Introduction
Interferon (IFN)-α is a member of the type I
interferon family, which is active as an antiviral and
immunomodulatory cytokine. Type I IFNs are able to modulate a
variety of cellular responses, including cell growth and apoptosis.
In humans, the production of IFN-α is most efficiently induced in
numerous types of immune cells by viral infection (1,2). IFN-α
has been used clinically in the therapy of certain malignancies and
viral diseases. Although the antitumor mechanism of IFN-α is not
entirely understood, cell cycle arrest and apoptosis have been
shown to be involved. These effects are likely to be independent of
each other and are partly dependent on the cells that are treated
with IFN-α (3,4). In particular, IFN-α has been shown to
induce autophagy (5).
Materials and methods
Cell culture
The human osteosarcoma MG-63 cell line was obtained
from the American type culture collection (Manassas, VA, USA). The
MG-63 cells were propagated in Dulbecco’s modified Eagle medium
(Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum
(Gibco) and antibiotics in a humidified incubator containing 5%
CO2 at 37°C. Human IFN-α (Sigma-Aldrich, St Louis, MO,
USA) was diluted in serum-free medium.
Matrigel invasion assay
The Matrigel invasion assay was performed to assess
the effects of IFN-α on the invasive properties of the MG-63 cells.
Transwell inserts (12-well, 12-mm with 12.0-μm pore size) from
Corning Inc. (Corning, NY, USA) were coated with 200 μl Matrigel
(final concentration, 1.0 mg/ml in ice-cold serum-free medium; BD
Biosciences, San Jose, CA, USA) and allowed to dry at 37°C for 3–5
h. The cells were treated for 48 h with IFN-α at concentrations of
1×102, 1×103 or 1×104 IU/ml. The
control and treated cells were washed twice with serum-free medium
and trypsinized. A 200-μl cell suspension (2×105 cells)
from each sample was added to each well in triplicate.
The filters were incubated for 48 h at 37°C in a
humidified incubator containing 5% CO2, fixed and
stained with 0.5% crystal violet in methanol. The non-migratory
cells were removed with a cotton tip and the migratory cells were
counted using a light microscope (Olympus, Tokyo, Japan) at ×400
magnification. Each experiment was run in duplicate. The results
are expressed as the mean number of cells that were counted in each
field, ± standard deviation (SD).
Cell proliferation assay
For the assessment of the cell growth, the MG-63
cells (5,000 cells/well) were treated with various concentrations
of IFN-α and cisplatin in 96-well plates. At 48 h post-treatment,
the cell growth was evaluated using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. MTT (Sigma-Aldrich) was added to the culture medium in each
well at a concentration of 500 μg/ml. Following incubation for 4 h
at 37°C, 100 μl dimethyl sulfoxide (DMSO) was added to each well
and the 550-nm absorption was measured. Each experiment was
reproduced in six wells and repeated at least three times.
Hoechst 33258 staining
The MG-63 cells were treated with various
concentrations of IFN-α and cisplatin in six-well plates. At 48 h
post-treatment, the cells were washed in cold phosphate-buffered
saline twice and fixed in 4% formaldehyde at 4°C for 10 min.
Following this, the fixed cells were washed and labeled with 5
μg/ml Hoechst 33258 (Sigma-Aldrich), and maintained at room
temperature in the dark for 10 min. The cells were then observed
and imaged using a fluorescence microscope (Olympus) with
excitation at 350 nm and emission at 460 nm. Apoptosis of the MG-63
cells was determined by the alteration of nuclear morphology and
fluorescent density that was observed subsequent to staining the
cells with Hoechst 33258.
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) double labeling for flow cytometry
(FCM)-assessed apoptosis
An Annexin V-FITC kit (BD Biosciences) was used to
detect apoptosis. The cells were cultured with various
concentrations of IFN-α and cisplatin for 48 h, harvested through
trypsinization and washed twice. The cells were then reacted with
FITC-conjugated Annexin V and PI for 15 min. The staining profiles
were determined using FACScan (BD Biosciences, Franklin Lakes, NJ,
USA) and Cell-Quest software (BD Biosciences). The early apoptotic
cells (Annexin-FITC-positive and PI-negative) were located in the
lower right quadrant. The late apoptotic or necrotic cells
(Annexin-FITC-positive and PI-positive) were located in the upper
right quadrant. The healthy cells (negative for the two probes)
were located in the lower left quadrant. The results are expressed
as the percentage of positively-stained cells among total cells.
Each group was repeatedly measured six times and each sample
included 1×104 cells.
Acridine orange staining for
autophagy
Autophagy is characterized by the formation and
promotion of acidic vesicular organelles. The MG-63 cells were
treated with various concentrations of IFN-α and cisplatin in
six-well plates. At 48 h post-treatment, the cells were incubated
with 1 mg/ml acridine orange (Sigma-Aldrich) for 15 min. Images
were obtained using a fluorescence microscope.
Green fluorescent protein (GFP)-LC3 dot
assay
The cells were transiently transfected with a
GFP-LC3 (Origene Tech., Inc., Rockville, MD, USA) vector using
Lipofectamine LTX and PLUS Reagents (Invitrogen Life Technologies,
Carlsbad, CA, USA) according to the manufacturer’s instructions.
After 24 h, the cells were exposed to IFN-α and/or cisplatin for 48
h as indicated and examined under a fluorescence microscope. The
induction of autophagy was quantified by counting the percentage of
cells in each group that contained LC3 aggregates.
Transmission electron microscopy
The cells were fixed with 3% glutaraldehyde in a
0.1-M cacodylate buffer for 1 h. Following fixation, the samples
were post-fixed in 1% OsO4 in the same buffer for 30
min. Ultra-thin sections were then observed under a transmission
electron microscope (JEOL, Tokyo, Japan).
