Introduction
Cellular schwannoma is a rare tumor of the
peripheral nerves. The tumor consists of cells that are
spindle-shaped in a fascicular or interfelted arrangement (Antoni
A), with high cell density and lacking the classic schwannoma
reticular zone (Antoni B) and structures in palisade arrangement
(Verocay bodies). The tumor cells are abundant, with coarse
chromatin and deeply stained nuclei. Therefore, the tumor may
easily be misdiagnosed as various different types of spindle cell
sarcoma. An intraperitoneal cellular schwannoma may also be
mistaken as a gastrointestinal stromal tumor (GIST). The current
study presents a rare case of a cellular schwannoma arising from
the gastric wall. In addition, the clinical characteristics,
diagnosis, differential diagnosis and treatment of the cellular
schwannoma in the gastric wall were analyzed. Written informed
consent was obtained from the patient.
Case report
A 66-year-old female was admitted to the Subei
People's Hospital of Jiangsu Province (Yangzhou, China) with the
chief complaint of a cervical epidermal cyst, on March 17, 2012.
The pre-operative physical examination and laboratory results
showed no positive abnormalities, with the exception of a 4×3-cm
lump in the right cervical area (Table
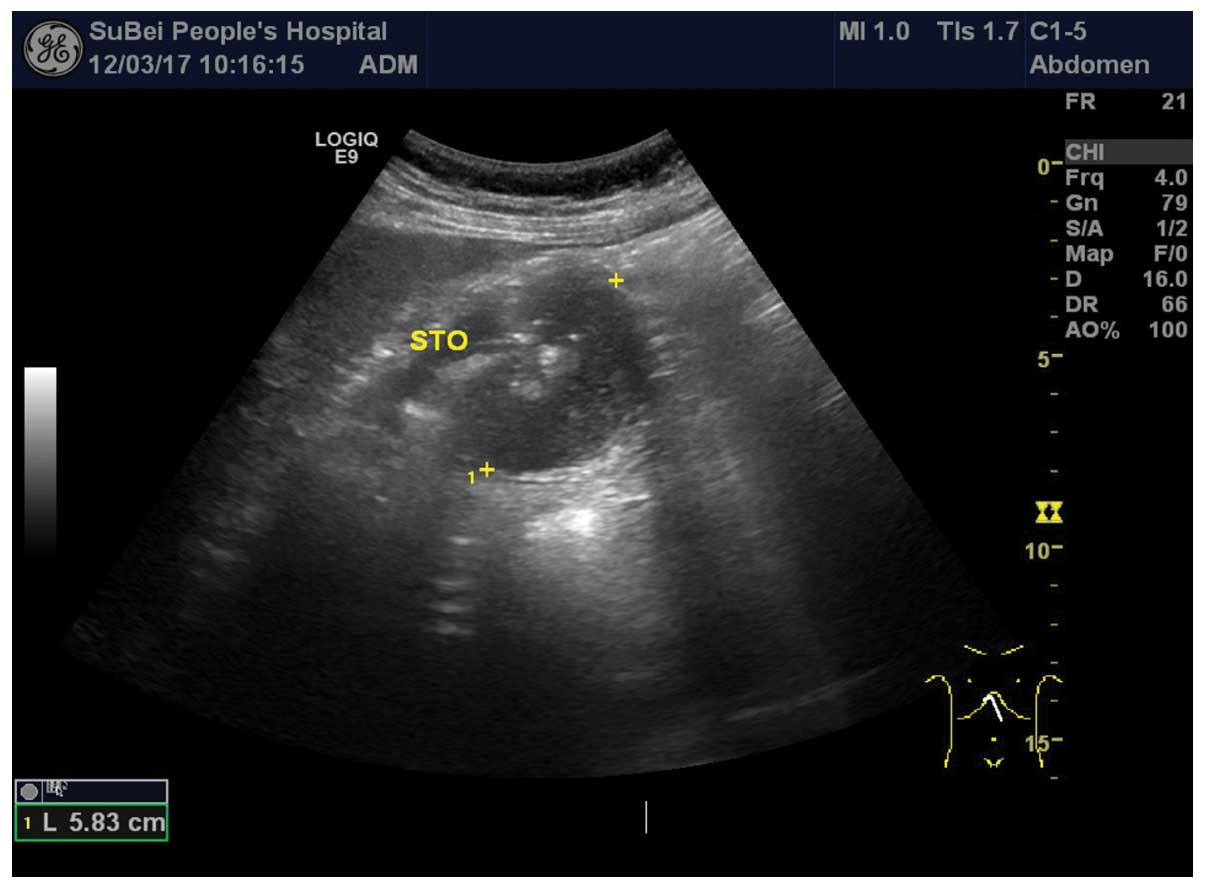
I). However, pre-operative abdominal ultrasound revealed a mass
in the posterior wall of the stomach (Fig. 1). In addition, abdominal
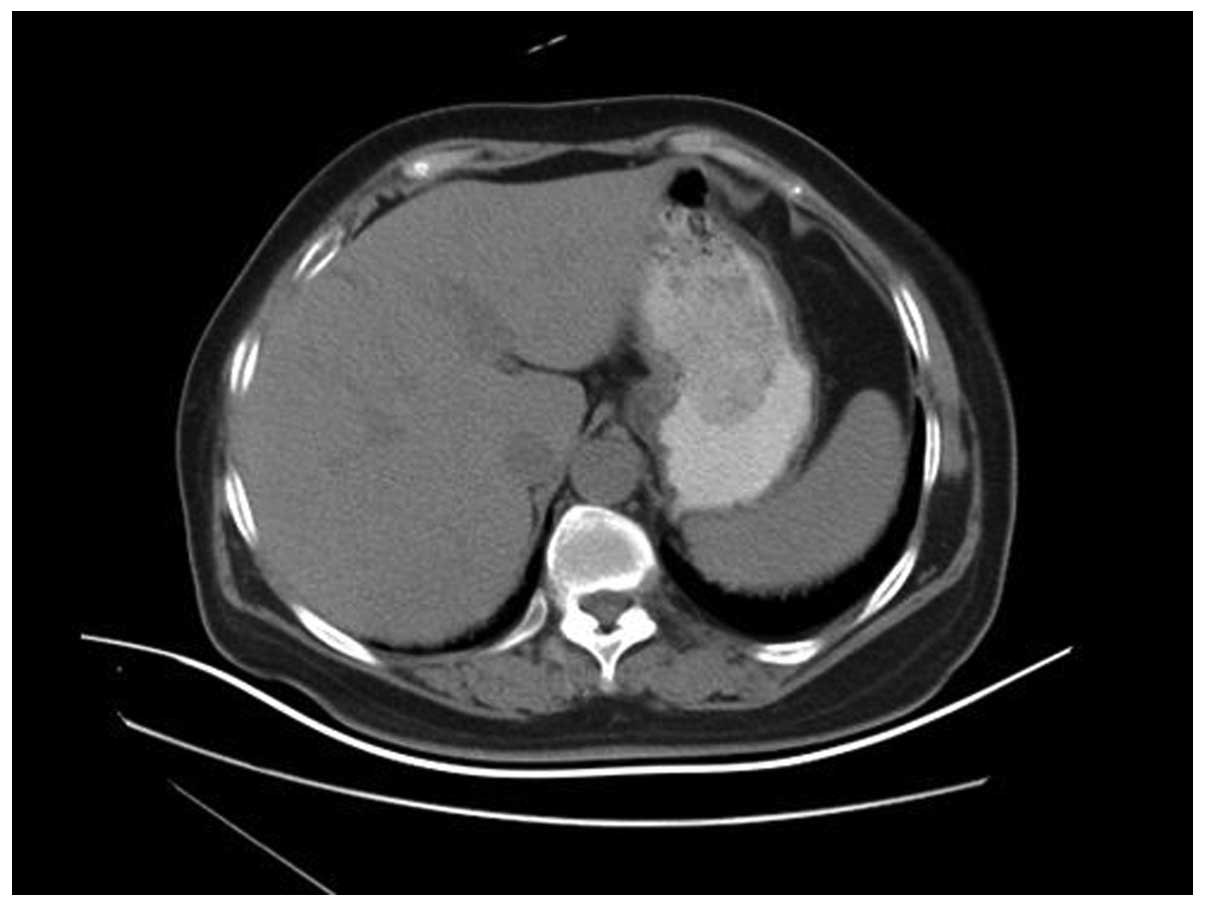
contrast-enhanced computed tomography (CT) showed a mass of soft
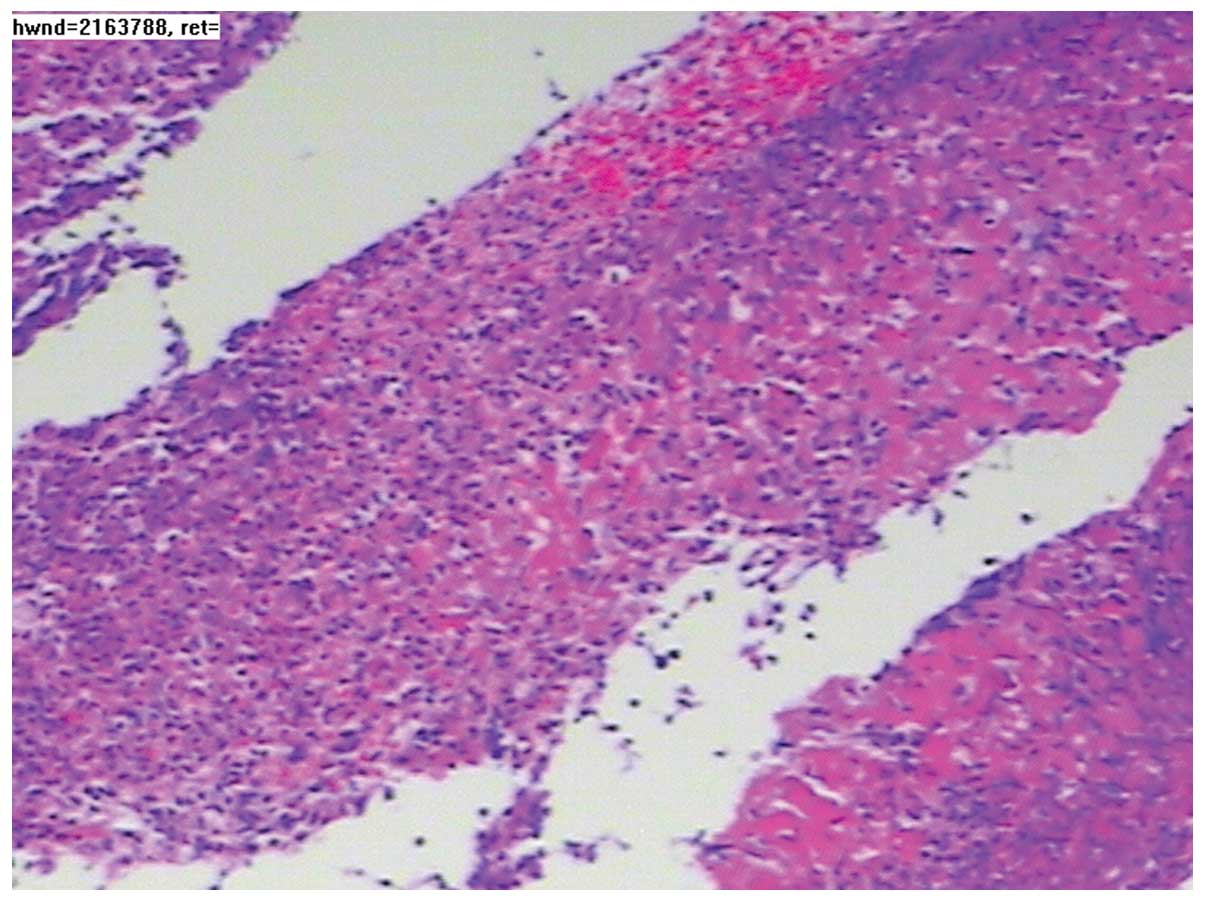
tissue lying behind the wall of stomach (Fig. 2). However, the fiberoptic
gastroscopy biopsy identified only moderate chronic superficial
