gastric polyp and 51 cases with
chronic atrophic gastritis) through endoscopy. In the normal
gastric mucosa group, there were nine males and eight females (mean
age ± SD, 42.2±9.8 years). In the chronic superficial gastritis
group, there were 27 males and 23 females (mean age ± SD, 40.4±10.4
years) and in the precancerous gastric lesion group (nine chronic
atrophic gastritis, 26 atrophic gastritis with intestinal
metaplasia, three chronic superficial gastritis with focal areas of
atrophic intestinal metaplasia and three atypical hyperplasia
patients), there were 35 males and 22 females (mean age ± SD,
47.2±12.4 years). Approval for this study was obtained from the
Ethics Review Committee of The First Affiliated Hospital of Wenzhou
Medical College. Written informed consent was obtained from each
patient. All tissue specimens were fixed in 10% formalin and
embedded in paraffin. The patients with gastric cancer were
followed up at our outpatient clinic until they succumbed to the
disease. The last follow-up appointment was on April 1, 2011.
Immunohistochemistry
For immunohistochemical staining, the paraffin
blocks of each patient were retrieved from the Pathology Department
and cut into 3-μm thick sections onto 1% polylysine-coated glass
slides. The first section of each block was stained with
hematoxylin and eosin to reconfirm the pathological diagnosis. The
sections were then stained immunohistochemically using a standard
biotin-streptavidin-peroxidase method according to a previous study
(16). The primary rabbit
anti-human NICD antibody was purchased from Merck-Millipore
(Darmstadt, Germany) and diluted at 1:100. The mouse anti-human p21
antibody was obtained from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA) and diluted at 1:50. The secondary antibody and the
universal immunohistochemical staining kit (PV6001 and PV9003,
respectively) were purchased from Zhongshan Goldenbridge
Biotechnology Company (Zhongshan, China).
Review and scoring of the immunostained
tissue sections
The immunostained tissue sections were independently
reviewed and scored under a microscope by two pathologists. A brown
color or light brown particles in the cytoplasm and/or the nucleus
of the cells was considered as positive staining. A total of 10
fields were randomly selected at low magnification (x40) and 100
epithelial cells from each field were counted. The fields were
scored as 0 (<1% of the cells stained), one (1–19% staining),
two (20–40% staining) or three (>40% staining), according to a
previous study (16). p21 protein
was reviewed using the same procedure as for NICD, and scored as 0
(<1% of the cells stained), one (1–24% staining), two (25–75%
staining) or three (>75% staining), as described previously
(16).
Statistical analyses
All data were analyzed using SPSS 16.0 statistical
