Introduction
Glioblastoma multiforme (GBM) is a malignant tumor
that responds poorly to radiotherapy and chemotherapy (1). Glioma stem cells (GSCs) are a small
percentage of glioma cells that demonstrate features of primitive
neural progenitor cells and tumor-initiating functions (2). It is hypothesized that GSCs promote
tumor progression in addition to being possible ‘seeds’ of
recurrence following conventional therapies for GBM (3,4). GSCs
are resistant to current therapeutic methods, including
radiotherapy, chemotherapy and anti-angiogenesis therapy (5–7), and
their capacity for anti-hypoxia is greater than that of other cells
in the GBM (8). In addition,
self-renewal and the undifferentiated state of GSCs are known to be
enhanced by hypoxia (9,10); however, the underlying mechanisms
have not been fully identified.
Nuclear erythroid 2-related factor 2 (Nrf2) is a
redox-sensitive, basic leucine zipper protein that regulates the
transcription of certain antioxidant genes. It is a key nuclear
transcription factor, which regulates antioxidant response element
(ARE)-containing genes (11).
Furthermore, Nrf2 constitutes a predominant detoxification system
in numerous types of cell (12,13)
and has been implicated in cancer prevention (13,14),
for example in GBM (15).
Therefore, we hypothesized that Nrf2 is significant within GSCs;
however, the expression and the role of Nrf2 in the anti-hypoxia
action of GSCs remains unclear.
Thus, the levels of Nrf2 in transcription and
translation were analyzed in the present study, and the
transcription of mRNA and the translation of proteins in GSCs was
investigated using real-time polymerase chain reaction (qPCR) and
western blot analysis. As a result, it was hypothesized that Nrf2
was significant in GSC resistance to environmental stress,
specifically in anti-hypoxia and metabolism therapies, which may be
beneficial to future studies. Therefore, Nrf2 may be a potential
target for the treatment of GBM.
Materials and methods
Ethical approval
The present study was conducted in accordance with
the ethics committee of Jinling Hospital (Jiangsu, China). All
patients provided informed written consent for involvement in this
study.
Cell direction and treatment
Primary human GBM cells (G1, G2 and G3) were derived
from freshly resected human surgical GBM specimens, which were
obtained from three patients at the Department of Neurosurgery in
Jinling Hospital. The samples were identified as GBM World Health
organization grade IV by the pathologists at Jinling Hospital. The
tumors were digested with collagenase type IV (Sigma-Aldrich, St.
Louis, MO, USA) and released to single cells by gentle pipetting,
and then filtered through a 70-μm cell strainer. The adherent
culture of GBM cells were seeded in Dulbecco’s modified Eagle’s
medium with Ham’s F12 medium (DMEM/F-12; Gibco-BRL, Carlsbad, CA,
USA) containing 10% fetal bovine serum (FBS; Hyclone, Waltham, MA,
USA) with a density of 2×105 live cells/ml. After 15
min, the nonadherent cells were seeded in DMEM/F-12 containing 10%
FBS at a density of 2×105 live cells/ml.
Flow cytometry
Fluorescence-activated cell sorting (FACS) was
performed to evaluate the number of CD133+ cells. The
GBM cells were collected and washed in phosphate-buffered saline
(PBS) three times and incubated with CD133-phycoerythrin (Miltenyi
Biotec, Gladbach, Germany) at 37°C for 40 min in a humidified
chamber, followed by an additional wash using PBS. The labeled
cells were analyzed using a BD FACSAria™ III system (BD
Biosciences, Franklin Lakes, NJ, USA). The data were analyzed using
FlowJo 7.6 software (Tree Star, Inc., Ashland, OR, USA).
Magnetic cell sorting (MACS) and cell
culture
MACS was conducted as described previously (16). Cells were dissociated and
resuspended in PBS containing 0.5% bovine serum albumin and 2
mmol/l EDTA. CD133 MicroBeads (Miltenyi Biotec) were used for
magnetic labeling and MACS was performed with the MiniMACS machine
(Miltenyi Biotec). Positive magnetic cells were separated using
several MACS columns in series.
The majority of the CD133+ cells (GSC1,
obtained from G1; GSC2, obtained from G2 and GSC3, obtained from
G3) were harvested for protein assaying and refinement of mRNA. The
remaining cells were used for immunofluorescence analysis.