Statistical analysis
The experimental data are expressed as the mean ±
SD. The group means were compared using a t-test via the
statistical software program, SPSS 13.0 (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
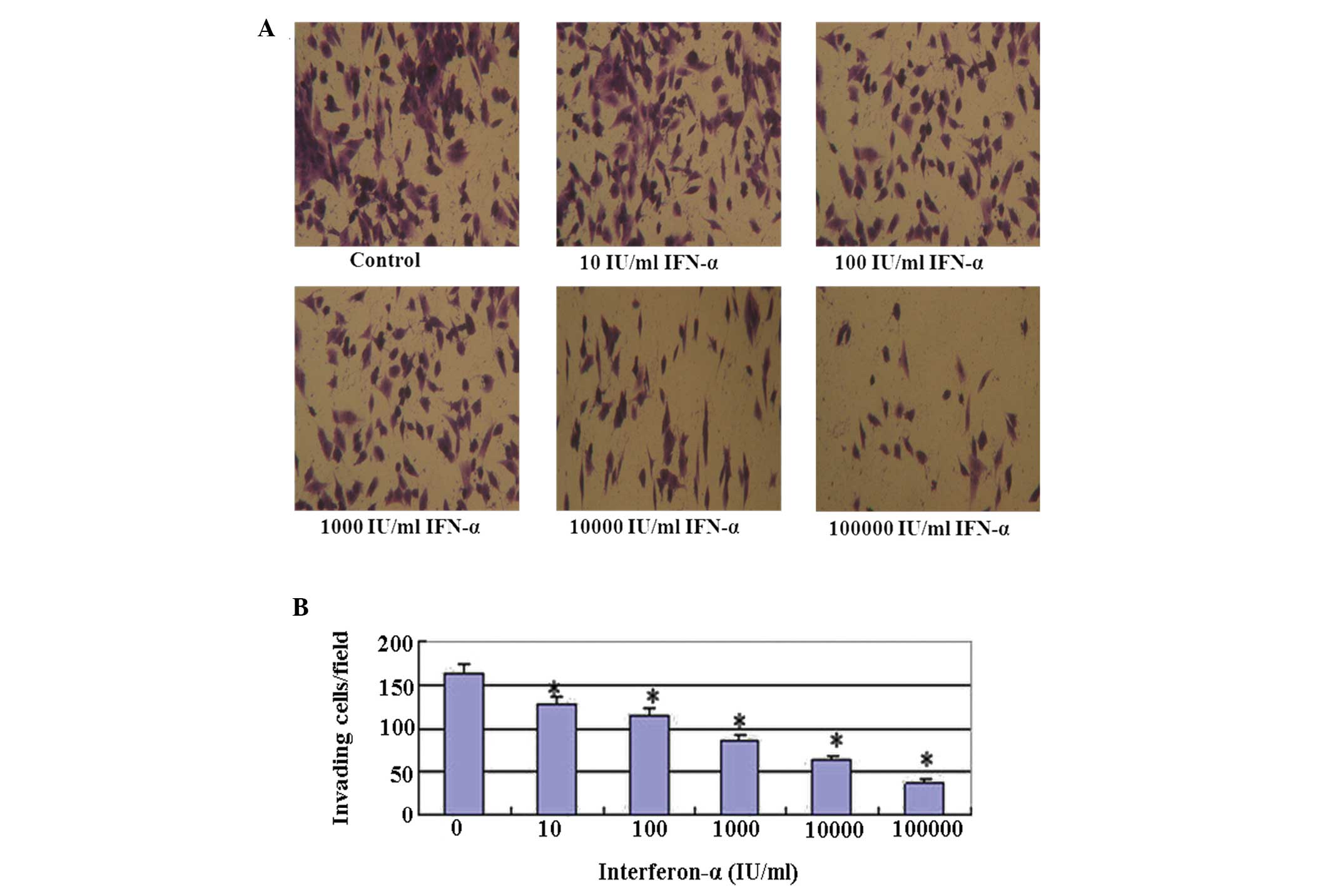
IFN-α markedly reduces tumor cell
invasion in MG-63 cells
Cell invasion was evaluated following the treatment
(Fig. 1). Marked reductions in the
invasive properties of the MG-63 cells were observed following the
treatment with IFN-α alone through the Matrigel invasion assays.
The staining of the invaded cells through the membrane demonstrated
that the number of invasive cells was significantly reduced in the
cells that were treated with IFN-α alone, compared with that of the
untreated control cells.
Anti-proliferative effects of IFN-α
and/or cisplatin in MG-63 cells
The effect of adding a low (100 IU/ml), middle
(1,000 IU/ml) or high (10,000 IU/ml) dose of IFN-α on the
antiproliferative activity of cisplatin was investigated. As shown
in Fig. 2, cell proliferation was
inhibited by exposing the cells to 100, 1,000 or 10,000 IU/ml IFN-α
and/or 5 μg/ml cisplatin for 48 h. Cells treated with a combination
of IFN-α and cisplatin exhibited more potent antiproliferative
activity compared with those treated with cisplatin alone, in a
dose-dependent manner.
