Introduction
According to the 2005 World Health Organization
classification, keratocystic odontogenic tumour (KCOT), which was
previously termed odontogenic keratocyst, is categorised as a
benign odontogenic tumour (1).
Several factors justified this decision (2), including the following: i) Clinically,
KCOT behaves as a locally destructive and highly recurrent lesion;
ii) histopathologically, it is typical to observe the basal
epithelial layer budding into the connective tissue and the absence
of mitotic figures in the suprabasal layer; and iii) genetically,
mutations of the tumour suppressor gene, Patched 1, in systemic or
sporadic KCOT were successively reported by Lench et al
(3) and Barreto et al
(4), indicating that dysregulation
of the Hedgehog signalling pathway is involved in the molecular
pathogenesis.
At present, marsupialisation or decompression
combined with two-stage curettage or enucleation is a common
treatment for large KCOT. This treatment has the advantage of
minimal invasiveness and the preservation of appearance and
function (1). This procedure
decreases the size of the lesion (5) and in specific circumstances, leads to
a lower recurrence rate (1),
although a final conclusion has not been reached with regard to
this potential benefit (2). To
improve the outcome of decompression, it is necessary to elucidate
the underlying molecular mechanisms of this therapy.
Podoplanin (PDPN), a 36–43-kDa mucin-like
transmembrane glycoprotein, is widely used as a marker for
lymphatic endothelial cells (6).
However, recent studies have shown that the protein is also
expressed in a variety of normal tissues, as well as neoplastic
tissues in pathological and physiological settings (6,7). This
molecule has diverse functions, including regulation of organ
development, cell motility, tumourigenesis and metastasis (6,8,9).
Previous studies (7,10) have reported that PDPN is markedly
expressed in KCOTs, as in the cases of ameloblastoma and
adenomatoid odontogenic and calcifying cystic odontogenic tumours,
but negative in orthokeratinised odontogenic cysts (OOCs) and
dentigerous cysts, suggestive of its neoplastic nature. It is
likely that the upregulated expression of PDPN has a role in the
processes of tumour proliferation and invasion.
Following decompression, the intracystic environment
of KCOTs change. Furthermore, several previous studies found that
the typical histological features were lost (11,12)
and that a variety of biomarkers associated with proliferation
(12), invasion, cell
differentiation (13) or apoptosis
(14) were downregulated following
the therapy. To the best of our knowledge, the manner in which the
expression of PDPN changes following decompression has not been
previously reported. Through the immunochemical detection of 16
cases, the present study is the first to report that the PDPN
expression in KCOTs is notably downregulated following this
therapy.
Materials and methods
Patients
The presents study analysed the cases of 16 patients
with histologically confirmed KCOTs from the Stomatology Hospital
of Jiangsu Province (Nanjing, China) and the Shanghai Ninth
People’s Hospital (Shanghai, China). Recurrent cases or those
associated with nevoid basal cell carcinoma syndrome were excluded.
All patients underwent decompression surgery followed by two-stage
cyst enucleation. The clinical information of the patients is shown
in Table I. Post-operative
follow-up was comprised of clinical and radiographic examinations
between January 2004 and September 2012. The average duration of
draining and irrigation prior to the two-stage surgery was 19.5
months (range, 6.5–44.0 months).
 | Table IPatient clinical information and
intensity of PDPN expression. |
Table I
Patient clinical information and
intensity of PDPN expression.