software (SPSS, Inc., Chicago, IL, USA). The comparison between the
groups was analyzed using the χ2 test. The correlation
of variables was analyzed using the Spearman’s rank correlation
test. The survival rates were calculated using the Kaplan-Meier
method and compared by the log-rank test. The Cox proportional
hazards regression model was used to measure the independent
contribution of each variable to the overall survival. P<0.05
was considered to indicate a statistically significant
difference.
Results
Differential expression of NICD and p21
proteins in gastric cancer, precancerous gastric lesions and normal
gastric tissues
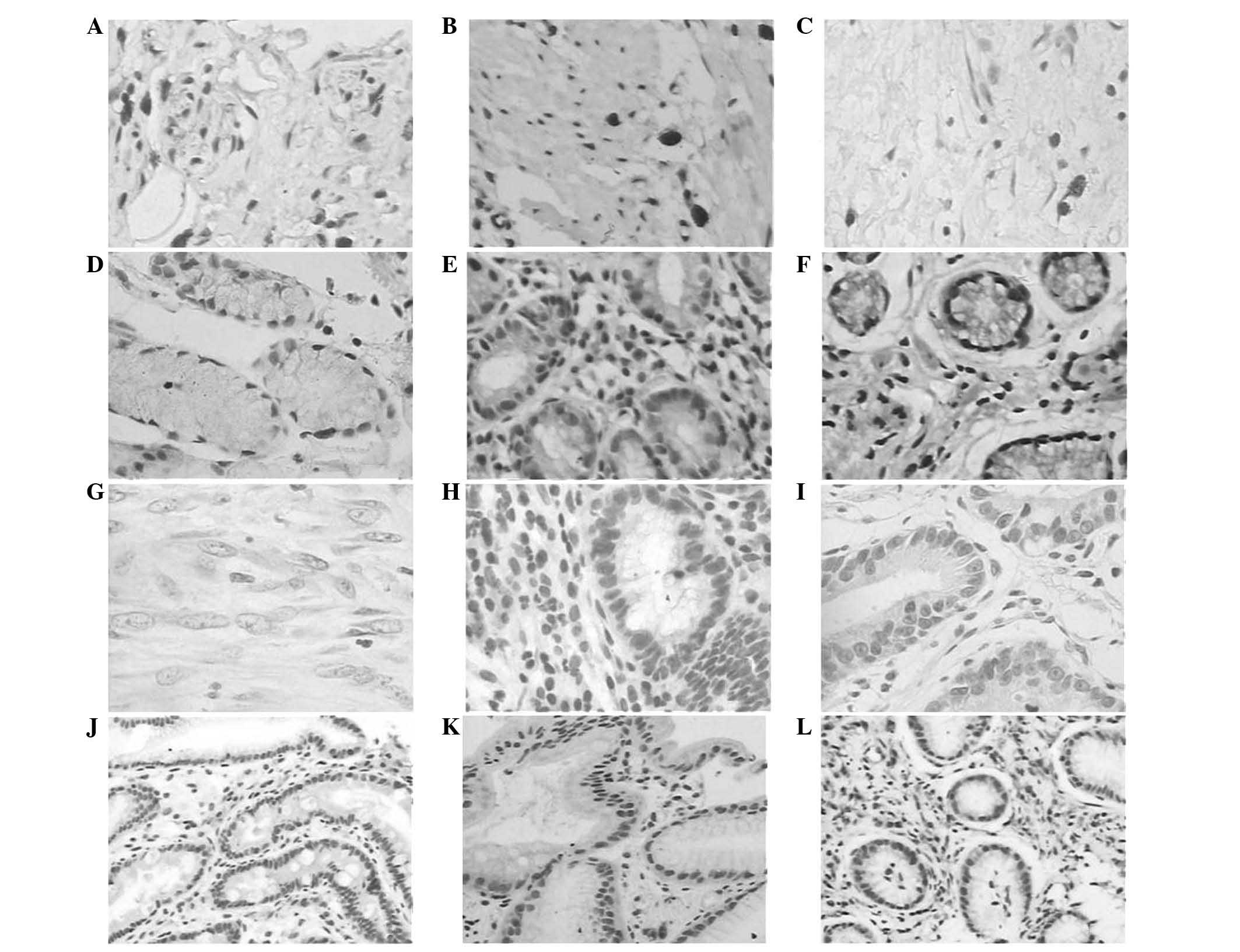
In the present study, NICD expression was first
detected in the gastric tissue specimens. The NICD protein was
observed to be mainly expressed in the nuclei of epithelial cells
and occasionally in the cytoplasm. The NICD protein was expressed
in 67.9% (74/109), 36.8% (21/57), 30.0% (15/50) and 23.5% (4/17) of
the gastric cancer, precancerous lesion, chronic superficial
gastritis and normal gastric mucosa samples, respectively,
suggesting that NICD expression was upregulated in the gastric
cancer and premalignant lesions. The difference was statistically
significant (χ2, 30.57; P<0.01). NICD+
expression was significantly greater in the gastric cancer samples
than in the precancerous lesion (χ2, 14.74; P<0.01),
chronic superficial gastritis (χ2, 19.97; P<0.01) and
normal gastric mucosa (χ2, 12.27; P<0.01) samples.
However, there was no statistically significant difference in NICD
expression between the precancerous lesion and chronic superficial
gastritis (χ2, 0.56; P>0.05) or normal gastric mucosa
(χ2, 1.04; P>0.05) samples, or between the chronic
superficial gastritis and normal gastric mucosa (χ2,
0.26; P>0.05) samples. Furthermore, p21 expression was also
analyzed in these tissues. The p21 protein was located in the
nuclei and was expressed in 38.5% (42/109), 75.4% (43/57), 82.0%
(41/50) and 82.4% (14/17) of the gastric cancer, precancerous
lesion, chronic superficial gastritis and normal gastric mucosa
samples, respectively, suggesting that p21 protein was
downregulated from normal mucosae through premalignant lesions to
gastric cancer (χ2, 40.24; P<0.01). p21+
expression was significantly lower in the gastric cancer than in
precancerous lesion (χ2, 20.40; P<0.01), chronic
superficial gastritis (χ2, 25.96; P<0.01) and normal
gastric mucosa (χ2, 11.44; P<0.01) samples. However,
there was no significant difference in p21 expression between the
precancerous lesion and chronic superficial gastritis samples
(χ2, 0.68; P>0.05), between the precancerous lesion
and normal gastric mucosa samples (χ2, 0.35; P>0.05)
or between the chronic superficial gastritis and normal gastric
mucosa samples (χ2, 0.01; P>0.05) (Table I; Fig.