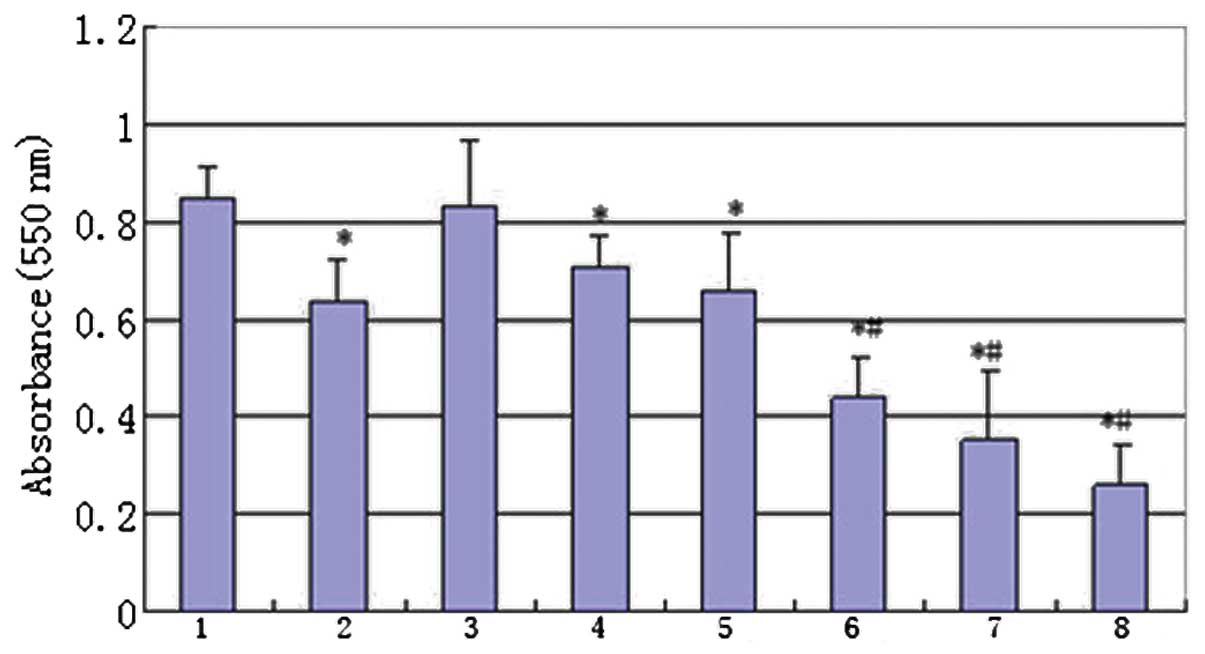 | Figure 2Antiproliferative effect of IFN-α and
cisplatin. The MG-63 cells were treated with IFN-α and/or
cisplatin. The cell viability was determined by an MTT assay. Group
1, control; 2, treated with 5 μg/ml cisplatin; 3, treated with 100
IU/ml IFN-α; 4, treated with 1,000 IU/ml IFN-α; 5, treated with
10,000 IU/ml IFN-α; 6, combination therapy of 100 IU/ml IFN-α and 5
μg/ml cisplatin; 7, combination therapy of 1,000 IU/ml IFN-α and 5
μg/ml cisplatin and 8, combination therapy of 10,000 IU/ml IFN-α
and 5 μg/ml cisplatin. (*P<0.01, compared with the
control mean values and #P<0.01 compared with group 2
mean values). IFN, interferon; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. |
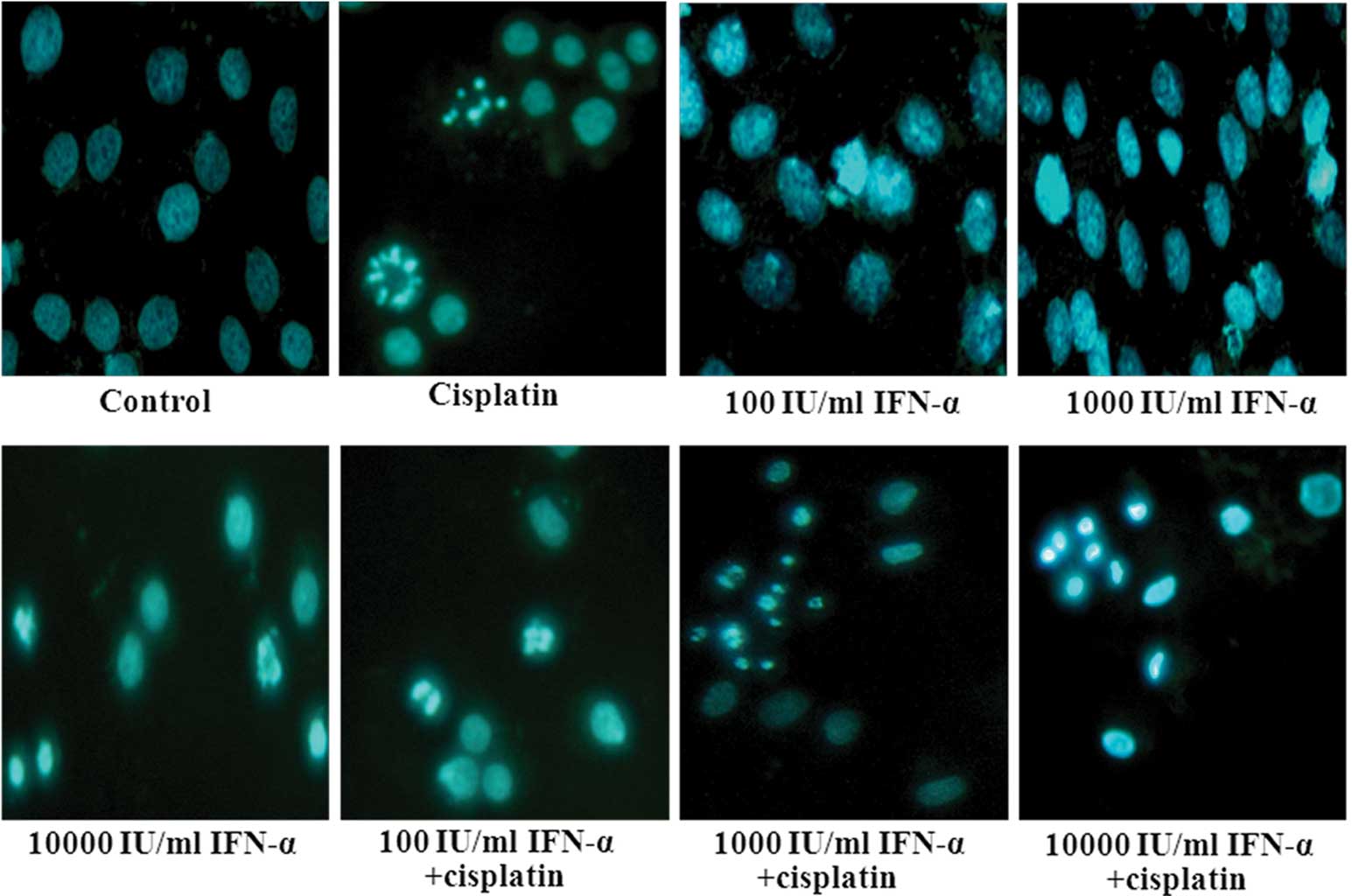
Effect of IFN-α and/or cisplatin on
apoptosis in MG-63 cells
The effect of adding a low (100 IU/ml), middle
(1,000 IU/ml) or high (10,000 IU/ml) dose of IFN-α on the apoptotic
action of cisplatin was investigated. The typical apoptotic