| Case | Age, years | Gender | Duration of
decompression, min | Location | Outcome (degree of
PDPN expression)a |
|---|
|
|---|
| Group I | Group II |
|---|
| 1 | 22 | M | 27 | Mandible;
Mol-Ram | 2 | 1 |
| 2 | 15 | M | 10 | Mandible;
Ang-Ram | 2 | 1 |
| 3 | 20 | M | 21 | Mandible;
Ang-Ram | 2 | 1 |
| 4 | 42 | M | 17 | Mandible;
Mol-Ram | 2 | 1 |
| 5 | 20 | F | 16 | Mandible;
Ang-Ram | 0 | 0 |
| 6 | 13 | F | 12 | Mandible;
Mol-Ram | 2 | 1 |
| 7 | 38 | F | 3 | Mandible;
Ang-Ram | 2 | 1 |
| 8 | 34 | F | 16 | Mandible;
Mol-Ram | 2 | 1 |
| 9 | 49 | F | 18 | Mandible;
Ang-Ram | 2 | 1 |
| 10 | 55 | M | 15 | Maxilla; Ant | 2 | 1 |
| 11 | 25 | F | 15 | Mandible;
Mol-Ram | 2 | 2 |
| 12 | 33 | F | 9 | Mandible;
Ang-Ram | 2 | 1 |
| 13 | 35 | M | 23 | Mandible;
Ang-Ram | 2 | 0 |
| 14 | 24 | F | 31 | Mandible;
Ang-Ram | 2 | 1 |
| 15 | 29 | M | 23 | Maxilla; Ant | 2 | 2 |
| 16 | 28 | F | 27 | Maxilla; Ant | 2 | 1 |
Paraffin specimens of the tissue samples that were
obtained at the time of decompression and enucleation were gathered
from the Division of Oral Pathology of the Stomatology Hospital of
Jiangsu Province and the Shanghai Ninth People’s Hospital. All
patients provided signed informed consent prior to enrollment and
the study was approved by the Ethics Committees of the Shanghai
Ninth People’s Hospital and the Jiangsu Stomatological
Hospital.
Immunohistochemical evaluation
Each of the 16 pairs of formalin-fixed,
paraffin-embedded samples were sectioned continuously into two 4-μm
slices and mounted onto glass microscope slides. One slice of each
pair was prepared for immunohistochemical (PDPN) analysis and the
other was used as a negative control by substituting
phosphate-buffered saline for the anti-PDPN as a primary antibody.
Positive staining for PDPN at the lymphatic vessels in connective
tissues was used as the positive control.
Briefly, the deparaffinised sections were immersed
in absolute methanol containing 0.3% H2O2 for
15 min at room temperature to block endogenous peroxidase activity.
Following washing, the sections were immersed in 0.01 M citrate
buffer (pH 6.0) and heated in a microwave oven at 95°C for 5 min. A
properly diluted (1:50) rabbit monoclonal anti-human D2–40 (PDPN)
antibody (Proteintech Group, Inc., Chicago, IL, USA) was then
applied to the sections at 4°C overnight, followed by a prediluted
anti-mouse IgG antibody conjugated with peroxidase (Nichirei
Corporation, Tokyo, Japan) for 1 h at room temperature. The
sections were immersed for 8 min in 0.05% 3,3′-diaminobenzidine
tetrahydrochloride in 0.05 M Tris-HCl buffer (pH 8.5) containing
0.01% H2O2 and then counterstained with
haematoxylin.
Criterion for immunohistochemical
evaluation
The immunohistochemical reactivity for PDPN was
evaluated using a semi-quantitative detection method, which is
referred to in the previous literature (10), and classified into three groups
according to the following intensity scores: 0, negative (−); 1,
weakly to moderately positive (+); and 2, strongly positive (++).
The evaluation was performed by two experienced pathologists who
were each blinded to the clinical information provided.
Statistical analysis
Student’s paired t-test was used to evaluate the
altered immunoreactivity of PDPN in KCOTs at the time of
enucleation compared with that at the time of decompression. SPSS
17.0 software (SPSS, Inc., Chicago, IL, USA) was used for
statistical analysis. Specific data are shown in Table I and P<0.05 was considered to
indicate a statistically significant difference.
Results
Immunohistochemistry analysis
In the present study, patients with samples obtained
at the time of decompression were assigned to group I and those
with samples obtained at the time of two-stage enucleation were
assigned to group II.