1).
 | Table IDifferential expression of NICD and
p21 proteins in gastric tissue specimens. |
Table I
Differential expression of NICD and
p21 proteins in gastric tissue specimens.
| A, Positive rate of
NICD and p21 proteins in differential specimens. |
|---|
|
|---|
| Group | n | NICD+, n
(%) | χ2 | P-value | p21+, n
(%) | χ2 | P-value |
|---|
| Gastric cancer | 109 | 74 (67.89) | 30.57 | P<0.01 | 42 (38.53) | 40.24 | P<0.01 |
| Precancerous
lesions | 57 | 21 (36.84) | | | 43 (75.44) | | |
| Chronic superficial
gastritis | 50 | 15 (30.00) | | | 41 (82.00) | | |
| Normal gastric
mucosa | 17 | 4 (23.53) | | | 14 (82.35) | | |
| B, Comparison
between differential groups using χ2 test. |
|---|
|
|---|
| Comparison | χ2 | P-value | χ2 | P-value |
|---|
| Cancer vs.
precancerous lesions | 14.47 | <0.01 | 20.40 | <0.01 |
| Cancer vs. chronic
superficial gastritis | 19.97 | <0.01 | 25.96 | <0.01 |
| Cancer vs. normal
gastric mucosa | 12.27 | <0.01 | 11.44 | <0.01 |
| Precancerous
lesions vs. chronic superficial gastritis | 0.56 | >0.05 | 0.68 | >0.05 |
| Precancerous
lesions vs. normal gastric mucosa | 1.04 | >0.05 | 0.35 | >0.05 |
| Chronic superficial
gastritis vs. normal gastric mucosa | 0.26 | >0.05 | 0.01 | >0.05 |
Association of NICD and p21 expression
with clinicopathological features of gastric cancer patients
To assess the clinical significance of NICD and p21
expression, the expression levels of the proteins were analyzed
against the clinicopathological features of the gastric cancer
patients. The data revealed that NICD protein expression was
significantly associated with a larger tumor size (χ2,
5.40; P<0.05), tumor dedifferentiation grade (χ2,
16.85; P<0.01), depth of tumor invasion (χ2, 14.77;
P<0.01), lymph node metastasis (χ2, 4.82; P<0.05),
surface morphology (χ2, 13.89; P<0.01) and Lauren
classification (χ2, 4.60; P<0.05). By contrast, no
association with age (χ2, 2.45; P>0.05), gender
(χ2, 1.28; P>0.05), tumor location (χ2,
2.53; P>0.05), vascular invasion (χ2, 1.13;
P>0.05) or distant metastasis (χ2, 0.31; P>0.05)
was identified. Furthermore, a loss of p21 expression was closely
associated with tumor dedifferentiation (χ2, 15.45;
P<0.01), depth of tumor invasion (χ2, 10.75;
P<0.01), vascular invasion (χ2, 5.12; P<0.05),
lymph node metastasis (χ2, 5.21; P<0.05), surface
morphology (χ2, 9.68; P<0.01) and Lauren
classification (χ2, 7.78; P<0.01). There was also no
association with age (χ2, 2.20; P>0.05), gender
(χ2, 0.00; P>0.05), tumor location (χ2,
0.80; P>0.05), tumor size (χ2, 0.23; P>0.05) or
distant metastasis (χ2, 0.01; P>0.05) (Table II).
 | Table IIAssociation of NICD and p21
expression with the clinicopathological features of gastric cancer
patients. |
Table II
Association of NICD and p21
expression with the clinicopathological features of gastric cancer
patients.
| Group | n | NICD+, n
(%) | χ2 | P-value | p21+, n
(%) | χ2 | P-value |
|---|
| Age (years) |
| <60 | 46 | 35 (76.9) | 2.45 | >0.05 | 14 (30.4) | 2.20 | >0.05 |
| ≥60 | 63 | 39 (61.9) | | | 28 (44.4) | | |
| Gender |
| Male | 83 | 54 (65.1) | 1.28 | >0.05 | 32 (38.6) | 0.00 | >0.05 |
| Female | 26 | 20 (76.9) | | | 10 (38.5) | | |
| Location |
| Cardia and
fundus | 18 | 12 (66.7) | 2.53 | >0.05 | 7 (38.9) | 0.80 | >0.05 |
| Gastric body | 39 | 29 (74.4) | | | 14 (35.9) | | |
| Angular
region | 12 | 6 (50.0) | | | 6 (50.0) | | |
| Antrum and
pylorus | 40 | 27 (67.5) | | | 15 (37.5) | | |
| Tumor size
(cm) |
| <3 | 42 | 23 (54.8) | 5.40 | <0.05 | 15 (35.7) | 0.23 | >0.05 |
| ≥3 | 67 | 51 (76.1) | | | 27 (40.3) | | |
|
Differentiation |
| Well and
moderate | 47 | 22 (46.8) | 16.85 | <0.01 | 28 (59.6) | 15.45 | <0.01 |
| Poor | 62 | 52 (83.9) | | | 14 (22.6) | | |
| Depth of tumor
invasion |
| T1+T2 | 30 | 20 (66.7) | 14.77 | <0.01 | 19 (63.3) | 10.75 | <0.01 |
| T3+T4 | 79 | 55 (69.6) | | | 23 (29.1) | | |
| Vascular
invasion |
| Positive | 64 | 46 (71.9) | 1.13 | >0.05 | 19 (29.7) | 5.12 | <0.05 |
| Negative | 45 | 28 (62.2) | | | 23 (51.1) | | |
| Lymph node
metastasis |
| Positive | 69 | 52 (75.4) | 4.82 | <0.05 | 21 (30.4) | 5.21 | <0.05 |
| Negative | 40 | 22 (55.0) | | | 21 (52.5) | | |
| Distant
metastasis |
| Positive | 10 | 6 (60.0) | 0.31 | >0.05 | 4 (40.0) | 0.01 | >0.05 |
| Negative | 99 | 68 (68.7) | | | 38 (38.3) | | |
| Surface
morphology |
| Early gastric
cancer | 11 | 2 (18.2) | 13.89 | <0.01 | 9 (81.8) | 9.68 | <0.01 |
| Progressive
gastric cancer | 98 | 72 (73.5) | | | 33 (33.7) | | |
| Lauren
Classification |
| Intestinal
type | 65 | 39 (60.0) | 4.60 | <0.05 | 32 (49.2) | 7.78 | <0.01 |
| Diffused type | 44 | 35 (79.6) | | | 10 (22.7) | | |
Association between NICD and p21
expression in gastric cancer
NICD expression was compared with p21 expression in
gastric cancer, and the results are provided in Table III. Spearman’s rank correlation
test showed that NICD protein expression was inversely associated
with p21 protein expression.