morphological changes, including condensed chromatin and shrunken,
crumpled and condensed nuclei, were observed in the MG-63 cells
that were treated with IFN-α and cisplatin following staining with
Hoechst 33258 (Fig. 3). A
quantitative determination of MG-63 cell apoptosis that was induced
by IFN-α and/or cisplatin was performed using Annexin V and PI
staining. The cisplatin-mediated apoptosis effect, which was
enhanced by IFN-α, was dose-dependent. The most evident apoptosis
effect occurred following the addition of a high dose (10,000
IU/ml) of IFN-α and 5 μg/ml cisplatin (Fig. 4A). The quantitative results are
shown in Fig. 4B.
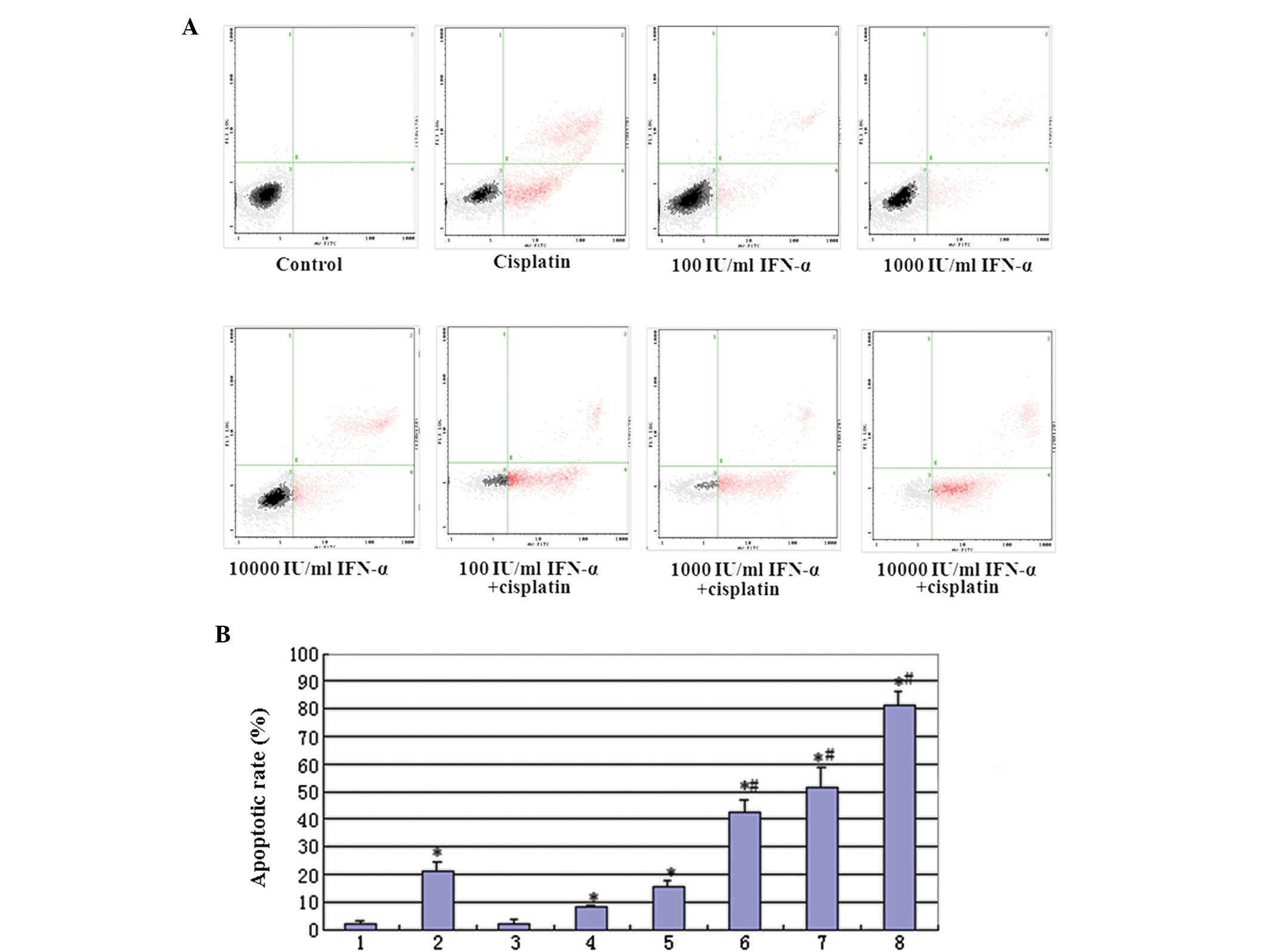 | Figure 4(A) Annexin V and PI staining for FACS
detection of apoptosis of MG-63 cells induced by IFN-α and/or
cisplatin. The MG-63 cells were incubated with various
concentrations of IFN-α and/or cisplatin for 48 h prior to being
stained with Annexin V and PI. (B) Quantitative evaluation of the