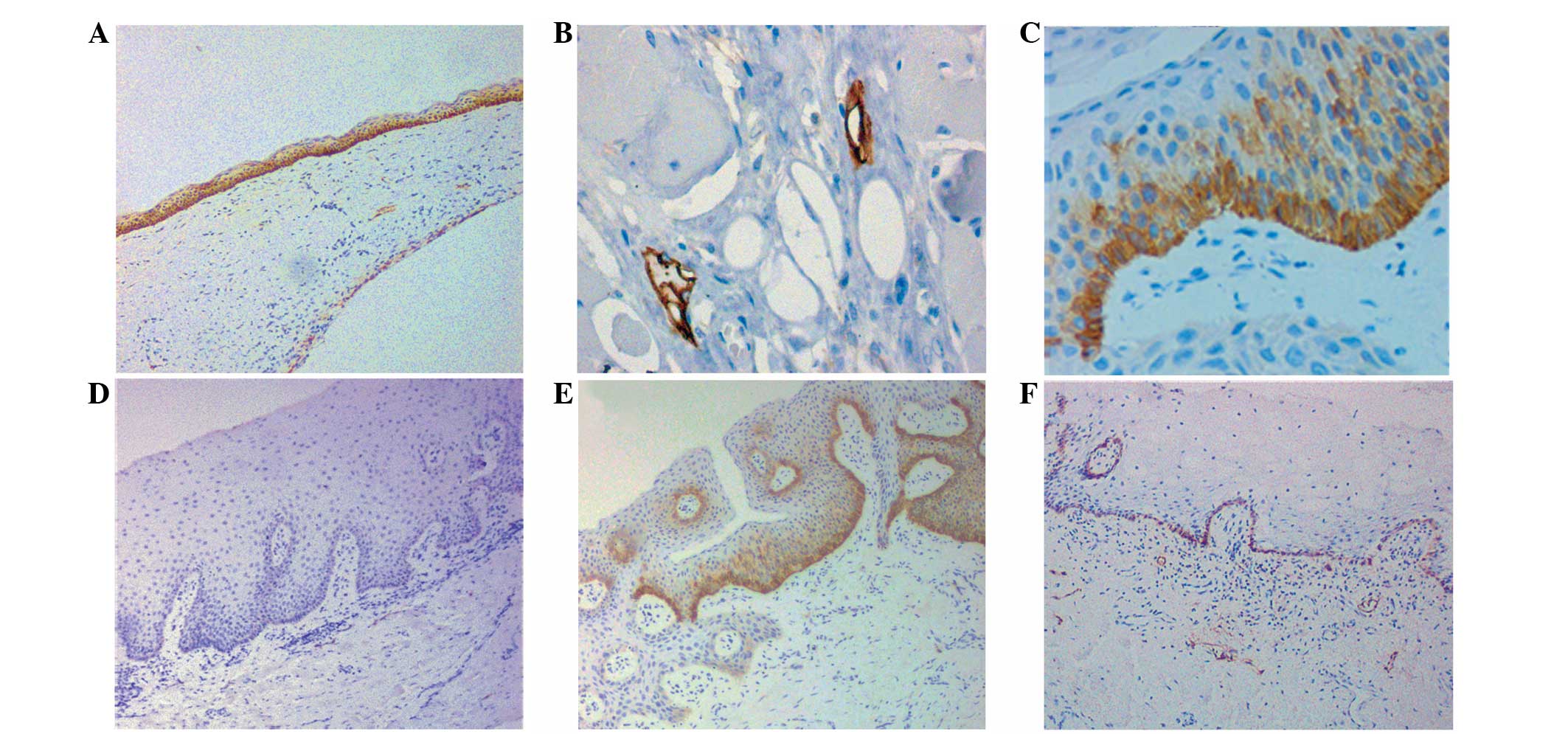
The immunohistochemical staining pattern of PDPN in
KCOTs was membranous and cytoplasmic (Fig. 1A). In group I, 15 of the 16 cases
(93.8%) showed strong PDPN expression in the epithelium, as with
the expression of the lymphatic endothelial cells in the positive
control (Fig. 1B), while 1 of the
16 (6.2%) cases was negative. All PDPN-positive cells were
restricted to the basal layer of the epithelial lining, with a
small amount of extension (2–3 layers of cells) toward the
parabasal or lower middle layers; the upper layers were negative
for this protein (Fig. 1C).
In group II, 3 of the 16 cases showed strong or
negative PDPN expression, each consistent with its corresponding
counterpart in group I. In the remaining cases, PDPN expression was
significantly decreased; one case was entirely negative in all
visual fields (Fig. 1D), 7 cases
showed localized positive staining for PDPN, indicating that the
segmental PDPN-positive areas were only found in sporadic regions
and coexisted with the PDPN-negative areas (Fig. 1E) and in 4 cases, the PDPN-positive
cells were restricted to a single layer (the basal layer), while
the adjacent prickle-cell layer was negative (Fig. 1F).
