Introduction
Human matrix metalloproteinases (MMPs) are
endopeptidases involved in the regulation of cell growth, migration
and remodeling of the extracellular matrix. MMPs degrade matrix and
non-matrix components and are regulated by several physiological
inhibitors and upregulators. MMP deregulation facilitates the
invasion of tumor cells into the surrounding connective tissue and
vessels (1). Previous studies
performed on several human neoplastic tissues have demonstrated a
correlation between increased MMP expression and tumor invasion,
metastatic spread, tumor recurrence and low survival rate (2). These proteolytic enzymes share a
similar structure and are classified based on their substrate
specificity. Accordingly, MMPs have been divided into collagenases,
gelatinases, stromelysins and matrilysins. MMP-9, a member of the
gelatinase group, not only readily digests denatured collagens and
gelatins, but also plays a particular role in angiogenesis since it
increases the bioavailability of proangiogenic factors (3–5).
The tissue inhibitors of metalloproteinases (TIMPs)
constitute a family of four members that regulate MMPs through
endogenous protease inhibition and cell surface activation
regulation (2,6,7). In
addition to this regulatory activity, TIMPs have multiple effects
on cell growth, apoptosis and differentiation (6) through an MMP-independent mechanism
(8). TIMP-2 induces apoptosis and
inhibits various stages of angiogenesis (9,10).
During cancer progression, high levels of TIMP-2 are associated
with the inhibition of tumor growth, angiogenesis, invasion and
metastasis, secondary to the inhibition of endothelial cell
migration (11,12). In a previous cell model, TIMP-2
overexpression was shown to be cytostatic and prevent local
invasion (13). As tumors progress,
TIMP-2 expression levels are decreased or absent in several types
of human cancer, particularly in invasive and metastatic tumors
(12).
Medullary thyroid carcinoma (MTC) arises from
parafollicular or C cells and accounts for 3–4% of all types of
thyroid cancer. MTC may occur sporadically (75%) or through a
hereditary mechanism caused by gain-of-function germline mutations
in the RET proto-oncogene. RET molecular analysis is now considered
essential in MTC management, since early diagnosis improves
prognosis and allows adequate genetic counseling (14–16).
The 10-year disease-specific survival rate of patients with MTC is
~75% (17). Currently, the only
curative approach for MTC is surgical resection of the tumor, as it
shows limited response to radiotherapy and/or chemotherapy. MTC
tends to metastasize early via angioinvasion and hematogenous
spread (16–19).
MMP and TIMP members have been shown to be
upregulated in differentiated thyroid carcinoma (7,20), but
little is known concerning their role in the pathogenesis or
clinical presentation of MTC. The search for alternative treatments
for metastatic disease has been intensified in the last decade
based on new knowledge of the molecular biology of these tumors.
Thus, characterizing these molecules may be useful in the
development of new therapeutic strategies. The present study
evaluated the expression of MMP-9 and TIMP-2 in MTC, and examined
the correlation between the clinical features and the expression
levels of these angiogenic factors.
Materials and methods
Thyroid tissue
The samples comprised of 77 specimens with
histopathological/immunohistochemical diagnosis of MTC, which were
obtained from patients attending the Endocrine or Head and Neck
Divisions at the Hospital de Clínicas de Porto Alegre
(university-based hospital; Porto Algre, Brazil) between 1997 and
2011. RET germline mutations were identified by standard procedure,
as previously described (21).
Sporadic MTC was diagnosed based on the absence of family history
and known germline RET point mutations in exons 8, 10, 11 or 13–16.
The clinical data in medical records were retrospectively reviewed.
The Ethics Committee at the Hospital approved the study protocol
(no. 10-0068).
For patients with clinical or biochemical evidence
of MTC, the surgical procedure consisted of total thyroidectomy
with varying cervical neck dissection procedures. For asymptomatic
gene carriers with no abnormalities on cervical ultrasonography
examination and normal serum calcitonin levels, prophylactic
thyroidectomy was recommended. Tumor staging was performed
according to the International Union Against Cancer
tumor-node-metastasis (TNM) classification (22). Patients with suspicious distant
metastasis (i.e., the presence of local metastases and/or serum
calcitonin levels >150 pg/ml) underwent imaging examination
(cervical, thoracic and abdomen CT or liver magnetic resonance
imaging, as well as bone scintigraphy). Individuals with
undetectable calcitonin and carcinoembryonic antigen (CEA) levels
and normal physical examinations were considered to be in complete
biochemical remission and were monitored annually without
additional imaging, unless a change in exam results, symptoms or
laboratory values was noted (16).