gastritis, accompanied by inflammatory exudate and necrotic tissue
(Fig. 3). Following cervical lump
resection, an abdominal laparotomy was performed and a
5.6×5.3×4.0-cm mass was found located in the posterior wall of the
stomach. The mass was prominent in the gastric wall, with a clear
border. The primary diagnosis was of a GIST due to the mesenchymal
origin. The surgery was successful and specimens were sent for
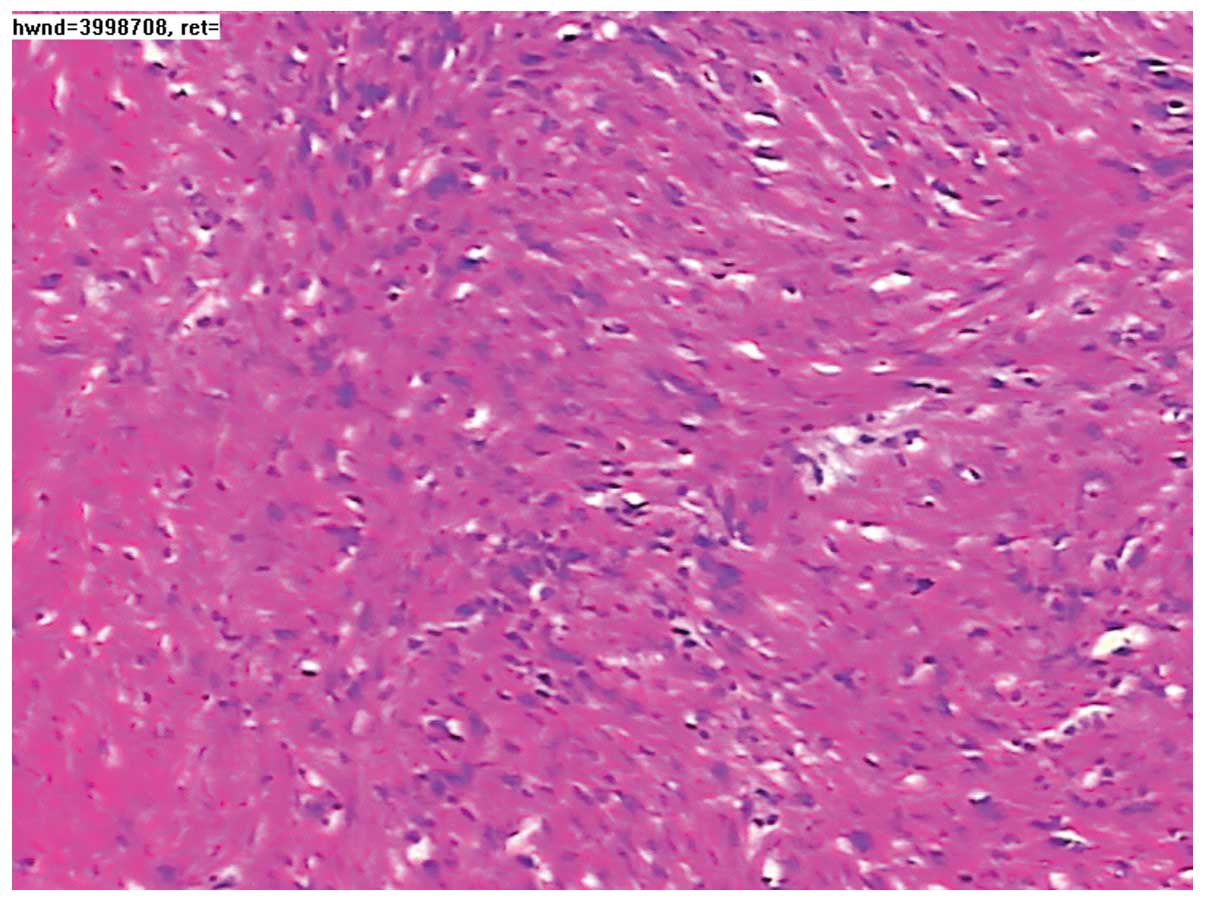
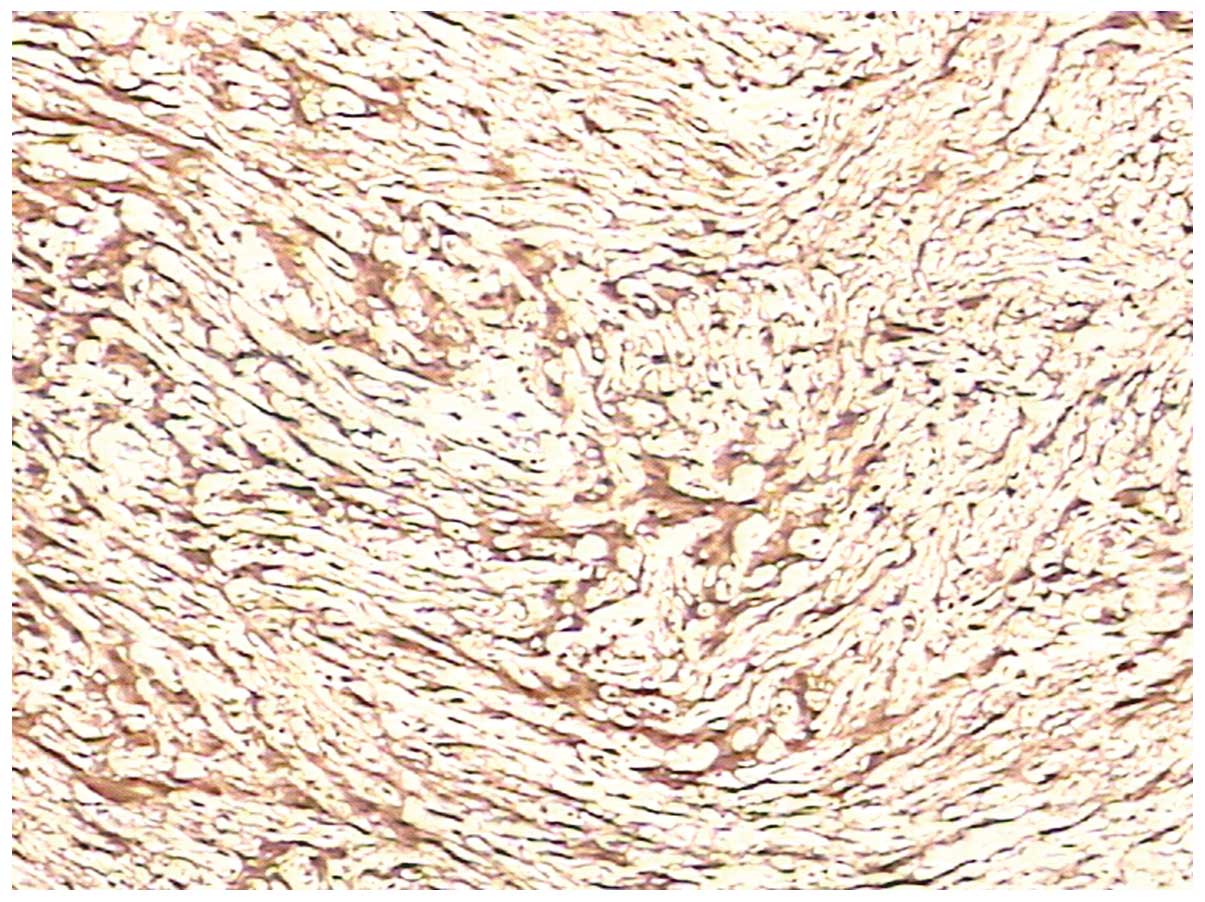
pathological examination. Microscopically, the excised tumor tissue
was composed of spindle-shaped and fascicularly-arranged cells, but
mitotic figures were rare (Fig. 4).
Immunohistochemical staining showed that the tumor was negative for
cluster of differentiation (CD)117, CD34, smooth muscle actin (SMA)