Intensity scoring
The mean intensity score in group I was 1.87 (0.27),
while the mean intensity score in group II was 1.00 (0.29). The
P-value of group I versus group II in PDPN staining intensity was
0.0001. The statistical analysis showed a significant decrease in
the expression of PDPN in group II compared with that in group
I.
Discussion
Marsupialisation or decompression is frequently used
as a conservative therapy for large KCOTs. It has been previously
reported that tumour size is evidently decreased following
decompression (5). For example,
Nakamura et al (12)
reported that 96% of cases showed a cystic reduction of ≥50%.
Histologically, the typical features of KCOTs were lost following
decompression and took the form of hyperplastic epithelium,
thickened fibrous lamina and increased inflammatory infiltration
(11,12). Similarly, in the present study, the
histological features of 13 samples in group II changed in the same
way. In the field of molecular biology, a series of biomarkers
highly expressed in KCOTs, including IL-1α-induced prostaglandins,
collagenase (15), Ki-67 (12,15),
bcl-2 (14) and keratinocyte growth
factor (16), have been reported to
notably decrease, indicating the attenuation of cell proliferation,
antiapoptosis and local invasion.
PDPN is a glycoprotein involved in the rearrangement
of the cytoskeleton. A number of previous in vitro and in
vivo studies have shown that PDPN is involved in cell motility
and tumour invasion and metastasis (8). In addition, PDPN reportedly promotes
the formation of elongated cellular extension and increased
adhesion and cell migration. As a regulator of cell morphology,
PDPN exhibits actin remodelling properties, including the
prevention of stress fibre and filopod formation (17).
The positive expression of PDPN in KCOTs may reflect
neoplastic activity or cell proliferation and invasion. A study by
Caetano et al (18) showed a
statistically significant correlation between PDPN expression and
the cellular proliferation index (Ki-67) of KCOT and OOC. The study
revealed that KCOTs demonstrated a higher proliferative activity
accompanied by stronger PDPN expression, while in its indolent
counterpart, OOC, the Ki-67 index and PDPN expression intensities
were lower. Similarly, Tsuneki et al (7) found that in KCOTs, PDPN-positive cells
were located within areas of proliferating cell nuclear antigen
(PCNA)-positive cells representative of the cell proliferation
centre. In addition, the study revealed that PDPN-positive cells
were overlapped by cells positive for the extracellular
matrix-related molecules, integrin-β1, fibronectin and matrix
metalloprotein-9 (MMP-9). Therefore, the authors hypothesised that
PDPN is involved in extracellular matrix remodelling and cell
growth in odontogenic tumours (7).
Notably, Caetano et al (18)
concluded that PDPN-positive staining was always found in the
peripheral regions of tumour cell nests, the basal layers of
epithelium and areas of high cellular activity, such as daughter
cysts of KCOTs and secreting ameloblasts of ameloblastic
fibro-odontomas. Above all, these results indicated that PDPN
possibly has a role in the process of tumoural invasion.
To the best of our knowledge, the present study is
the first report to compare the altered expression of PDPN in KCOTs
between one-stage decompression and two-stage enucleation. In the
current study, 93.8% of cases (15/16) showed a markedly positive
expression in the epithelium that was restricted to the basal layer
and extended into the stratum spinosum for two to three cell
layers, consistent with the observations of previous studies. At
the time of enucleation, only three cases showed the same level of
PDPN expression as that prior to decompression, and in all the
remaining samples, PDPN expression was significantly decreased. The
evidence that the immunoreactivity of PDPN correlates with a series
of proteins involved in cell proliferation and tumourous invasion,
including Ki-67 (18), PCNA
(7) and MMP-9 (7), further confirms that the process of
cell proliferation and the local destruction of KCOTs are
attenuated by decompression. However, due to the small sample size
of the present study, this conclusion requires further
investigation.
The concrete mechanisms of PDPN in the pathogenesis
and progression of KCOTs remain unclear. Few previous studies have
analysed the correlation between PDPN expression and the clinical
behaviour of KCOTs, such as the tendency to recur. In the present
study, although a notable change was observed in PDPN expression
following decompression, the potential correlation between this
change and the clinical features of KCOTs, including the duration
of decompression, tumour location and recurrence rate, were not
analysed due to the small sample size and short follow-up period.