Somatic M918T mutation analysis
For sporadic MTC patients, the frequency of somatic
M918T mutation was analyzed. The MTC samples were material
paraffin-embedded formalin-fixed tissue blocks. DNA was extracted
using the Magnesil Genomic Fixed Tissue system (Promega
Corporation, Madison, WI, USA) according to the manufacturer’s
instructions. Exon 16 was amplified by polymerase chain reaction
using 100–300 ng/ml DNA in a reaction mix (25 ml) containing 20 mM
Tris-HCl (pH 8.0), 50 mM KCl, 2 mM MgCl2, 0.2 mM dNTPs,
0.2 mM each primer and 1.25 U Platinum Taq DNA Polymerase
(Invitrogen Life Technologies, Carlsbad, CA, USA). The running
profile of the amplifications and restriction fragment length
polymorphism analysis were similar to those described previously
for genomic DNA (21).
Immunohistochemistry (IHC) analysis
IHC was performed on thin sections (3 μm) of
previously formalin-fixed and paraffin-embedded tissues. The
antibodies used were polyclonal rabbit antihuman vascular
endothelial growth factor (VEGF)-A (clone VG1; M7273; Dako,
Carpinteria, CA, USA) and monoclonal mouse anti-human VEGF receptor
(VEGFR)-2 (A-3; SC-6251), TIMP-2 (YY6; sc80366) and MMP-9 (2C3;
sc-21733) antibodies (Santa Cruz Biotechnology Inc., Santa Cruz,
CA, USA). MTC samples were submitted to a routine
immunohistochemical technique, which included deparaffinization and
rehydration, antigenic recovery, inactivation of endogenous
peroxidase and blockage of non-specific reactions. Primary
antibodies were incubated overnight at 4°C at dilutions of 1:400
(VEGF-A), 1:200 (VEGFR-2) and 1:100 (TIMP-2 and MMP-9), followed by
the application of streptavidin-horseradish peroxidase conjugate
(LSAB; Dako) and diaminobenzidine tetrahydrochloride (DAB kit;
Dako). The positive controls were human tissues, including skeletal
muscle for VEGF-A, intestinal tumor for VEGFR-2, lung for TIMP-2
and heart for MMP-9. The negative control was obtained by omission
of the primary antibody.
The intensity of VEGF-A, VEGFR-2, MMP-9 and TIMP-2
staining in each lesion was determined and quantified according to
the following grades: 0, absent (−); 1, weak (+); 2, moderate (++);
and 3, strong (+++). Grading was based on the predominant staining
characteristics of the tumor. The slides were examined using an
Olympus BX51 microscope with an Olympus QColor 5 camera (Olympus
America Inc., Melville, NY, USA). The slides were independently
read by two blinded and experienced pathologists, who were unaware
of the respective clinicopathological data. When the two experts
differed in their interpretations, they consulted with each other
to reach a consensus.
Statistical analysis
The data are presented as the median and
interquartile range (IQR). Baseline characteristics were compared
using the χ2 test for qualitative variables and the
Mann-Whitney U test for quantitative variables. Spearman’s
coefficient or the Mann-Whitney U tests were used to assess the
correlation between angiogenic marker expression (VEGF-A, VEGFR-2,
TIMP-2 or MMP-9) and age at surgery, tumor size, TNM stage and
disease outcome. P<0.05 was considered to indicate a
statistically significant difference. The Statistical Package for
the Social Sciences 18.0 professional software (SPSS, Inc.,
Chicago, IL, USA) was used for statistical analysis.
Results
Patients
The clinical and oncological features of the 77
patients included in this study are shown in Table I. The median age at diagnosis was
35.6 years (IQR, 2.5–83.3 years) and 45 (58.4%) of the patients
were female. In total, 36 (46.8%) patients had hereditary MTC,
whereas 41 (53.2%) patients had the sporadic form of the disease.
Of these patients, 34 had MEN 2A and two were found to have MEN 2B
syndrome. The RET mutations identified in MEN 2A patients were as
follows: C634Y (25 individuals; 73.5%), C634R (four individuals;
11.7%), C618R (three individuals; 8.8%) and E768D (two individuals;
5.8%). The two patients with MEN 2B presented with the
characteristic phenotype and a mutation in codon 918.
 | Table IClinical characteristics of 77
patients with MTC. |
Table I
Clinical characteristics of 77
patients with MTC.