FACS assay. Group 1, control; 2, treated with 5 μg/ml cisplatin; 3,
treated with 100 IU/ml IFN-α; 4, treated with 1,000 IU/ml IFN-α; 5,
treated with 10,000 IU/ml IFN-α; 6, combination therapy of 100
IU/ml IFN-α and 5 μg/ml cisplatin; 7, combination therapy of 1,000
IU/ml IFN-α and 5 μg/ml cisplatin and 8, combination therapy of
10,000 IU/ml IFN-α and 5 μg/ml cisplatin. (*P<0.01,
compared with the control mean values and #P<0.01,
compared with treated with group 2 mean values). PI, propidium
iodide; FACS, fluorescence-activated cell sorting; IFN,
interferon. |
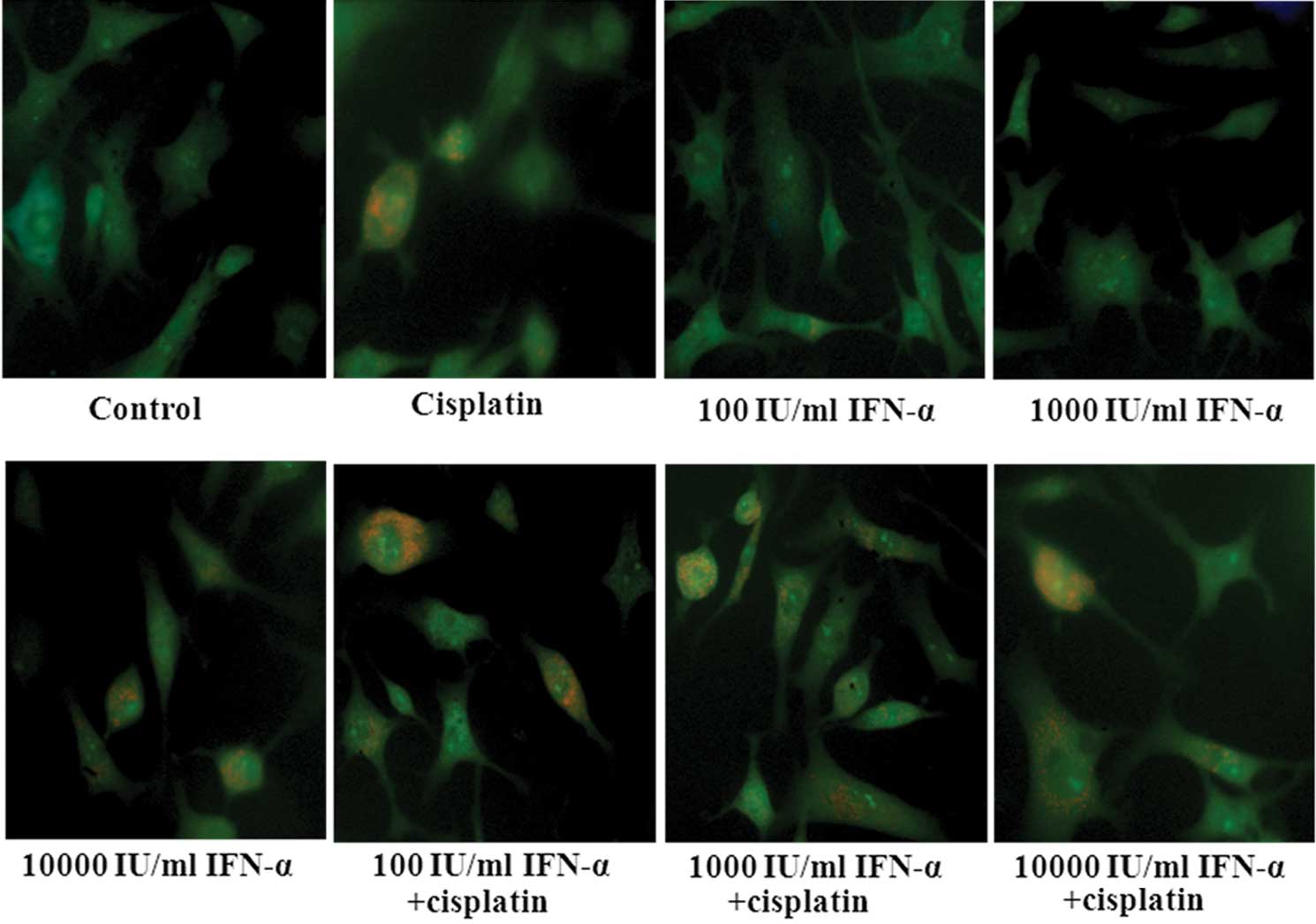
Effect of IFN-α and/or cisplatin on
autophagy in MG-63 cells
Following treatment with cisplatin or high-dose
(10,000 IU/ml) IFN-α, the cells revealed an intracellular
accumulation of acidic vesicular and autolysosomes, implying that
cisplatin or a high-dose of IFN-α may induce autophagic responses.
Co-treatment with IFN-α increased the cisplatin-induced acidic
vesicular in the MG-63 cells. (Fig.