The CD133− cells were seeded in DMEM/F-12
containing 10% FBS at a density of 2×105 live cells/ml.
The cells were maintained in a standard tissue culture incubator at
37°C with 5% CO2 in air and 100% relative humidity.
Contribution of GSC spheres
A small quantity of CD133+ cells were
cultured in serum-free DMEM/F-12, in addition to a neural
supplement from the Neural Stem Cell kit (Invitrogen Life
Technologies, Carlsbad, CA, USA), 20 ng/ml recombinant human
epidermal growth factor (Invitrogen Life Technologies), 20 ng/ml
recombinant human basic fibroblast growth factor (Invitrogen Life
Technologies), 100 IU/ml penicillin G and 100 μg/ml streptomycin;
this step identified the generation of GSC spheres.
Immunofluorescence analysis of GSCs
CD133+ and CD133− cells were
incubated in DMEM/F-12 containing 10% FBS for 4 h. The cells were
fixed in 4% paraformaldehyde (PFA) and incubated at 4°C with a
mouse polyclonal primary antibody against nestin (1:500; Abcam,
Cambridge, UK) and a rabbit polyclonal primary antibody against
Nrf2 (1:500; Abcam), according to the manufacturer’s
instructions.
For identifying the generation of the GSC spheres,
certain CD133+ cells were cultured in neural stem cell
medium, fixed in 4% PFA and incubated at 4°C with a rabbit
polyclonal primary antibody against CD133 (1:500; Biorbyt,
Cambridge, UK).
After 12 h, Dylight 594-AffiniPure goat anti-rabbit
secondary antibody (red fluorescence; 1:100; EarthOx LLC, San
Francisco, CA, USA) and Dylight 488 AffiniPure goat anti-mouse
secondary antibody (green fluorescence; 1:100; EarthOx) were added
and incubated for 2 h at room temperature. The cells were mounted
with mounting media containing 4′,6-diamino-2-phenylindole (Vector
Laboratories, Burlingame, CA, USA) and were observed under a
fluorescence microscope (Axio Observer A1; Carl Zeiss, Oberkochen,
Germany).
Western blot analysis
Western blot analysis was performed on the protein
isolated from the cell lysates of CD133+ and
CD133− GBM cells (GSC1, GSC2, GSC3 and G1, G2, G3,
respectively) using the rabbit polyclonal antibodies against Nrf2
(1:500), the rabbit polyclonal antibodies against nestin (1:500)
and the rabbit polyclonal antibodies against glial fibrillary
acidic protein (1:1,000). Nestin is an important GSC marker
(17); therefore, antibodies
against nestin were used to indicate the stem cell characteristics
of CD133+ cells. The blots were stripped and reprobed
with anti-GAPDH (rabbit polyclonal; Bio-World, Dublin, OH, USA) to
determine the equivalent loading, as described in previous studies
(18). The cells were lysed in
lysis buffer that comprised of 50 mM Tris-HCl, pH 7.5; 150 mM NaCl,
0.5% TX-100, 5% glycerol, 1% SDS, 1 mM
Na3VO4, 10 mM NaF and 1 mM
phenylmethanesulfonyl fluoride. The protein concentrations were
determined using a DC Protein assay (Bio-Rad, Hercules, CA, USA)
and 5X SDS sample buffer (0.5 M Tris-HCl, pH 6.8; 28% glycerol, 9%
SDS, 5% 2-mercaptoethanol and 0.01% bromphenol blue) was added. The
lysates were electrophoresed on an 8% SDS-PAGE gel and transferred
to a nitrocellulose membrane (Merck Millipore, Darmstadt, Germany).
The membranes were incubated with primary antibodies at 4°C
overnight. The horseradish peroxidase-conjugated secondary
antibodies were exposed to radiographic film (Kodak Film,
Rochester, NY, USA) using an enhanced chemiluminescence reagent
(Merck Millipore).
RNA preparation and qPCR
Total RNA was prepared using TRIzol (Takara Bio,
Inc., Shiga, Japan), according to the manufacturer’s instructions.
Approximately 400 ng total RNA was used as a template for cDNA
synthesis using the Verso cDNA synthesis kit (Takara Bio, Inc.).
qPCR was conducted using a SYBR®-Green Master mix
(Takara Bio, Inc.), according to the manufacturer’s instructions.
qPCR was conducted using the ABI 7900HT sequence detection system
(Applied Biosystems, Foster City, CA, USA). The primers of the
target genes are listed in Table.