 | Table IIIAssociation of NICD and p21 protein
expression. |
Table III
Association of NICD and p21 protein
expression.
| Group | n (%) | χ2 | P-value |
|---|
|
NICD+/p21+ | 20 (18.35) | 7.40 | P<0.01 |
|
NICD−/p21− | 13 (11.93) | | |
|
NICD+/p21− | 54 (49.54) | | |
|
NICD−/p21+ | 22 (20.18) | | |
Association of NICD and p21 expression
with overall survival of gastric cancer patients
In the present study, gastric cancer patients were
followed up for overall survival until April 1, 2011. The overall
survival was defined as the time from the surgery to April 1, 2011,
provided that the patient survived until that date, or the date of
mortality. The 109 gastric cancer patients were followed up for
5–40 months with a mean follow-up time of 21.09±6.82 months, among
which there were 55 patients who succumbed to the disease prior to
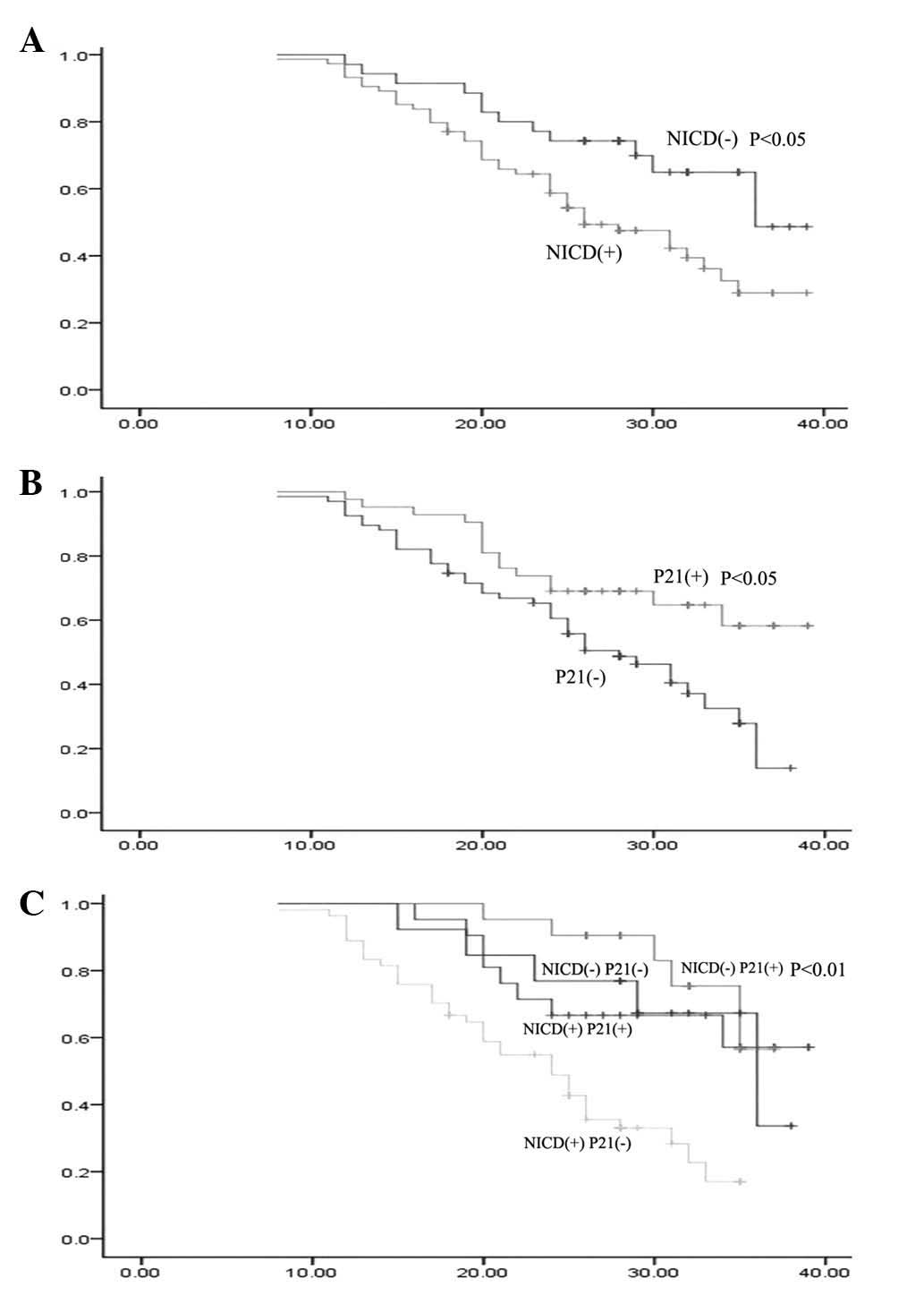
the last follow-up. The two- and three-year survival rates of
NICD+ (58.7 and 28.9%, respectively) gastric cancer
patients were significantly lower than those that were
NICD− (74.30 and 48.70%, respectively; χ2,
6.01; P<0.05; Fig. 2A). The
three-year survival rate of gastric cancer patients with
p21+ (58.3%) expression was significantly greater than
that of p21− patients (13.90%) (χ2, 6.84;
P<0.05; Fig. 2B). The survival
rates were determined using the expression data of NICD and p21,
and the two- and three-year survival rates were 90.5 and 57.1%,
67.0 and 33.7%, 66.7 and 56.5% and 48.8 and 17.0% in
NICD−/p21+, NICD−/p21−,
NICD+/p21+ and
NICD+/p21− patients, respectively.
Furthermore, the survival rate in NICD−/p21+
patients was significantly higher than that of
NICD+/p21− patients (χ2, 15.57;
P<0.01; Fig. 2C).
Univariate and multivariate analyses of
prognostic factors for overall survival of gastric cancer
patients
Univariate and multivariate analyses of prognostic
factors were performed for overall survival of gastric cancer
patients using the Cox proportional hazards regression model. Among
the 11 factors that were analyzed (age, gender, tumor location,