| Patient
characteristics | Values |
|---|
| Age, years | 35.6
(2.5–83.3)a |
| Females, n (%) | 45 (58.4) |
| Tumor stage, n
(%) |
| I | 17 (22.1) |
| II | 24 (31.2) |
| III | 22 (28.6) |
| IV | 14 (18.2) |
| Hereditary/sporadic,
n (%) | 36 (46.8)/41
(53.2) |
| Calcitonin,
pg/ml | 262.0
(28.0–953.6)a |
| CEA, ng/ml | 14.6
(2.4–52.6)a |
| Persistent disease, n
(%) | 38 (49.4) |
At the time of surgery, 34 (46.6%) patients
presented with lymph node disease and 14 (18.9%) exhibited distant
metastases. The median calcitonin level was 262.0 pg/ml (IQR,
28.0–953.6 pg/ml) and the median CEA level was 14.6 ng/ml (IQR,
2.4–52.6 ng/ml). In total, 17 patients were diagnosed with tumor
stage I (22.1%), 24 with stage II (31.2%), 22 with stage III
(28.6%) and 14 with stage IV (18.2%). In addition, 39 (61.9%)
patients were considered free of disease following a follow-up
period of 6.96±4 years.
MMP-9 and TIMP-2 expression in MTC
Immunohistochemical staining for MMP-9 and TIMP-2
were detected in 69 (89.6%) and 72 (93.5%) out of the 77 samples
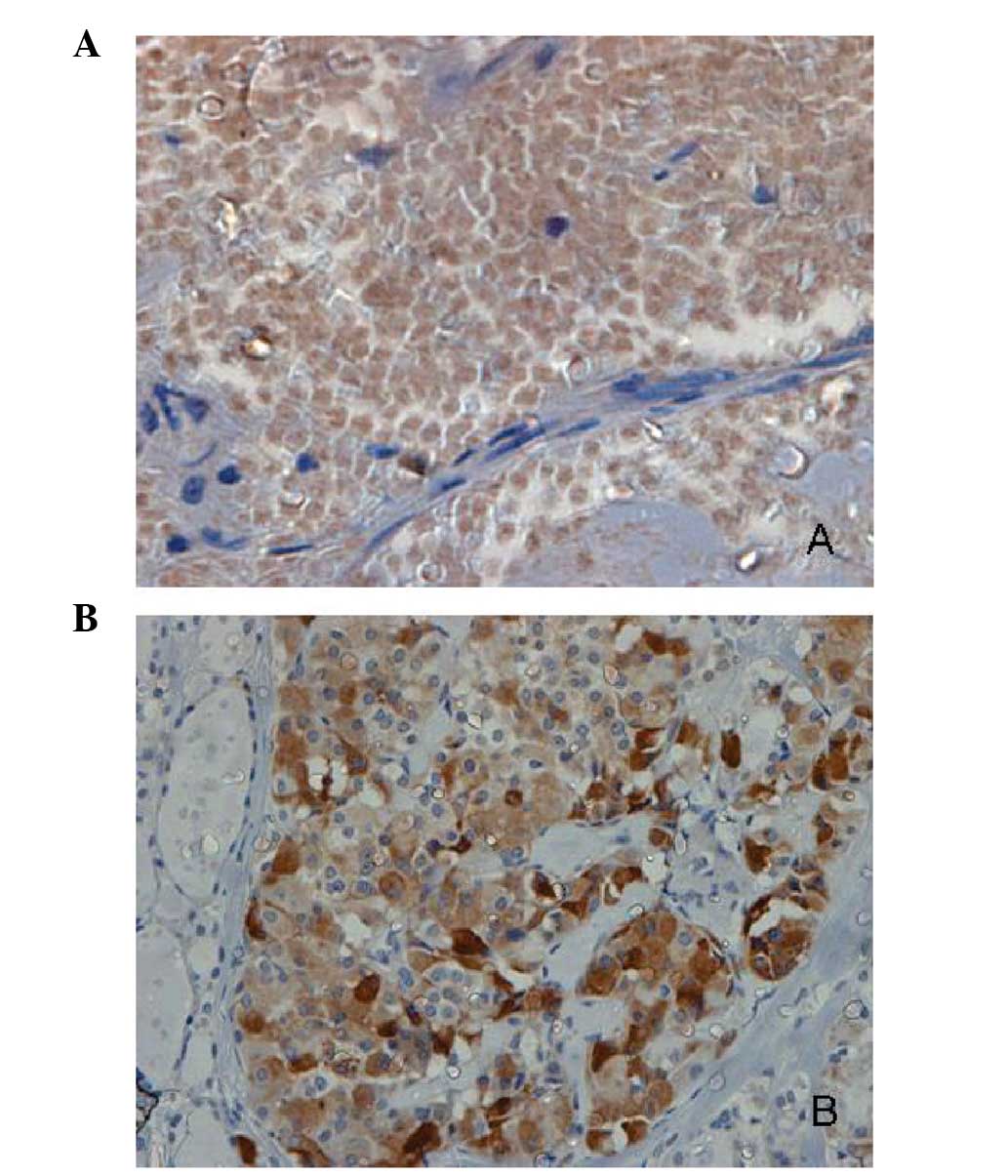
analyzed, respectively (Fig. 1A and
B). As predicted, positive MMP-9 and TIMP-2 immunoreactivity
was detectable in the cytoplasm of thyroid cancer cells, but rarely
in stromal cells or surrounding healthy thyroid tissue.