I, which indicates the amplicon length and annealing
temperature.
 | Table IPrimers of target genes. |
Table I
Primers of target genes.
| Gene | Primer
sequence | Temperature
(°C) | Length (bp) | Amplicon size
(bases) |
|---|
| Nrf2 | F:
5′-TCAGCGACGGAAAGAGTATGA-3′ | 60.6 | 21 | 174 |
| R:
5′-CCACTGGTTTCTGACTGGATGT-3′ | 61.9 | 22 |
| GAPDH | F:
5′-GAAATCCCATCACCATCTTC-3′ | 59.6 | 20 | 226 |
| R:
5′-CCACTGGTTTCTGACTGGATGT-3′ | 61.3 | 22 |
Statistical analysis
The values are supplied as the mean ± SD. The data
were analyzed for significance using Student’s t-test. The
differences between the groups were analyzed using analysis of
variance followed by a post hoc test using the SPSS 19.0 package
(SPSS Inc., Chicago, IL, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
GSCs in GBM
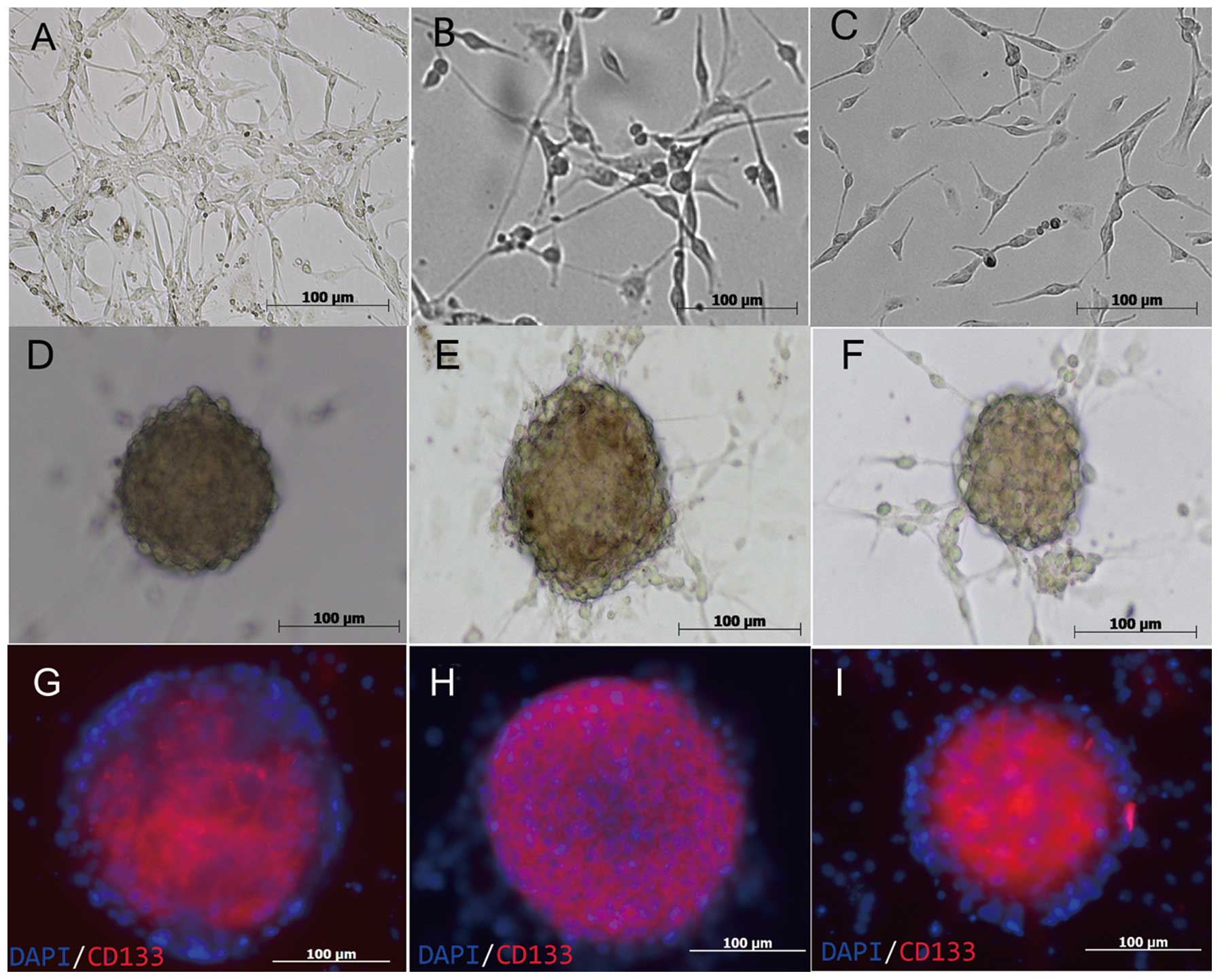
In the present study, the ratio of CD133+
to CD133− cells in GBM was analyzed using a flow
cytometry assay. The GBM samples (GSC1, GSC2 and GSC3) contained
~0.91±0.07%, 0.83±0.11% and 0.49±0.06% CD133+ cells,
respectively, under the culture condition of a 5% CO2
atmosphere (Fig. 1); this result
was similar to that of previous studies (19).