5). GFP-LC3 exhibited a diffuse pattern, which became
punctuated when treated with cisplatin. Co-treatment with IFN-α
increased the cisplatin-induced punctuated pattern in the MG-63
cells and the cisplatin-mediated autophagy effects were enhanced by
IFN-α in a dose-dependent manner (Fig.
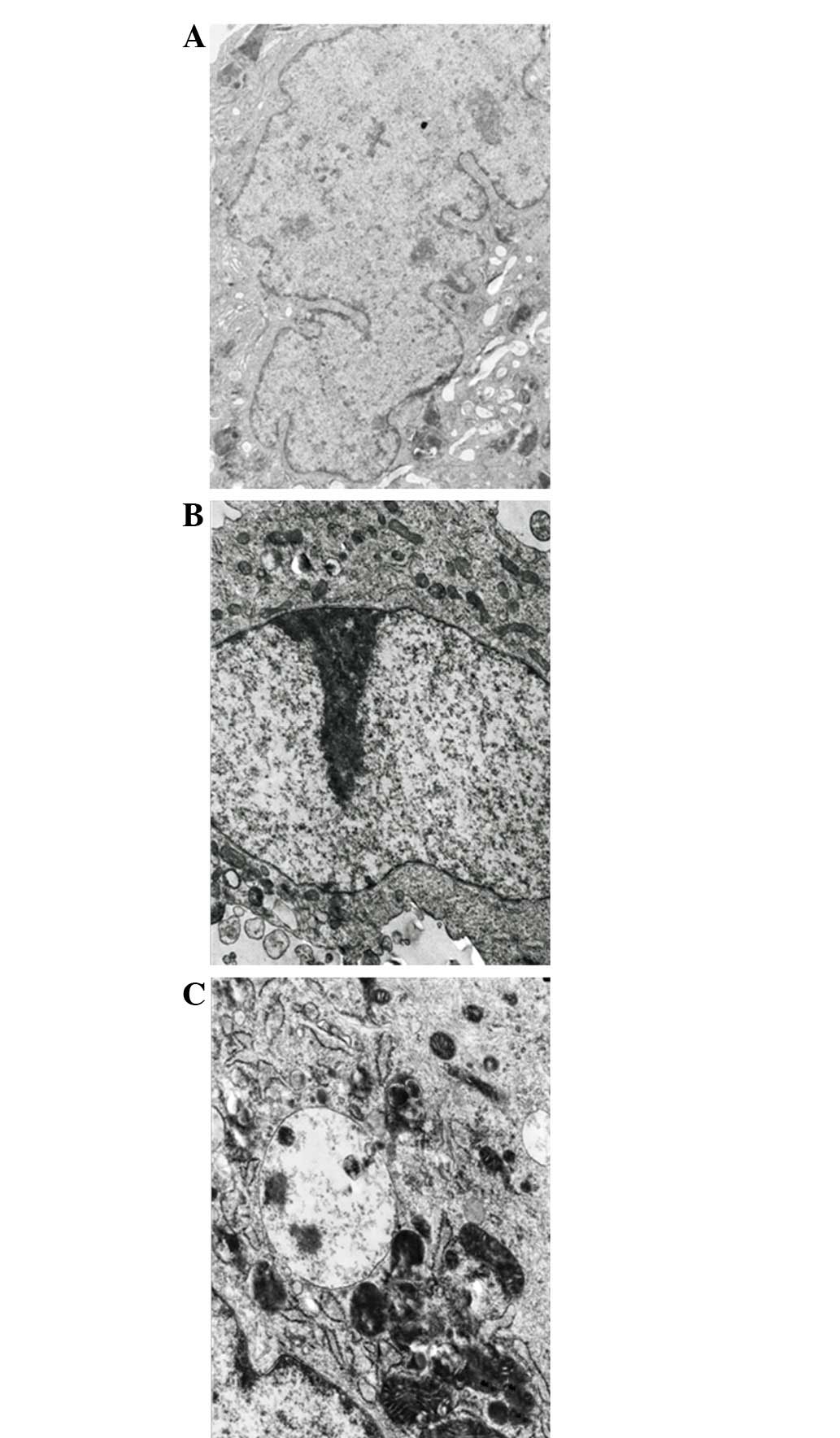
6A). The quantitative results are shown in Fig. 6B. Additionally, the co-treatment of
IFN-α and cisplatin for 48 h developed autophagosome-like
characteristics, including single- and double-membrane vacuoles
containing intact and degraded cellular debris (Fig. 7). These data confirmed that the
co-treatment with IFN-α increased cisplatin-induced autophagy in
the MG-63 cells.