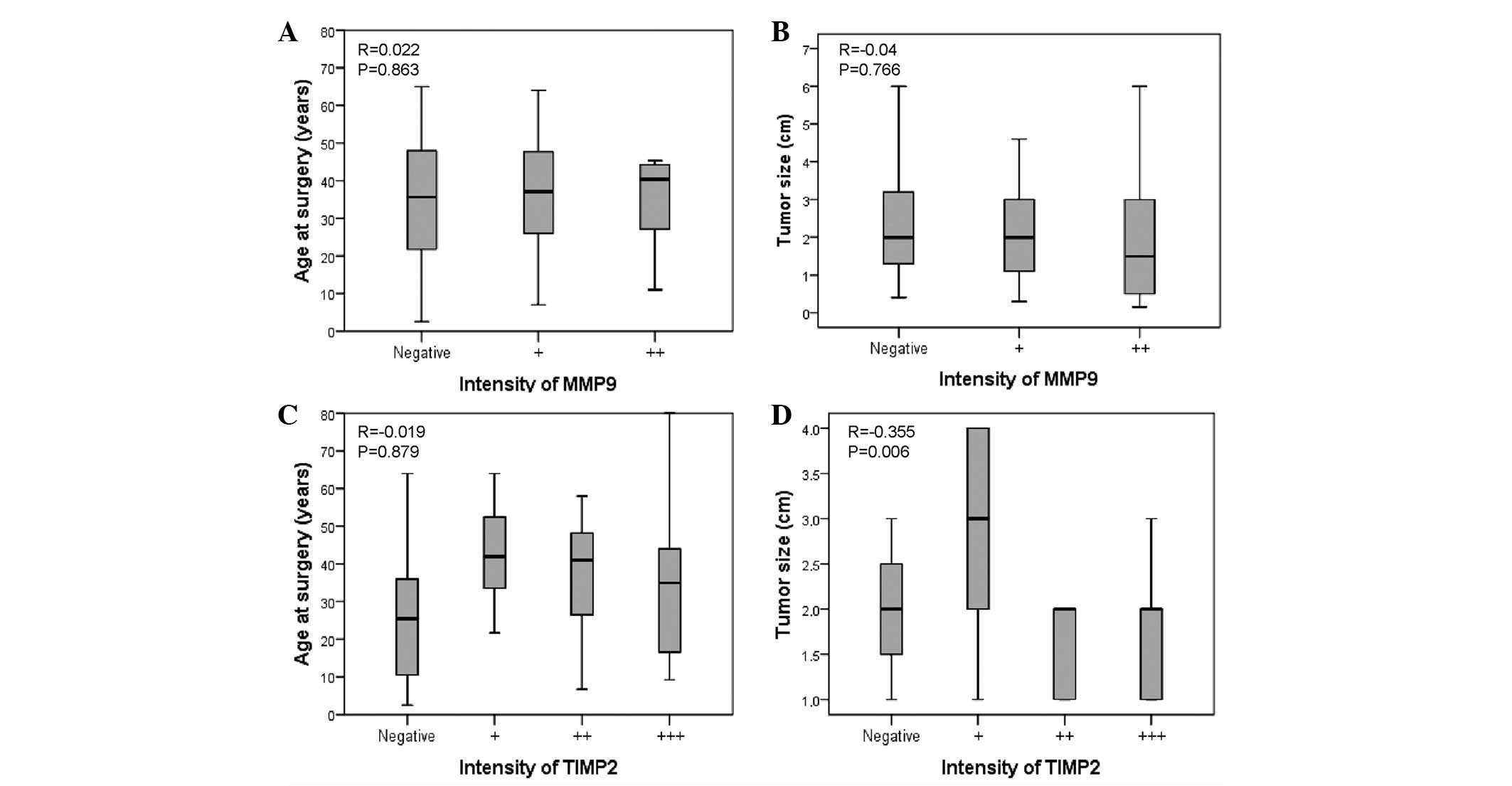
The expression of MMP-9 was not found to correlate
with age or tumor size (P=0.8 and P=0.76, respectively; Fig. 2) or TNM stage (P=0.37; Table II). However, a trend toward an
association, although not statistical significant, was observed
between MMP-9 and baseline levels of calcitonin (r=0.296; P=0.06)
and distant metastasis (P=0.053) (Table III).