Culture of GSC spheres
The GSC spheres were cultured in non-adherent
growth, serum-free neural stem cell medium and spheres formed after
two days (Fig. 2). Following ~10
days of rapid growth, the cell spheres attained a diameter of ~100
μm.
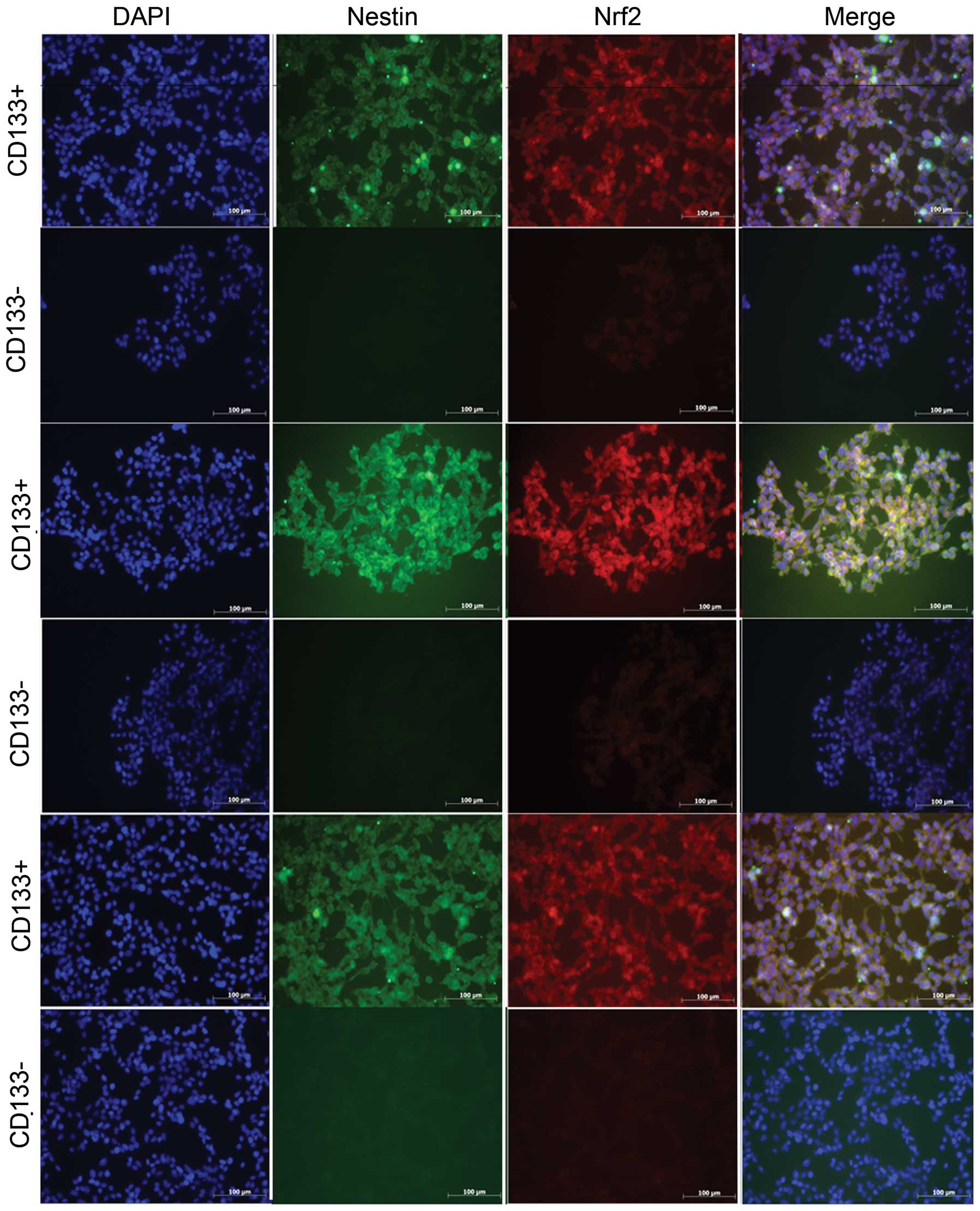
Immunofluorescence of CD133+
GSCs and CD133− cells
Nrf2 was significantly expressed in
CD133+ GBM cells, regardless of which patient they were
derived from. The CD133+ and CD133− cells
from GBM were cultured in the same medium for ~4 h, which did not
significantly influence the differentiation of GSCs.