tumor size, tumor differentiation, depth of tumor invasion,
vascular invasion, lymph node and distant metastasis, and NICD and
p21 protein expression; Table IV),
the univariate analysis showed that tumor differentiation, depth of
tumor invasion, vascular invasion, lymph node metastasis, and
NICD+ and p21+ protein expression were
eligible for the multivariate analysis (Table V). The multivariate analysis
revealed that only NICD+ or p21+ protein
expression, depth of tumor invasion and lymph node metastasis had
statistical significance. NICD+ or p21−
protein expression, depth of tumor invasion and lymph node
metastasis were independent prognostic factors of gastric cancer
(Tables III and IV).
 | Table IVUnivariate analysis of prognostic
factors for the overall survival of gastric cancer patients. |
Table IV
Univariate analysis of prognostic
factors for the overall survival of gastric cancer patients.
| | | | | | 95% confidence
bounds |
|---|
| | | | | |
|
|---|
| Variable | β | SE | Wald | P-value | OR | Lower limit | Upper limit |
|---|
| Age (years) | −0.15 | 0.28 | 0.29 | 0.59 | 0.86 | 0.50 | 1.47 |
| Gender (n) | 0.12 | 0.32 | 0.13 | 0.71 | 1.12 | 0.60 | 2.09 |
| Tumor size
(cm) | −0.34 | 0.30 | 1.29 | 0.26 | 0.71 | 0.40 | 1.28 |
| Tumor location
(n) | −0.01 | 0.13 | 0.00 | 0.96 | 0.99 | 0.77 | 1.29 |
| Differentiation
(n) | 0.60 | 0.29 | 4.36 | 0.04 | 1.83 | 1.04 | 3.22 |
| Depth of tumor
invasion (n) | 1.34 | 0.29 | 21.34 | 0.00 | 3.80 | 2.16 | 6.71 |
| Vascular invasion
(n) | 0.71 | 0.30 | 5.71 | 0.02 | 2.04 | 1.14 | 3.65 |
| Lymph node
metastasis (n) | 1.04 | 0.33 | 9.74 | 0.00 | 2.82 | 1.47 | 5.41 |
| Distant metastasis
(n) | 0.45 | 0.41 | 1.22 | 0.27 | 1.57 | 0.71 | 3.47 |
| NICD protein
(n) | 1.19 | 0.35 | 11.34 | 0.00 | 3.27 | 1.64 | 6.53 |
| p21 protein
(n) | −1.14 | 0.32 | 12.58 | 0.00 | 0.32 | 0.17 | 0.60 |
 | Table VMultivariate analysis of prognostic
factors for the overall survival of gastric cancer patients. |
Table V
Multivariate analysis of prognostic
factors for the overall survival of gastric cancer patients.