These questions remain to be further investigated.
Acknowledgements
The current study was supported as a project funded
by the Priority Academic Program Development of Jiangsu Higher
Education Institutions.
References
|
1
|
Bhargava D, Deshpande A and Pogrel MA:
Keratocystic odontogenic tumour (KCOT) - a cyst to a tumour. Oral
Maxillofac Surg. 16:163–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Madras J and Lapointe H: Keratocystic
odontogenic tumour: reclassification of the odontogenic keratocyst
from cyst to tumour. Tex Dent J. 125:446–454. 2008.
|
|
3
|
Lench NJ, Telford EA, High AS, et al:
Characterisation of human patched germ line mutations in naevoid
basal cell carcinoma syndrome. Hum Genet. 100:497–502. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barreto DC, Gomez RS, Bale AE, et al: PTCH
gene mutations in odontogenic keratocysts. J Dent Res.
79:1418–1422. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shudou H, Sasaki M, Yamashiro T, et al:
Marsupialisation for keratocystic odontogenic tumours in the
mandible: longitudinal image analysis of tumour size using 3D
visualised CT scans. Int J Oral Maxillofac Surg. 41:290–296. 2012.
View Article : Google Scholar
|
|
6
|
Astarita JL, Acton SE and Turley SJ:
Podoplanin: emerging functions in development, the immune system,
and cancer. Front Immunol. 3:2832012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsuneki M, Maruyama S, Yamazaki, et al:
Podoplanin expression profiles characteristic of odontogenic
tumour-specific tissue architectures. Pathol Res Pract.
208:140–146. 2012. View Article : Google Scholar
|
|
8
|
Wicki A, Lehembre F, Wick N, et al: Tumour
invasion in the absence of epithelial-mesenchymal transition:
podoplanin-mediated remodeling of the actin cytoskeleton. Cancer
Cell. 9:261–272. 2006. View Article : Google Scholar
|
|
9
|
Martín-Villar E, Megías D, Castel S, et
al: Podoplanin binds ERM proteins to activate RhoA and promote
epithelial-mesenchymal transition. J Cell Sci. 119:4541–4553.
2006.PubMed/NCBI
|
|
10
|
Okamoto E, Kikuchi K, Miyazaki Y, et al:
Significance of podoplanin expression in keratocystic odontogenic
tumour. J Oral Pathol Med. 39:110–114. 2010. View Article : Google Scholar
|
|
11
|
Ninomiya T, Kubota Y, Koji T and Shirasuna
K: Marsupialization inhibits interleukin-1alpha expression and
epithelial cel proliferation in odontogenic keratocysts. J Oral
Pathol Med. 31:526–533. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakamura N, Mitsuyasu T, Mitsuyasu Y, et
al: Marsupialization for odontogenic keratocysts: long-term
follow-up analysis of the efects and changes in growth
characteristics. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
94:543–553. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
August M, Faquin WC, Troulis MJ, et al:
Dedifferentiation of odontogenic keratocyst epithelium after cyst
decompression. J Oral Maxillofac Surg. 61:678–683. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pogrel MA and Jordan RC: Marsupialization
as a definitive treatment for the odontogenic keratocyst. J Oral
Maxillofac Surg. 62:651–655. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oka S, Kubota Y, Yamashiro T, et al:
Effects of positive pressure in odontogenic keratocysts. J Dent
Res. 84:913–918. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suyama Y, Kubota Y, Yamashiro T, et al:
Expression of keratinocyte growth factor and its receptor in
odontogenic keratocysts. J Oral Pathol Med. 38:476–480. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koop K, Eikmans M, Wehland M, et al:
Selective loss of podoplanin protein expression accompanies
proteinuria and precedes alterations in podocyte morphology in a
spontaneous proteinuric rat model. Am J Pathol. 173:315–326. 2008.
View Article : Google Scholar
|
|
18
|
Caetano AS, Tjioe KC and Faustino SE:
Immunolocalization of podoplanin in benign odontogenic tumours with
and without ectomesenchyme. Arch Oral Biol. 58:408–415. 2013.
View Article : Google Scholar : PubMed/NCBI
|