Conversely, GSC spheres grew rapidly in the
serum-free medium. The present study, therefore, demonstrated that
the majority of the cells, which were derived from MACS, expressed
the neural stem cell biomarker CD133 (Fig. 3) (20,21)
and were capable of cultivating GSC spheres.
Nrf2 expression in CD133−
cells and CD133+ GSCs at the transcriptional level
qPCR was conducted to assess the relative quantities
of Nrf2 mRNA in CD133+ GSCs and CD133− GBM
cells. The qPCR data identified that the expression of Nrf2 was
greater in CD133+ GSCs than that observed in
CD133− GBM cells (P<0.05; Fig. 4).
Western blot analysis
Western blot analysis was conducted and a similar
difference in expression levels was observed. The western blot
analysis showed the total Nrf2 protein was expressed to a greater
extent in CD133+ GSCs compared with that in
CD133− GBM cells (Fig.
5).
Discussion
GBM is considered to be the central nervous system
tumor with the highest rate of malignancy (22) and numerous studies have identified
that GSCs are a significant factor (23,24).
Furthermore, previous studies have identified that Nrf2 is
important in GBM genesis in vivo and in vitro
(15,25). As Nrf2 was hypothesized to be
significant in GSC and tumor genesis, it was necessary to
understand whether transcription and translation rates varied in
GSCs (26).
The present study required a stable and reliable
source of GSCs for analysis. Three predominant sources of GSCs have
been identified: The tissue of GBM patients (3), cell lines (27) or xenografts of nude mice. In our
preliminary experiments, there were complications with obtaining
GSCs from GBM cell lines using the neural stem cell medium.
Previous studies have adopted the MACS system to extract GSCs from
GBM cell lines (16,27). Thus, MACS was conducted in the
present study to obtain pure, stable GSCs from the GBM. CD133 and
nestin antibodies were used for cell identification (21,28).
The transcriptional and translational protein levels
observed in the present study demonstrated that the expression of
Nrf2 in CD133+ GSCs was significantly higher than that
in CD133− cells (from the GBM). Western blot analysis
suggested that the quantity of Nrf2 in CD133− GBM cells
was lower than that in CD133+ GSCs. This was confirmed
by qPCR, as the expression of Nrf2 in CD133+ GSCs was
greater than that observed in CD133− GBM cells
(P<0.05). Immunofluorescence and immunocytochemical analysis
demonstrated a high level of Nrf2 in CD133+ GSCs, which
was observed in the nucleus as well as the cytoplasm (Fig. 3). Nrf2 was capable of regulating ARE
following its translocation into the nucleus. Numerous studies have
identified that GSCs were more resistant to hypoxia and
anti-angiogenesis therapies compared with CD133− GGM
cells (22); furthermore, hypoxia
induces the invasion of GSCs into the underlying tissue (8). Nrf2 is one of the primary
transcriptional regulators of anti-hypoxic and antioxidant
reactions, which alter gene transcription following translocation
into the nucleus and connected to the ARE region. The present study
identified an increased expression of Nrf2 in GSCs; therefore, it
was hypothesized that Nrf2 was critical in GSC genesis. However,
this may be a result of the increased expression of an anti-hypoxia
gene, as well as additional genes and molecules, which contribute
to cell proliferation, differentiation, apoptosis, necrosis,
self-renewal and the cell cycle (14,29).