 | Table IICorrelation between MMP-9 and TIMP-2
staining and TNM in patients with MTC. |
Table II
Correlation between MMP-9 and TIMP-2
staining and TNM in patients with MTC.
| TNM stage (n) | | |
|---|
|
| | |
|---|
| I | II | III | IV | rs | P-value |
|---|
| MMP-9 | | | | | −0.116 | 0.37 |
| − | 3 | 10 | 4 | 7 | | |
| + | 7 | 11 | 7 | 4 | | |
| ++ | 2 | 1 | 3 | 1 | | |
| Total | 12 | 22 | 14 | 12 | | |
| TIMP-2 | | | | | −0.395 | 0.001 |
| − | 2 | 1 | 3 | 3 | | |
| + | 0 | 3 | 3 | 5 | | |
| ++ | 2 | 4 | 3 | 2 | | |
| +++ | 8 | 16 | 7 | 1 | | |
| Total | 12 | 24 | 15 | 12 | | |
 | Table IIICorrelation between MMP-9 and TIMP-2
staining with local or distant metastasis. |
Table III
Correlation between MMP-9 and TIMP-2
staining with local or distant metastasis.
| A, MMP-9 |
|---|
|
|---|
| Metastasis | n | −, n | +, n | ++, n | +++, n | rs | P-value |
|---|
| Lymph node | | | | | | 0.005 | 0.96 |
| N0 | 34 | 13 | 17 | 4 | 0 | | |
| N1 | 31 | 13 | 13 | 5 | 0 | | |
| Distant | | | | | | −0.239 | 0.053 |
| M0 | 52 | 17 | 27 | 8 | 0 | | |
| M1 | 14 | 9 | 4 | 1 | 0 | | |
|
| B, TIMP-2 |
|
| Metastasis | n | −, n | +, n | ++, n | +++, n | | P-value |
|
| Lymph node | | | | | | 0.423 | 0.0001 |
| N0 | 14 | 2 | 3 | 6 | 24 | | |
| N1 | 24 | 9 | 9 | 6 | 9 | | |
| Distant | | | | | | −0.416 | 0.001 |
| M0 | 14 | 7 | 7 | 9 | 32 | | |
| M1 | 22 | 5 | 5 | 3 | 1 | | |
TIMP-2 intensity was not found to correlate with age
(P=0.8; Fig. 2C), but was found to
negatively correlate with baseline levels of calcitonin (r=−0.327;
P=0.036), tumor size (r=−0.355; P=0.006; Fig. 2D) and tumoral stage (r=−0.395;
P=0.001; Table II). The highest
TIMP-2 expression was observed in samples from patients without
local (r=−0.423; P=0.0001) or distant (r=−0.416; P=0.001)
metastasis (Table III).
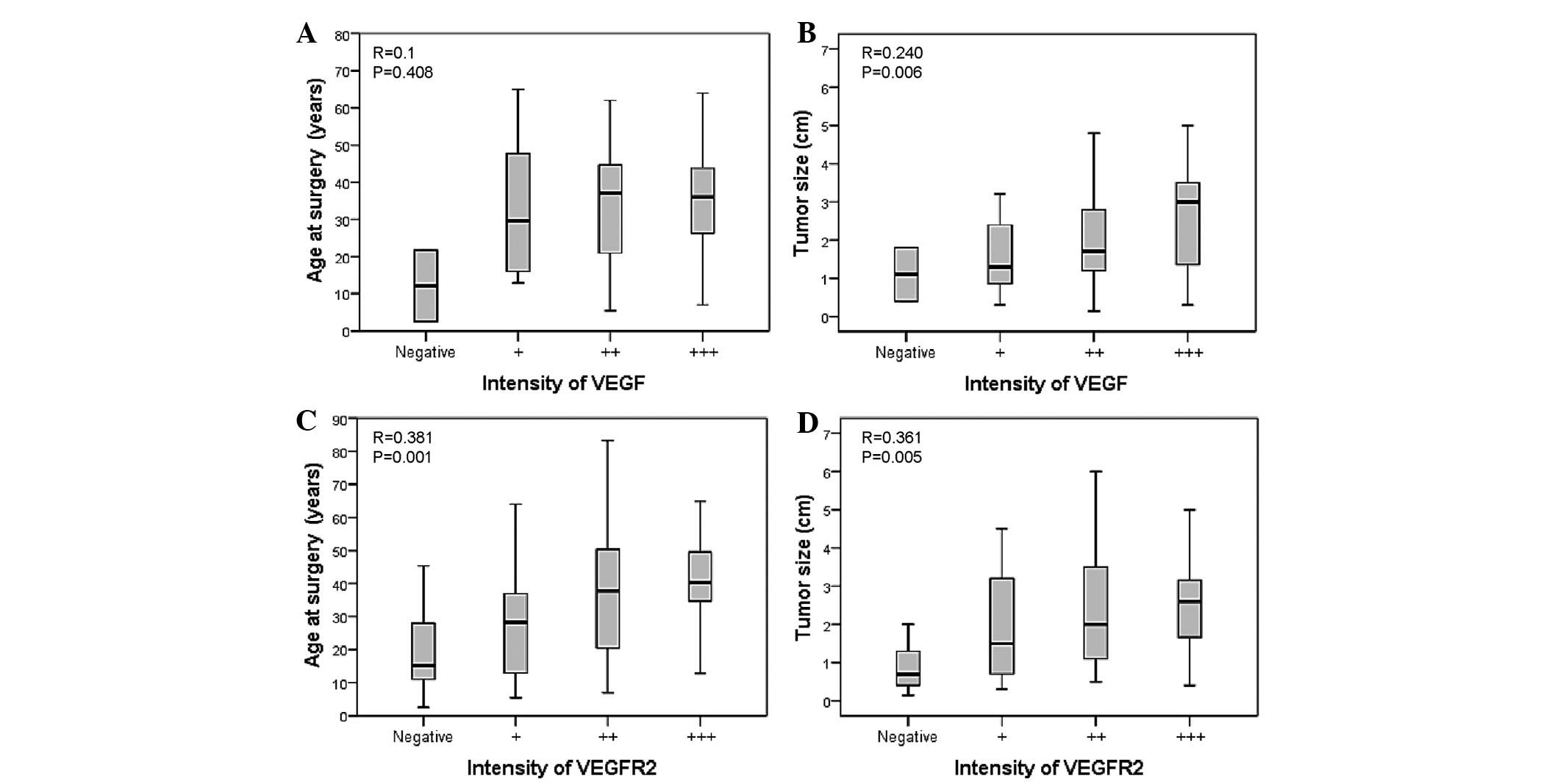
VEGF and VEGFR-2 expression in MTC
The expression of VEGF and its receptor was also
investigated. The analysis of the expression of these angiogenic
molecules with clinical parameters did not demonstrate a
correlation with age (P=0.4; Fig.