| | | | | | 95% confidence
bounds |
|---|
| | | | | |
|
|---|
| Variable | β | SE | Wald | P-value | OR | Lower limit | Upper limit |
|---|
| Tumor
differentiation | 0.10 | 0.31 | 0.11 | 0.74 | 1.11 | 0.60 | 2.03 |
| Depth of tumor
invasion | 1.10 | 0.30 | 14.29 | 0.00 | 3.00 | 1.70 | 5.31 |
| Vascular
invasion | −0.10 | 0.32 | 0.10 | 0.76 | 0.91 | 0.49 | 1.69 |
| Lymph node
metastasis | 1.66 | 0.41 | 16.12 | 0.00 | 5.23 | 2.33 | 11.73 |
| NICD protein | 0.83 | 0.39 | 4.39 | 0.04 | 2.28 | 1.06 | 4.94 |
| p21 protein | −0.70 | 0.34 | 4.37 | 0.04 | 0.50 | 0.26 | 0.96 |
Discussion
The present study identified differential expression
of the NICD and p21 proteins in gastric cancer tissue specimens
compared with in normal mucosa, gastritis and precancerous lesion
samples. NICD was upregulated, but p21 protein was downregulated,
in the gastric cancer tissues, and the two proteins were shown to
be inversely associated. Furthermore, increased NICD expression,
but a loss of p21 expression, was closely associated with tumor
dedifferentiation, depth of tumor invasion, lymph node metastasis,
surface morphology and Lauren classification in gastric cancer. The
overall survival rate of gastric cancer patients was greater in
those with NICD− as opposed to NICD+ tumors,
and in p21+ rather than in p21− tumors. The
altered expression of these two proteins was also associated with
the overall survival of the patients. The COX-regression
multivariate analysis showed that NICD+,
p21−, depth of tumor invasion and lymph node metastasis
were all independent prognostic factors for gastric cancer
patients. Future studies will further evaluate these two proteins
as novel prognostic markers for gastric cancer patients.
Using a pancreatic cancer mouse model
(Rosa26NICD), De La et al(18) demonstrated that the abnormal
activation of Notch1 signaling leads to excessive epithelial cell
proliferation, decreased apoptosis and malignant transformation of
the epithelial phenotype, consequently resulting in the development
of pancreatic intraepithelial neoplasms and cancer in the mice. The
present study showed that NICD protein expression was significantly
greater in poorly-differentiated gastric cancer compared with that
in well- and moderately differentiated tumors. Furthermore, NICD
expression was closely associated with tumor size, depth of tumor
invasion, lymph node metastasis, surface morphology and Lauren
classification of tumors. These ex vivo data are consistent
with the previously mentioned data on pancreatic cancer in mice.
Similarly, Fre et al(19)
identified that the overexpression of NICD through transgenic
technology significantly inhibited the differentiation of crypt
progenitor cells in the mouse intestine. In glioma, Fan et
al(20) demonstrated that the
inhibition of Notch1 signaling activation reduced the proportion of
glioma stem cells, inhibited tumor cell colony formation and
increased tumor cell differentiation and apoptosis. In gastric
cancer, Yeh et al(21)
revealed that the overexpression of the NICD protein in gastric
adenocarcinoma SC-M1 cells using gene transfection techniques
resulted in a marked increase in tumor cell colony formation,
migration, invasion, xenograft formation and growth. Recently,
Notch1 protein expression has been shown to regulate stem cells and
cancer stem cells. The constitutive activation of Notch1 signaling
in Sertoli cells has been shown to cause gonocytes to exit from
quiescence (22). Notch
overexpression has been demonstrated to preserve stem cell
characteristics and confer stem cell characteristics upon a subset
of progenitor cells (23).
Furthermore, Notch1 is able to promote T cell leukemia-initiating
activity by RUNX-mediated regulation of PKC-θ and reactive oxygen
species (24). However, Notch1
inhibition in vivo results in mammary tumor regression and
reduces mammary tumor sphere-forming activity in
vitro(25). The inhibition of
the Notch1 pathway has been shown to allow glioblastoma cells to
overcome apoptosis resistance and become sensitized to apoptosis
that is induced by ionizing radiation, the death ligand tumor
necrosis factor-related apoptosis-inducing ligand or the
Bcl-2/Bcl-XL inhibitor ABT-737 (26). In conclusion, Notch1 may be a novel
target for gastric cancer therapy.