and desmin, but positive for S-100 and Ki67 (<1%) (Fig. 5). As a result, the pathological
diagnosis was of a cellular schwannoma. Since no post-operative
complications occurred, the patient was treated and discharged 10
days after surgery. No evidence of recurrence was identified during
12 months of follow-up by ultrasonography or CT.
 | Table ILaboratory observations upon
admission. |
Table I
Laboratory observations upon
admission.
| Tumor markers | Index | Normal range |
|---|
| CA19-9, KU/l | 8.96 | <35.00 |
| NSE, ng/ml | 5.74 | <13.00 |
| CEA, ng/ml | 2.03 | <5.00 |
| CA242, KU/l | 5.47 | <20.00 |
| Ferritin, ng/ml | 16.58 | <322.00 |
| β-HCG, MIU/ml | 0.39 | <3.00 |
| AFP, ng/ml | 0.32 | <20.00 |
| Free-PSA, ng/ml | 0.06 | <1.00 |
| PSA, ng/ml | 0.57 | <5.00 |
| CA125, KU/l | 1.06 | <35.00 |
| HGH, ng/ml | 1.18 | <7.50 |
| CA15-3, KU/l | 3.07 | <35.00 |
Discussion
Cellular schwannoma, first reported by Woodruff
et al in 1981 (1), is a rare
pseudosarcoma (2) that is mainly
found in middle-aged individuals, with no significant difference in
incidence between males and females (3). The predilection is for the paraspinal
region, particularly the mediastinal, retroperitoneal, pelvic and
sacral areas, followed by the neck and limbs (4–6).
Gastric cellular schwannoma is a rare mesenchymal tumor that arises
from the nerve plexus of the gut wall. Clinically, its main
manifestations are gastrointestinal bleeding, chronic abdominal
pain and an abdominal mass, but it is often detected incidentally.
The majority of cases show a solitary painless mass and are
occasionally multifocal (7).
Pre-operative investigation is not pathognomonic, as a number of
cases are diagnosed as GISTs (8).