3A). Nevertheless, a positive correlation was identified
between VEGF-A expression and tumor size (r= 0.240; P=0.006;
Fig. 3B). In addition, VEGFR-2 was
found to positively correlate with age at surgery (r=0.381;
P=0.001; Fig. 3C) and tumor size
(r=0.361; P=0.005; Fig. 3D). A
positive correlation was also identified between VEGFR-2 and TNM
stage (r=0.397; P=0.002). Notably, an inverse correlation was
identified between TIMP-2 and VEGF-A expression (r=−0.270;
P=0.024).
Sporadic and hereditary MTC tumors
Since there are hereditary and sporadic forms of
MTC, the two groups were analyzed separately. Patients with the
hereditary form of the disease had a mean age of 26.2±16.7 years.
In this group, 39.9% of the patients were diagnosed with stage I of
the disease, 33.3% with stage II, 22.2% with stage III and 2.8%
with stage IV. Compared with the hereditary group, patients with
sporadic MTC were older (41.9±15 years old; P<0.0001) and
presented with more advanced disease at diagnosis (stage I, 2.4%;
stage II, 31.7%; stage III, 24.4%; and stage IV, 31.7%;
P<0.0001). No significant difference was identified between the
groups with regard to MMP-9 staining (P=0.654; Table IV). Nonetheless, higher TIMP-2
expression was observed in patients with hereditary disease
(P=0.001; Table IV).
 | Table IVMMP-9 and TIMP-2 expression in
hereditary and sporadic medullary thyroid carcinoma. |
Table IV
MMP-9 and TIMP-2 expression in
hereditary and sporadic medullary thyroid carcinoma.
| Variable | Hereditary, n | Sporadic, n | P-value |
|---|
| MMP-9 | | | 0.654 |
| n | 32 | 37 | |
| − | 14 | 14 | |
| + | 14 | 18 | |
| ++ | 4 | 5 | |
| TIMP-2 | | | 0.001 |
| n | 34 | 38 | |
| − | 2 | 10 | |
| + | 3 | 9 | |
| ++ | 5 | 8 | |
| +++ | 24 | 11 | |
In the sporadic form of the disease, the analysis of
clinical parameters demonstrated an inverse correlation between
TIMP-2 staining and tumor size (r=−0.429; P=0.02) and tumor stage
(r=−0.475; P=0.006) (Table IV). In
addition, patients with the sporadic form and tumors restricted to
the thyroid were found to present the highest levels of TIMP-2
(P=0.018).