Furthermore, p21 expression has been shown to be
reduced or lost in a variety of cancer types (27,28,29). A
possible explanation is that p21 functions as a regulator of cell
cycle progression at S phase, therefore preventing cell
proliferation. In addition, p21 expression is controlled by the
tumor suppressor protein, p53, which is frequently mutated in a
number of human cancers, thus significantly contributing to a loss
of p21 expression in various cancer tissues. In the present study,
a gradual reduction of p21 protein expression from normal gastric
mucosa, chronic superficial gastritis and precancerous gastric
lesions to gastric cancer was observed. The loss of p21 expression
was associated with tumor dedifferentiation, depth of tumor
invasion, vascular invasion, lymph node metastasis, surface
morphology and Lauren classification of gastric cancer. These data
suggest that p21 plays a suppressor role in the development and
progression of gastric cancer, the expression of which may aid in
controlling a variety of malignant behaviors of gastric cancer.
Furthermore, the effect of activated Notch1 signaling (NICD) on the
regulation of p21 expression may differ in various tumor cell
types. However, the majority of studies support that Notch1
expression inhibits p21 expression and activation or vice versa
(30). p21WAF1/Cip1 is a negative
transcriptional regulator of Wnt4 expression downstream of Notch1
activation (31). The adult stem
cell marker Musashi-1 modulates endometrial carcinoma cell cycle
progression and apoptosis via Notch1 and p21 (32). Silencing of SKP2 by RNA interference
in G1 stabilizes p27 and p21 but abolishes the Notch1 effect on
G1-S progression (33). Kim et
al(12) also observed that the
overexpression of NICD inhibits p53 phosphorylation and the
expression of the p53 target gene, p21, therefore inhibiting
ultraviolet-induced apoptosis. These data indicate that Notch1 may
function by regulating p21 expression. The present study supports
this notion. However, further studies are required to clarify Notch
regulation of p21 expression in gastric cancer cells.
The present study demonstrated that a combination of
aberrant expression of NICD and p21 proteins was able to predict
overall survival of gastric cancer patients, which is more
efficient than that of an individual protein. The present data are
consistent with the data reported by Li et al(34). Thus, the NICD and p21 proteins may
be useful as prognostic indicators for gastric cancer. However, the
present data showed that the expression of the two proteins was
significantly altered in gastric cancer tissues, although they were
not significantly altered in the early stages of malignancy,
including precancerous lesions versus chronic superficial gastritis
or normal gastric mucosae, or chronic superficial gastritis versus
normal gastric mucosae, indicating that they may be late events
during stomach carcinogenesis. Thus, they are not useful for early
detection or as tumorigenesis markers of gastric cancer.
Acknowledgements
This study was supported by a grant from the Natural
Science Foundation of Zhejiang Province (grant no. Y2101458) and
the Innovation Technology Project for High-Level Personnel of
Wenzhou City (201011). The authors would like to thank the
Pathology Department of The First Hospital Affiliated with Wenzhou
Medical College for the analysis of the pathological data.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Lin Y, Ueda J, Kikuchi S, et al:
Comparative epidemiology of gastric cancer between Japan and China.
World J Gastroenterol. 17:4421–4428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koch U and Radtke F: Notch and cancer: a
double-edged sword. Cell Mol Life Sci. 64:2746–2762. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stanley P: Regulation of Notch signaling
by glycosylation. Curr Opin Struc Biol. 17:530–535. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Katoh M and Katoh M: Notch signaling in
gastrointestinal tract (review). Int J Oncol. 30:247–251.
2007.PubMed/NCBI
|
|
7
|
Sriuranpong V, Borges MW, Ravi RK, et al:
Notch signaling induces cell cycle arrest in small cell lung cancer
cells. Cancer Res. 61:3200–3205. 2001.PubMed/NCBI
|
|
8
|
Duan L, Yao J, Wu X and Fan M: Growth
suppression induced by Notch1 activation involves Wnt-beta-catenin
down-regulation in human tongue carcinoma cells. Biol Cell.
98:479–490. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA
and Chen H: Overexpression of the NOTCH1 intracellular domain
inhibits cell proliferation and alters the neuroendocrine phenotype
of medullary thyroid cancer cells. J Biol Chem. 281:39819–39830.
2006. View Article : Google Scholar
|
|
10
|
Cayo MA, Cayo AK, Jarjour SM and Chen H:
Sodium butyrate activates Notch1 signaling, reduces tumor markers,
and induces cell cycle arrest and apoptosis in pheochromocytoma. Am
J Transl Res. 1:178–183. 2009.PubMed/NCBI
|
|
11
|
Greblatt DY, Vaccaro AM, Jaskula-Sztul R,
et al: Valproic acid activates notch-1 signaling and regulates the
neuroendocrine phenotype in carcinoid cancer cells. Oncologist.
12:942–951. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim SB, Chae GW, Lee J, et al: Activated
Notch1 interacts with p53 to inhibit its phosphorylation and
transactivation. Cell Death Differ. 14:982–991. 2007.PubMed/NCBI
|
|
13
|
Ning L, Wentworth L, Chen H and Weber SM:
Down-regulation of Notch1 signaling inhibits tumor growth in human
hepatocellular carcinoma. Am J Transl Res. 1:358–366.