Thus the present study indicated that Nrf2 may be a potential
biomarker and rational therapeutic target for the diagnosis and
treatment of GSCs.
However, there were limitations in the present
study; the influence of Nrf2 on GSC genesis, self-renewal and
differentiation was not investigated. Although immunofluorescence
assays identified that Nrf2 translocated into the cell nucleus, the
associated genes regulated by this nuclear factor, such as quinone
oxidoreductase1 and hemoxygenase-1 (30), were not analyzed; these may be key
genes within GSCs. Furthermore, the Nrf2 expression was only
assayed in vitro and as the environment within humans is
complex, the interaction of cells and tissue fluid in vivo
may alter the expression of Nrf2. Therefore, further study is
required.
The mechanism behind the variable Nrf2 levels that
exist between the GSCs and other GBM cells requires further
investigation. In addition, the Nrf2 signaling pathway may be a
novel target for the regulation and suppression of the
proliferation and invasion of GSCs into the underlying tissue. In
conclusion, Nrf2 may be considered as a potential target for the
treatment of tumors in humans.
Acknowledgements
The authors would like to thank Dr Feng Genbao and
Dr He Jin for their technical assistance. This study was supported
by grants from the National Natural Science Foundation of China
(grant nos. 81070974 and 81271377), the Jiangsu Provincial Key
Subject (grant no. X4200722) and the Jinling Hospital of Nanjing,
China (grant no. 2010Q017).
Abbreviations:
|
GSCs
|
glioma stem cells
|
|
GBM
|
glioblastoma multiforme
|
|
Nrf2
|
nuclear factor erythroid 2-related
factor 2
|
|
ARE
|
antioxidant response element
|
References
|
1
|
Van Meir EG, Hadjipanayis CG, Norden AD,
Shu HK, Wen PY and Olson JJ: Exciting new advances in
neuro-oncology: the avenue to a cure for malignant glioma. CA
Cancer J Clin. 60:166–193. 2010.PubMed/NCBI
|
|
2
|
Vescovi AL, Galli R and Reynolds BA: Brain
tumour stem cells. Nat Rev Cancer. 6:425–436. 2006. View Article : Google Scholar
|
|
3
|
Huang Q, Zhang QB, Dong J, Wu YY, Shen YT,
Zhao YD, Zhu YD, Diao Y, Wang AD and Lan Q: Glioma stem cells are
more aggressive in recurrent tumors with malignant progression than
in the primary tumor, and both can be maintained long-term in
vitro. BMC Cancer. 8:3042008. View Article : Google Scholar
|
|
4
|
He H, Li MW and Niu CS: The pathological
characteristics of glioma stem cell niches. J Clin Neurosci.
19:121–127. 2012. View Article : Google Scholar
|
|
5
|
Johannessen TC, Bjerkvig R and Tysnes BB:
DNA repair and cancer stem-like cells - potential partners in
glioma drug resistance? Cancer Treat Rev. 34:558–567. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kang MK and Kang SK: Tumorigenesis of
chemotherapeutic drug-resistant cancer stem-like cells in brain
glioma. Stem Cells Dev. 16:837–847. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Z, Bao S, Wu Q, Wang H, Eyler C,
Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, et al:
Hypoxia-inducible factors regulate tumorigenic capacity of glioma
stem cells. Cancer Cell. 15:501–513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heddleston JM, Wu Q, Rivera M, Minhas S,
Lathia JD, Sloan AE, Iliopoulos O, Hjelmeland AB and Rich JN:
Hypoxia-induced mixed-lineage leukemia 1 regulates glioma stem cell
tumorigenic potential. Cell Death Differ. 19:428–439. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Soeda A, Park M, Lee D, Mintz A,
Androutsellis-Theotokis A, McKay RD, Engh J, Iwama T, Kunisada T,
Kassam AB, et al: Hypoxia promotes expansion of the CD133-positive
glioma stem cells through activation of HIF-1alpha. Oncogene.