The following step was to identify the somatic RET
M918T mutation in the sporadic group, since it has been shown that
the presence of the missense somatic RET mutation correlates with
aggressive disease. In total, 31 DNA samples were available for
analysis and 25 samples (80.6%) were found to exhibit somatic
M918T. The presence of the somatic mutation was not found to
correlate with the expression of MMP-9 (P=1.00) or TIMP-2
(P=0.88).
Discussion
The current study examined the expression of
pro-invasive factors in MTC. MMP-9 and TIMP-2 expression were
observed in ~90% of the samples. While MMP-9 did not show any
correlation with clinical parameters, TIMP-2 immunoreactivity was
found to inversely correlate with tumor size and stage of the
disease at diagnosis. Notably, the samples with more intense TIMP-2
staining were from patients without local or distant
metastasis.
Cancer cells degrade basement membranes and invade
tissues. TIMPs, secreted proteins that complex with individual
MMPs, regulate the functional activity and activation of individual
MMPs (23). MMP-9 is a functional
component of the angiogenic switch during carcinogenesis as it
increases the bioavailability of pro-angiogenic growth factors,
including VEGF-A (24). Conversely,
TIMP-2 inhibits not only VEGF-induced VEGFR-2 phosphorylation, but
also endothelial cell growth in response to fibroblast growth
factor-2 (FGF-2), likely through the inhibition of FGF-2-induced
ERK1/2 signaling (2,25).
Previously, several studies have shown augmented
MMP-9 expression in differentiated thyroid carcinoma, demonstrating
a correlation between MMP-9 levels and lymph node metastasis
(20,26). Nevertheless, other studies have
failed to demonstrate increases in MMP-9 expression in papillary
thyroid carcinoma (PTC) (27).
Studies analyzing TIMP-2 have also shown controversial results.
While the expression of this molecule has been found to positively
correlate with tumor size, tumoral stage and vascular invasion in
PTC (20), contradictory results
have been observed in other carcinomas, in which TIMP-2 has been
shown to be more frequently associated with localized tumors than
regional or distant metastases (28,29).
Few studies have analyzed MMP-9 and TIMP-2
expression in MTC. A previous small study that evaluated 10 cases
of MTC showed weak MMP and TIMP immunostaining (30). An additional study that evaluated 37
MTC samples found that TIMP-2 expression did not correlate with any
clinical parameter at diagnosis (31). This is consistent with the results
of the current study, which did not identify a correlation between
MMP-9 expression and clinical parameters. However, the trend toward
a correlation between MMP-9 with baseline calcitonin levels and
distant metastasis suggests that the lack of correlation may be due
to the large number of patients in the early stages of disease (I
and II). Furthermore, the various histological origins between
tumors may also be implicated in a putative and different action of
MMP-9 in MTC.
As aforementioned, TIMPs have been implicated in
promoting and inhibiting cell growth, suggesting that the specific
effects of TIMPs on cell fate depend on the cell context and
specific model system under study. Although TIMP-2 has been
associated with large tumor size and high invasiveness in PTC
(20), positive TIMP-2 staining is
significantly higher in localized colorectal tumors and negative in
the invasive forms (29). This also
appears to be the case in MTC, in which an inverse correlation was
observed in the current study between TIMP-2 staining and baseline
calcitonin, tumor size, tumor stage and distant metastases,
suggesting that increased levels of TIMP-2 may be a marker of low
metastatic potential in medullary cancer. These results are
consistent with previous observations that demonstrated weaker
TIMP-2 staining in neoplastic cells of invasive MTC (30). The observed inverse correlation
between MMP-9 and TIMP-2 expression may indicate that these
molecules are coregulated by a currently unknown mechanism.
Previous studies have shown that MTC exhibits
moderate to strong staining of VEGF-A (32–34).
In addition, the VEGF-A-mediated stimulation of VEGFR-2
autophosphorylation is crucial in mediating the effects of VEGF-A,
including vasodilatation, endothelial cell migration and
proliferation, and it has been considered as the key mediator of
VEGF-induced angiogenesis (35).
The current study extended previous studies on the role of VEGF-A
and its receptors in MTC. A positive correlation was observed
between VEGFR-2 staining, tumor size and TNM stages and an inverse
correlation was identified between TIMP-2 expression and VEGFR-2
levels. This observation is particularly significant, since it
appears to be a link between the TIMP pathways and the VEGF cascade
in MTC. Previously, it has been shown, in other tumors, that
endothelial cells may respond to angiogenic factors, such as VEGF,
by decreasing the synthesis of TIMP-2 to facilitate tumor
angiogenesis and metastasis (8,36).