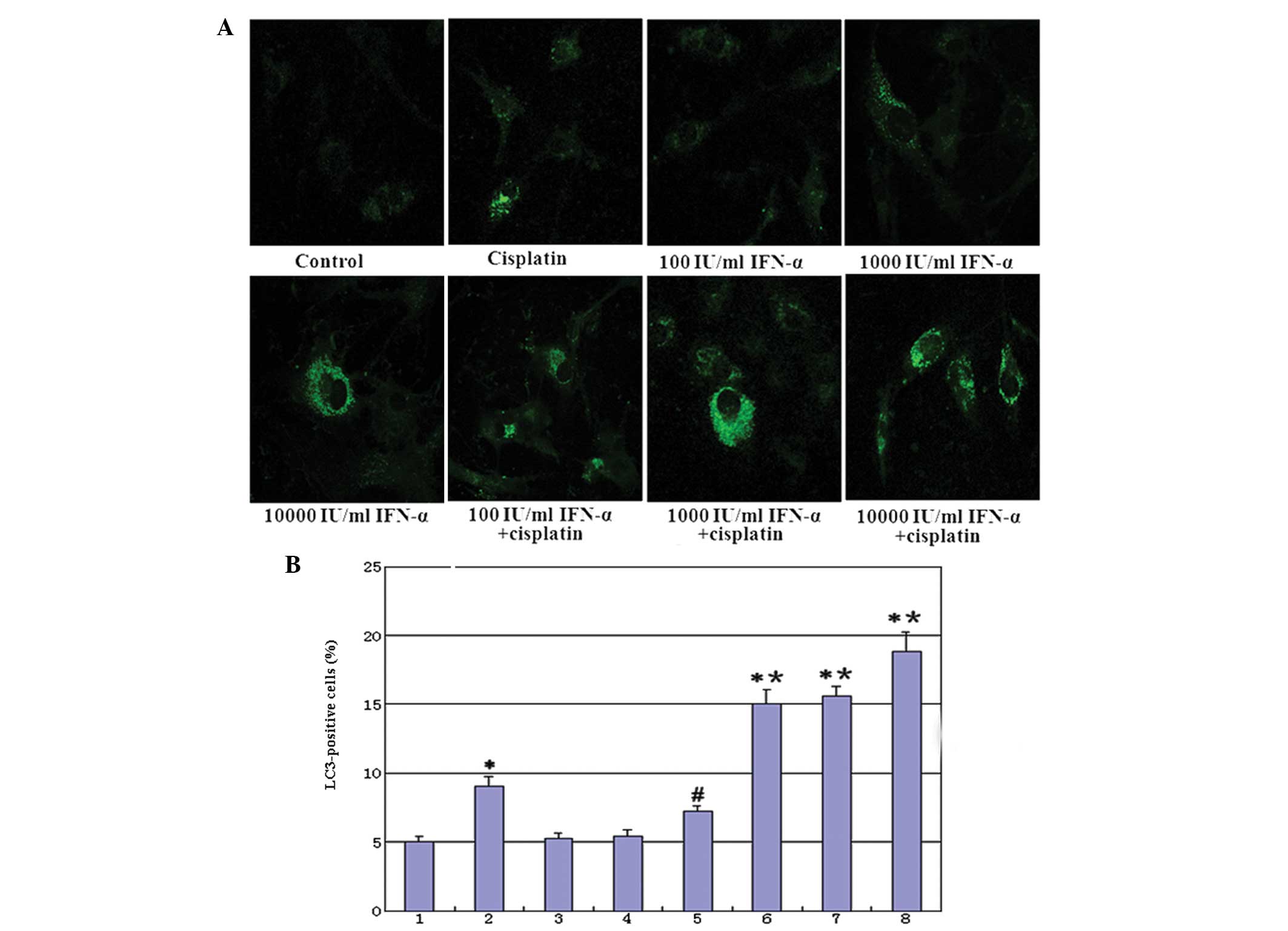 | Figure 6(A) IFN-α and/or cisplatin induced
punctuated GFP-LC3 distribution in cells. At 24 h following the
transient transfection of GFP-LC3, the cells were treated with
IFN-α and/or cisplatin for 48 h and analyzed for fluorescence. The
images were captured using a fluorescence microscope
(magnification, ×400). (B) Quantitative assay: The induction of
autophagy was quantified by counting the percentage of cells in
each group that contained LC3 aggregates. Group 1, control; 2,
treated with 5 μg/ml cisplatin; 3, treated with 100 IU/ml IFN-α; 4,
treated with 1,000 IU/ml IFN-α; 5, treated with 10,000 IU/ml IFN-α;
6, combination therapy of 100 IU/ml IFN-α and 5 μg/ml cisplatin; 7,
combination therapy of 1,000 IU/ml IFN-α and 5 μg/ml cisplatin and
8, combination therapy of 10,000 IU/ml IFN-α and 5 μg/ml cisplatin.
(*P<0.01, compared with the control mean values;
#P<0.05, compared with the control mean values and
*P<0.01, compared with group 2 mean values). IFN,
interferon; GFP, green fluorescence protein. |
Discussion
Osteosarcoma is the most common primary tumor of the
bone and predominantly occurs in the second decade of life. The
condition is the most frequently occurring pediatric
non-hematological tumor of the bone and the fifth most prevalent
malignancy of adolescence (6).
Osteosarcoma is notable for locally aggressive behavior and early
metastasis formation. High-dose cytotoxic chemotherapy and surgical
resection have improved the prognosis of patients with
osteosarcoma. The long-term survival for patients with localized
(non-metastatic) disease is ~70%, but at the cost of considerable
therapy-related morbidity (7,8). At
present, ~20% of patients have metastases and almost all patients
with recurrent osteosarcoma have metastatic disease. The cure rate
for patients with metastatic or recurrent disease remains poor
(9,10). Effective post-operative adjuvant
therapies, therefore, are extremely significant for improving the
overall survival of osteosarcoma patients.
IFN-α is a cytokine that belongs to type I IFNs and
exerts multiple effects on cellular functions (1,11,12).
The IFN-α family is composed of at least 13 functional IFN
subtypes, which share the same receptor system and exert similar
biological activities (13,14). In particular, 50 years of research
on IFN-α have revealed that these cytokines exhibit a variety of
biological effects, which differ from those that are present during
viral replication, including antitumor activity. IFN-α is a widely
expressed cytokine that is secreted as the first line of defense
against several tumors (15). It
has been used in >40 countries for the treatment of >14 types
of cancer, including certain hematological malignancies (hairy cell
leukemia, chronic myeloid leukemia and certain B- and T-cell
lymphomas) and certain solid tumors, including melanoma, renal
carcinoma and Kaposi’s sarcoma. However, despite numerous years of
intense work in animal tumor models and considerable experience in
the clinical use of IFN-α, the significance of the various
mechanisms of action underlying the response in patients remains a
matter of debate. It was previously considered that the direct
inhibitory effects on tumor cell growth were the main mechanisms
that were significant in the antitumor response in IFN-treated
patients. However, it has been shown that IFN-α is able to directly
inhibit the proliferation of normal and tumor cells in vitro
and in vivo, and may exert other direct effects on tumor
cells (16,17,18).