28:3949–3959. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kensler TW, Wakabayashi N and Biswal S:
Cell survival responses to environmental stresses via the
Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 47:89–116.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao F, Wu T, Lau A, Jiang T, Huang Z,
Wang XJ, Chen W, Wong PK and Zhang DD: Nrf2 promotes neuronal cell
differentiation. Free Radic Biol Med. 47:867–879. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kato K, Takahashi K, Monzen S, Yamamoto H,
Maruyama A, Itoh K and Kashiwakura I: Relationship between
radiosensitivity and Nrf2 target gene expression in human
hematopoietic stem cells. Radiat Res. 174:177–184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bobilev I, Novik V, Levi I, Shpilberg O,
Levy J, Sharoni Y, Studzinski GP and Danilenko M: The Nrf2
transcription factor is a positive regulator of myeloid
differentiation of acute myeloid leukemia cells. Cancer Biol Ther.
11:317–329. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou Y, Wang HD, Zhu L, Cong ZX, Li N, Ji
XJ, Pan H, Wang JW and Li WC: Knockdown of Nrf2 enhances autophagy
induced by temozolomide in U251 human glioma cell line. Oncol Rep.
29:394–400. 2013.PubMed/NCBI
|
|
16
|
Beier D, Hau P, Proescholdt M, Lohmeier A,
Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U and Beier
CP: CD133(+) and CD133(−) glioblastoma-derived cancer stem cells
show differential growth characteristics and molecular profiles.
Cancer Res. 67:4010–4015. 2007.
|
|
17
|
Strojnik T, Røsland GV, Sakariassen PO,
Kavalar R and Lah T: Neural stem cell markers, nestin and musashi
proteins, in the progression of human glioma: correlation of nestin
with prognosis of patient survival. Surg Neurol. 68:133–144. 2007.
View Article : Google Scholar
|
|
18
|
Närvä E, Rahkonen N, Emani MR, Lund R,
Pursiheimo JP, Nästi J, Autio R, Rasool O, Denessiouk K, Lähdesmäki
H, et al: RNA-binding protein L1TD1 interacts with LIN28 via RNA
and is required for human embryonic stem cell self-renewal and
cancer cell proliferation. Stem Cells. 30:452–460. 2012.PubMed/NCBI
|
|
19
|
Sharma V, Dixit D, Ghosh S and Sen E:
COX-2 regulates the proliferation of glioma stem like cells.
Neurochem Int. 59:567–571. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mizrak D, Brittan M and Alison MR: CD133:
molecule of the moment. J Pathol. 214:3–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shmelkov SV, St Clair R, Lyden D and Rafii
S: AC133/CD133/Prominin-1. Int J Biochem Cell Biol. 37:715–719.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Folkins C, Shaked Y, Man S, Tang T, Lee
CR, Zhu Z, Hoffman RM and Kerbel RS: Glioma tumor stem-like cells
promote tumor angiogenesis and vasculogenesis via vascular
endothelial growth factor and stromal-derived factor 1. Cancer Res.
69:7243–7251. 2009. View Article : Google Scholar
|
|
23
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
24
|
Kondo T, Setoguchi T and Taga T:
Persistence of a small subpopulation of cancer stem-like cells in
the C6 glioma cell line. Proc Natl Acad Sci USA. 101:781–786. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan H, Wang H, Zhu L, Wang X, Cong Z, Sun
K and Fan Y: The involvement of Nrf2-ARE pathway in regulation of
apoptosis in human glioblastoma cell U251. Neurol Res. 35:71–78.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alam J, Stewart D, Touchard C, et al:
Nrf2, a Cap’n’Collar transcription factor, regulates induction of
the heme oxygenase-1 gene. J Biol Chem. 274:26071–26078. 1999.
|
|
27
|
Fan H, Guo H, Zhang IY, Liu B, Luan L, Xu
S, Hou X, Liu W, Zhang R, Wang X and Pang Q: The different HMGA1
expression of total population of glioblastoma cell line U251 and
glioma stem cells isolated from U251. Brain Res. 1384:9–14. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang M, Song T, Yang L, Chen R, Wu L,
Yang Z and Fang J: Nestin and CD133: valuable stem cell-specific
markers for determining clinical outcome of glioma patients. J Exp
Clin Cancer Res. 27:852008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Motohashi H and Yamamoto M: Nrf2-Keap1
defines a physiologically important stress response mechanism.
Trends Mol Med. 10:549–557. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang L, Lin C, Wang L, Guo H and Wang X:
Hypoxia and hypoxia-inducible factors in glioblastoma multiforme
progression and therapeutic implications. Exp Cell Res.
318:2417–2426. 2012. View Article : Google Scholar : PubMed/NCBI
|