Imaging studies, such as barium meal, ultrasound and CT, are used
only for diagnosing the position of gastric cellular schwannoma,
but the qualitative diagnosis is often extremely difficult. The use
of magnetic resonance imaging is justified, since the tumor is
hypointense on T1 scans; however, the hypointensity is more evident
on T2 scans (9). The rate of
omissions and incorrect diagnoses by gastrofiberscope is higher,
mainly affected by the level of skill of the gastroscopy physician,
the location and depth of the biopsy, and other factors. However,
biopsy extraction may improve the diagnostic rate. Adopting the
intraoperative frozen section procedure is feasible if the
conditions are permissible. The majority of cases are confirmed by
pathology.
Gastric cellular schwannomas are easily misdiagnosed
as various types of sarcoma in pathology, which leads to
unnecessarily excessive treatments. We believe that the following
criteria may aid the diagnosis of cellular schwannomas. In general,
a cellular schwannoma is circular or elliptical in shape, with a
diameter between 1–23 cm and an average diameter of 5.2 cm. The
incisional surface is greyish to sallow. In addition, a mosaic-like
distribution of yellow nodules is visible in specific cases, with
focal bleeding, but no cystic changes. Histologically, a cellular
schwannoma exhibits thick fibrous capsules, gathered subcapsular or
extracapsular lymphocytes, visible foam cells and hyaline
degeneration of the thick-walled blood vessels within the tumor.
The tumor cells are spindle-shaped, with a fascicular or
interfelted arrangement (Antoni A) and high cell density, lacking
the classic schwannoma reticular zone (Antoni B) and palisade
arrangement structures (Verocay bodies). The vortex-like structure
that is used as an indicator of neuroal differentiation was
occasionally visible. In specific cases, the tumor cells display a
certain degree of pleomorphism and a small number of mitotic
figures (<4/10 HPF), but do not exhibit pathological mitotic
figures or coagulation necrosis. Immunohistochemical staining is of
great value in the differential diagnosis of this tumor (Table II) (10); the tumor is negative for S-100,
glial fibrillary acidic protein (GFAP) and CD57 in the cellular
schwannoma, and positive for CK (AE1/AE3), desmin, SMA, CD34, CD117
and discovered on GIST1 (DOG1), with a low positive rate for
Ki67.
 | Table IIImmunohistochemical indices for the
differential diagnosis of mesenchymal tumors. |
Table II
Immunohistochemical indices for the
differential diagnosis of mesenchymal tumors.
| Tumor | CD34 | bcl-2 | CD99 | S-100 | Cytokeratin | EMA | Calretinin | Desmin | SMA |
|---|
| Cellular
schwannoma | ± | + | | + | | | | | |
| Neurofibroma | + | + | | + | | | | | |
| Spindle cell
lipoma | + | + | | | | | | | |
| Synovial sarcoma | − | + | ± | | + | + | | | |
| Desmoid tumor | − | | | | − | − | | + | + |
|
Hemangiopericytoma | + | − | ± | | | | | − | − |
| Malignant peripheral
nerve sheath tumor | ± | ± | | + | ± | | | ± | ± |
| Sarcomatoid
mesothelioma | − | ± | ± | | + | + | + | | |
| SFT | + | + | + | | − | − | − | ± | ± |
| Calcifying fibrous
pseudotumor | ± | | | | − | | − | − | |
| Smooth muscle
tumor | ± | ± | ± | | | | | + | + |
According to the pathological characteristics,
gastric cellular schwannomas are primarily identified with the
following tumors: A GIST is the most common gastrointestinal
mesenchymal tumor, with clinical imageology characteristics
extremely close to that of gastric cellular schwannoma (11). The gastric cellular schwannoma cells
exhibit a short spindle or a spindle similar to that of GISTs with
a predominance of spindle cells, generally intertwined in a short
article bundle or swirling arrangement. Therefore, gastric cellular
schwannoma is occasionally difficult to identify.
Immunohistochemically, gastric cellular schwannoma has been found
to be positive for CD117, CD34 and DOG1 and negative for GFAP.
Previously, it has been reported that gastric cellular schwannoma
may be contaminated with small focal GISTs (12), therefore, a biopsy from numerous
positions is necessary. Gastric myogenic tumors are rare. The
sections of leiomyomas and leiomyosarcomas are often gray, exhibit
braided structures, red cytoplasm, rod- and cigar-shaped visible
nuclei and visible vacuoles. The tumor is positive for myogenic
markers, not diffusely positive for S-100 and negative for GFAP.
Solitary fibrous tumors (SFTs) are composed of rich and sparse
areas of cells in alternating distributions. Among the tumor cells
containing collagen, there are fibers of varying thickness and
shape, with long spindle cells, but no Antoni A and B areas.