2009.PubMed/NCBI
|
|
14
|
Tanaka M, Setoguchi T, Hirotsu M, et al:
Inhibition of Notch pathway prevents osteosarcoma growth by cell
cycle regulation. Br J Cancer. 100:1957–1965. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo D, Ye J, Dai J, et al: Notch-1
regulates Akt signaling pathway and the expression of cell cycle
regulatory proteins cyclin D1, CDK2 and p21 in T-ALL cell lines.
Leuk Res. 33:678–685. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Massi D, Tarantini F, Franchi A, et al:
Evidence for differential expression of Notch receptors and their
ligands in melanocytic nevi and cutaneous malignant melanoma. Mod
Pathol. 19:246–254. 2006. View Article : Google Scholar
|
|
17
|
Tarakji B and Nassani MZ:
Immunohistochemical expression of p21 in normal tissues of salivary
gland, pleomorphic adenoma and carcinoma ex pleomorphic
adenoma-(undifferentiated and adenocarcinoma types). Med Oral Patol
Oral Cir Bucal. 15:e697–e703. 2010. View Article : Google Scholar
|
|
18
|
De La OJP, Emerson LL, Goodman JL, et al:
Notch and Kras reprogram pancreatic acinar cells to ductal
intraepithelial neoplasia. Proc Natl Acad Sci USA. 105:18907–18912.
2008.PubMed/NCBI
|
|
19
|
Fre S, Huyghe M, Mourikis P, et al: Notch
signals control the fate of immature progenitor cells in the
intestine. Nature. 435:964–968. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fan X, Matsui W, Khaki L, et al: Notch
pathway inhibition depletes stem-like cells and blocks engraftment
in embryonal brain tumors. Cancer Res. 66:7445–7452. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yeh TS, Wu CW, Hsu KW, et al: The
activated Notch1 signal pathway is associated with gastric cancer
progression through cyclooxygenase-2. Cancer Res. 69:5039–5048.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garcia TX, Defalco T, Capel B and Hofmann
MC: Constitutive activation of NOTCH1 signaling in Sertoli cells
causes gonocyte exit from quiescence. Dev Biol. 377:188–201. 2013.
View Article : Google Scholar
|
|
23
|
Piccin D, Yu F and Morshead C: Notch
signaling imparts and preserves neural stem characteristics in the
adult brain. Stem Cells Dev. 22:1541–1550. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Giambra V, Jenkins CR, Wang H, et al:
NOTCH1 promotes T cell leukemia-initiating activity by
RUNX-mediated regulation of PKC-θ and reactive oxygen species. Nat
Med. 18:1693–1698. 2012.PubMed/NCBI
|
|
25
|
Simmons MJ, Serra R, Hermance N and
Kelliher MA: NOTCH1 inhibition in vivo results in mammary tumor
regression and reduced mammary tumorsphere-forming activity in
vitro. Breast Cancer Res. 14:R1262012. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fassl A, Tagscherer KE, Richter J, et al:
Notch1 signaling promotes survival of glioblastoma cells via
EGFR-mediated induction of anti-apoptotic Mcl-1. Oncogene.
31:4698–4708. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Skirnisdottir I and Seidal T: Association
of p21, p21 p27 and p21 p53 status to histological subtypes and
prognosis in low-stage epithelial ovarian cancer. Cancer Genomics
Proteomics. 10:27–34. 2013.PubMed/NCBI
|
|
28
|
Place RF, Wang J, Noonan EJ, et al:
Formulation of small activating RNA into lipidoid nanoparticles
inhibits xenograft prostate tumor growth by inducing p21
expression. Mol Ther Nucleic Acids. 1:e152012. View Article : Google Scholar
|
|
29
|
Jee H, Lee SH, Park JW, et al: Connexin32
inhibits gastric carcinogenesis through cell cycle arrest and
altered expression of p21Cip1 and p27Kip1. BMB Rep. 46:25–30. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rowland BD and Peeper DS: KLF4, p21 and
context-dependent opposing forces in cancer. Nat Rev Cancer.
6:11–23. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Devgan V, Mammucari C, Millar SE, et al:
p21WAF1/Cip1 is a negative transcriptional regulator of Wnt4
expression downstream of Notch1 activation. Genes Dev.
19:1485–1495. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Götte M, Greve B, Kelsch R, et al: The
adult stem cell marker Musashi-1 modulates endometrial carcinoma
cell cycle progression and apoptosis via Notch-1 and p21WAF1/CIP1.
Int J Cancer. 129:2042–2049. 2011.PubMed/NCBI
|
|
33
|
Sarmento LM, Huang H, Limon A, et al:
Notch1 modulates timing of G1-S progression by inducing SKP2
transcription and p27 Kip1 degradation. J Exp Med. 202:157–168.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li DW, Wu Q, Peng ZH, et al: Expression
and significance of Notch1 and PTEN in gastric cancer. Ai Zheng.
26:1183–1187. 2007.(In Chinese).
|