The present study on human osteosarcoma MG-63 cells
demonstrated that IFN-α treatment suppressed human osteosarcoma
cell invasion. The Matrigel invasion assays demonstrated marked
reductions in the invasive properties of the MG-63 cells following
treatment with IFN-α. The osteosarcoma cells were also treated with
cisplatin and/or IFN-α. Apoptosis and autophagy were assessed using
MTT assay, Hoechest 33258 staining, FCM assay, acridine orange
staining, GFP-LC3 dotted assay and transmission electron
microscopy. Further analysis demonstrated that the effects of
cisplatin may be enhanced by combining the drug with IFN-α.
In conclusion, the present study has shown that
IFN-α is able to suppress invasion and enhance cisplatin-mediated
apoptosis and autophagy in human osteosarcoma MG-63 cells. The
combination therapy of chemotherapeutics and IFN-α may be a novel
approach for osteosarcoma, and further evidence is required by
experiments in vivo.
Acknowledgements
This study was supported by grants from the
Scientific Support Project of the Anhui Provincial Education
Department of China (nos. KJ2012ZD08 and KJ2012Z162), the National
Scientific and Technological Support Projects of China
(no.81101273), the Key Basic Research and Development Program of
China (973; no.2007CB513108) and the National Natural Science
Foundation of China (no. 30872253).
References
|
1
|
Zhang W, Rao HY, Feng B, Liu F and Wei L:
Effects of interferon-alpha treatment on the incidence of
hyperglycemia in chronic hepatitis C patients: a systematic review
and meta-analysis. PLoS One. 7:e392722012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tough DF: Modulation of T-cell function by
type I interferon. Immunol Cell Biol. 90:492–497. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yim HY, Yang Y, Lim JS, Lee MS, Zhang DE
and Kim KI: The mitochondrial pathway and reactive oxygen species
are critical contributors to interferon-α/β-mediated apoptosis in
Ubp43-deficient hematopoietic cells. Biochem Biophys Res Commun.
423:436–440. 2012.PubMed/NCBI
|
|
4
|
Fishman AI, Johnson B, Alexander B, Won J,
Choudhury M and Konno S: Additively enhanced antiproliferative
effect of interferon combined with proanthocyanidin on bladder
cancer cells. J Cancer. 3:107–112. 2012. View Article : Google Scholar
|
|
5
|
Harris J: Autophagy and cytokines.
Cytokine. 56:140–144. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
7
|
Anninga JK, Gelderblom H, Fiocco M, Kroep
JR, Taminiau AH, Hogendoorn PC and Egeler RM: Chemotherapeutic
adjuvant treatment for osteosarcoma: where do we stand? Eur J
Cancer. 47:2431–2445. 2011. View Article : Google Scholar
|
|
8
|
Dai X, Ma W, He X and Jha RK: Review of
therapeutic strategies for osteosarcoma, chondrosarcoma, and
Ewing’s sarcoma. Med Sci Monit. 17:177–190. 2011.
|
|
9
|
Loeb DM: Is there a role for immunotherapy
in osteosarcoma? Cancer Treat Res. 152:447–457. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jaffe N: Osteosarcoma: review of the past,
impact on the future. The American experience. Cancer Treat Res.
152:239–262. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tarhini AA, Gogas H and Kirkwood JM: IFN-α
in the treatment of melanoma. J Immunol. 189:3789–3793. 2012.
|
|
12
|
Pasquali S and Mocellin S: The anticancer
face of interferon alpha (IFN-alpha): from biology to clinical
results, with a focus on melanoma. Curr Med Chem. 17:3327–3336.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moll HP, Maier T, Zommer A, Lavoie T and
Brostjan C: The differential activity of interferon-α subtypes is
consistent among distinct target genes and cell types. Cytokine.
53:52–59. 2011.
|
|
14
|
Miwa S, Kadono Y, Sugata T, Mizokami A and
Namiki M: Successful treatment for metastases from renal cell
carcinoma with alternation of interferon-alpha subtypes. Int J Clin
Oncol. 15:97–100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mahmutović S and Beslagić E: Significance
of the interferon (IFN) in the therapy. Bosn J Basic Med Sci.
4:42–44. 2004.
|
|
16
|
de Vries-van Leeuwen IJ,
Kortekaas-Thijssen C, Nzigou Mandouckou JA, Kas S, Evidente A and
de Boer AH: Fusicoccin-A selectively induces apoptosis in tumor
cells after interferon-alpha priming. Cancer Lett. 293:198–206.
2010.PubMed/NCBI
|
|
17
|
Ota K, Matsumiya T, Sakuraba H, Imaizumi
T, Yoshida H, Kawaguchi S, Fukuda S and Satoh K: Interferon-alpha2b
induces p21cip1/waf1 degradation and cell proliferation in HeLa
cells. Cell Cycle. 9:131–139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang T, Sun HC, Zhou HY, Luo JT, Zhang
BL, Wang P, Wang L, Qin LX, Ren N, Ye SL, et al: Interferon alpha
inhibits hepatocellular carcinoma growth through inducing apoptosis
and interfering with adhesion of tumor endothelial cells. Cancer
Lett. 290:204–210. 2010. View Article : Google Scholar
|