However, a certain degree of CD34 and bcl-2 expression is likely to
be evident. Gastric malignant peripheral nerve sheath tumors are
extremely rare. Atypia of the tumor cells, necrosis and the common
presence of mitotic figures may aid diagnosis. Gastric malignant
peripheral nerve sheath tumors lack subcapsular or extracapsular
lymphocyte sets, foamy histiocyte aggregates between the spindle
cells, perivascular lymphocytic infiltration and vascular wall
hyalinosis, which gastric cellular schwannomas display
morphologically. The majority of gastric malignant peripheral nerve
sheath tumors are associated with neurofibromatosis (13). However, certain studies have
speculated that malignant peripheral nerve sheath tumors of the
stomach are GISTs (14).
Neurofibromas do not exhibit Antoni A and B areas when using
microscopy. The immunophenotype of neurofibroma is often not
diffusely positive for S-100 expression. A solitary neurofibroma of
the stomach is extremely rare (15). Fibrosarcoma cells express only
vimentin and are negative for S-100, GFAP and neurogenic markers.
Gastrointestinal autonomic nerve tumors are more common in the
small intestine, followed by the stomach, and are prone to being
classified as a stromal tumor with neuronal differentiation
(11). In addition to gastric
cellular schwannomas, malignant fibrous histiocytomas, inflammatory
myofibroblastomas and other types of tumor occasionally exhibit
lymphoid hyperplasia and follicles at the tumor edge. However,
their histological morphologies are extremely different compared
with schwannoma, so are not difficult to identify.
Cellular schwannomas are not sensitive to
radiotherapy or chemotherapy (16).
Currently, a complete surgical resection of the tumor is the only
effective method of treatment (17). The final diagnosis of gastric
cellular schwannoma relies on the pathology and
immunohistochemistry (18–19). The type of benign or malignant tumor
is based mainly on the nuclear mitotic figures and heteromorphisms
of the tumor cells. The majority of gastric cellular schwannomas
are benign, with good prognosis and a low recurrence rate, but
10–15% of the tumors are malignant (20). According to the location, size and
peculiarity of the tumor, the appropriate surgical procedure may be
selected. Gastric cellular schwannomas rarely exhibit lymph node
metastasis, so a regional lymph node dissection is not required.
For the growth of the tumor in the gastric lumen, which is small,
endoscopic removal may be performed or combined with laparoscopic
surgery for the tumor resection. For the growth of the tumor
outside of the gastric lumen, a laparoscopic removal, gastric wedge
resection or subtotal gastrectomy may be performed. Minimally
invasive surgery has the advantages of a rapid post-operative
recovery and little trauma, and following the establishment of
pneumoperitoneum, there is no requirement to consider the
metastasis of tumor cells in the abdominal cavity. Therefore,
minimally invasive surgery may be used as the preferred surgical
treatment for gastric cellular schwannomas. For larger, suspected
malignant tumors and in the minimally invasive treatment of a
difficult situation, conventional open surgery is recommended.
Radical gastrectomy may be performed on malignant tumors, but these
often exhibit distant metastasis, resulting in a poor efficacy
(21). The specific substances
expressed in gastric cellular schwannoma must be identified to
highlight a theoretical basis for molecular-targeted therapy
(22). The patient in the current
case exhibited no abdominal abnormalities, but a pre-operative
routine examination for a neck mass identified the abdominal mass.
Therefore, the patient was diagnosed and treated early. The patient
did not exhibit any specific symptoms or signs. In addition, a
radiological examination did not reveal any specific signs, as the
clinical physician's diagnostic focus was too narrow and the
examination was not comprehensive. Furthermore, the biopsy position
of the gastrofiberscope was too superficial and the intraoperative
biopsy did not undergo rapid freezing, resulting in a misdiagnosis.
The performed radical resection resulted in the excessive treatment
of the patient. The current report consequently provides a warning
to clinicians when diagnosing cellular schwannoma.
References
|
1
|
Woodruff JM, Godwin TA, Erlandson RA, et
al: Cellular schwannoma: a variety of schwannoma sometimes mistaken
for a malignant tumor. Am J Surg Pathol. 5:733–744. 1981.
View Article : Google Scholar
|
|
2
|
Fletcher CD, Davies SE and McKee PH:
Cellular schwannoma: a distinct pseudosarcomatous entity.
Histopathology. 11:21–35. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ozkan EE, Guldur ME and Uzunkoy A: A case
report of benign schwannoma of the liver. Intern Med. 49:1533–1536.
2010. View Article : Google Scholar
|
|
4
|
White W, Shiu MH, Rosenblum MK, et al:
Cellular schwannoma. A clinicopathologic study of 57 patients and
58 tumors. Cancer. 66:1266–1275. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lodding P, Kindblom LG, Angervall L and
Stenman G: Cellular schwannoma. A clinicopathologic study of 29
cases. Virchows Arch A Pathol Anat Histopathol. 416:237–248.
1990.PubMed/NCBI
|
|
6
|
Casadei GP, Scheithauer BW, Hirose T, et
al: Cellular schwannoma. A clinicopathologic, DNA flow cytometric,
and proliferation marker study of 70 patients. Cancer.
75:1109–1119. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ogose A, Hotta T, Hatano H, et al:
Presacral multiple cellular schwannomas. Spine (Phila Pa 1976).
28:E426–E429. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Williamson JM, Wadley MS, Shepherd NA and
Dwerryhouse S: Gastric schwannoma: a benign tumour often mistaken
clinically, radiologically and histopathologically for a
gastrointestinal stromal tumor - a case series. Ann R Coll Surg
Engl. 94:245–249. 2012. View Article : Google Scholar
|
|
9
|
Sarlomo-Rikala M, El-Rifai W, Lahtinen T,
et al: Different patterns of DNA copy number changes in
gastrointestinal stromal tumors, leiomyomas, and schwannomas. Hum
Pathol. 29:476–481. 1998. View Article : Google Scholar
|
|
10
|
Zong L, Chen P, Wang GY and Zhu QS: Giant
solitary fibrous tumor arising from greater omentum. World J
Gastroenterol. 18:6515–6520. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kwon MS, Lee SS and Ahn GH: Schwannomas of
the gastrointestinal tract: clinicopathological features of 12
cases including a case of esophageal tumor compared with those of
gastrointestinal stromal tumors and leiomyomas of the
gastrointestinal tract. Pathol Res Pract. 198:605–613. 2002.
View Article : Google Scholar
|
|
12
|
Cho H, Watanabe T, Aoyama T, et al: Small
bud of probable gastrointestinal stromal tumor within a
laparoscopically-resected gastric schwannoma. Int J Clin Oncol.
17:294–298. 2012. View Article : Google Scholar
|
|
13
|
Tozbikian G, Shen R and Suster S: Signet
ring cell gastric schwannoma: report of a new distinctive
morphological variant. Ann Diagn Pathol. 12:146–152. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Agaimy A, Märkl B, Kitz J, et al:
Peripheral nerve sheath tumors of the gastrointestinal tract: a
multicenter study of 58 patients including NFl-associated gastric
schwannoma and unusual morphologic variants. Virchows Arch.
456:411–422. 2010. View Article : Google Scholar
|
|
15
|
Magro G, Piana M, Venti C, et al: Solitary
neurofibroma of the mesentery: report of a case and review of the
literature. Pathol Res Pract. 196:713–718. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sordillo PP, Helson L, Hajdu SI, et al:
Malignant schwannoma - clinical characteristics, survival, and
response to therapy. Cancer. 47:2503–2509. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Imao T, Seki M, Amano T and Takemae K:
Laparoscopic resection of retroperitoneal schwannoma: report of
three cases and review of 22 cases in Japanese literature.
Hinyokika Kiyo. 57:491–495. 2011.(In Japanese).
|
|
18
|
Daimaru Y, Kido H, Hashimoto H and Enjoji
M: Benign schwannoma of the gastrointestinal tract: a
clinicopathologic and immunohistochemical study. Hum Pathol.
19:257–264. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takemura M, Yoshida K, Takii M, et al:
Gastric malignant schwannoma presenting with upper gastrointestinal
bleeding: a case report. J Med Case Rep. 6:372012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fukuchi M, Naitoh H, Shoji H, et al:
Schwannoma of the stomach with elevated preoperative serum
carbohydrate antigen 19-9: report of a case. Surg Today.
42:788–792. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Euanorasetr C and Suwanthanma W: Gastric
schwannoma presenting with perforation and abscess formation: case
report and literature review. J Med Assoc Thai. 94:1399–1404.
2011.
|
|
22
|
Altuna X, Lopez JP, Yu MA, et al:
Potential role of imatinib mesylate (Gleevec, STI-571) in the
treatment of vestibular schwannoma. Otol Neurotol. 3:163–170. 2011.
View Article : Google Scholar : PubMed/NCBI
|