The present study also compared the expression of
angiogenic markers in sporadic and hereditary MTC groups. The
marked TIMP-2 staining observed in the hereditary group may be due
to an earlier diagnosis of the disease in these patients due to
molecular screening in contrast to a more advanced tumor at the
time of diagnosis in the sporadic group of patients. The inverse
correlation between tumor size and stage also suggested that the
presence of TIMP in the hereditary form of the disease is a marker
of a less aggressive tumor.
In conclusion, the results of the current study
suggest that MMPs are implicated in the development and maintenance
of sporadic and hereditary MTC. Notably, TIMP-2 has been found to
correlate with a less invasive MTC presentation and may be a marker
of less aggressive disease. Consequently, these observations
reinforce the potential advantage of compounds that inhibit the
tumor angiogenic activity in MTC.
Acknowledgements
The authors would like to thank the physicians who
referred patients for molecular analysis and the surgeons at the
Hospital de Clínicas de Porto Alegre (Porto Alegre, Brazil), Dr
Alceu Migliavacca and Dr José Ricardo Guimarães, for surgical
management of the patients. The current study was supported by
grants from the Conselho Nacional de Desenvolvimento Científico e
Tecnológico (CNPq), Fundação de Amparo Pesquisa do Estado do Rio
Grande do Sul (FAPERGS), Fundo de Incentivo à Pesquisa do Hospital
de Clínicas de Porto Alegre (FIPE), Programa de Apoio a Núcleos de
Excelência (PRONEX) and Coordenação de Aperfeiçoamento de Pessoal
de Nível Superior (CAPES), Brazil.
References
|
1
|
Roy R, Yang J and Moses MA: Matrix
metalloproteinases as novel biomarkers and potential therapeutic
targets in human cancer. J Clin Oncol. 931:5287–5297.
2000.PubMed/NCBI
|
|
2
|
Seo DW, Li H, Guedez L, Wingfield PT, Diaz
T, Salloum R, Wei BY and Stetler-Stevenson WG: TIMP-2 mediated
inhibition of angiogenesis: an MMP-independent mechanism. Cell.
114:171–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coussens LM, Fingleton B and Matrisian LM:
Matrix metalloproteinase inhibitors and cancer trials and
tribulations. Science. 295:2387–2392. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Massi D, Franchi A, Ketabchi S, Paglierani
M, Pimpinelli N and Santucci M: Expression and prognostic
significance of matrix metalloproteinases and their tissue
inhibitors in primary neuroendocrine carcinoma of the skin. Hum
Pathol. 34:80–88. 2003. View Article : Google Scholar
|
|
5
|
Bergers G, Brekken R, McMahon G, et al:
Matrix metalloproteinase-9 triggers the angiogenic switch during
carcinogenesis. Nat Cell Biol. 2:737–744. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baker AH, Edwards DR and Murphy G:
Metalloproteinase inhibitors: biological actions and therapeutic
opportunities. J Cell Sci. 115:3719–3727. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rydlova M, Holubec L Jr and Ludvikova M,
Kalfert D, Franekova J, Povysil C and Ludvikova M: Biological
activity and clinical implications of the matrix
metalloproteinases. Anticancer Res. 28:1389–1398. 2008.PubMed/NCBI
|
|
8
|
Stetler-Stevenson WG and Seo DW: TIMP-2:
an endogenous inhibitor of angiogenesis. Trends Mol Med. 11:97–103.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang H, Wen Y, Mooney S, Li H, Behr B and
Polan ML: Matrix metalloproteinase and tissue inhibitor of matrix
metalloproteinase expression in human preimplantation embryos.
Fertil Steril. 80(Suppl 2): S736–S742. 2003. View Article : Google Scholar
|
|
10
|
Dimo B, Ioannidis I, Karameris A, Vilaras
G, Tzoumakari P, Nonni A, Patsouris E and Lazaris AC: Comparative
study of the Immunohistochemical expression of tissue inhibitors of
metalloproteinases 1 and 2 between clearly invasive carcinomas and
‘in situ’ trophoblast invasion. Med Oncol. 29:2270–2275.
2012.PubMed/NCBI
|
|
11
|
Imren S, Kohn DB, Shimada H, Blavier L and
DeClerk YA: Overexpression of tissue inhibitor of
metalloproteinases-2 retroviral-mediated gene transfer in vivo
inhibits tumor growth and invasion. Cancer Res. 56:2891–2895.
1996.PubMed/NCBI
|
|
12
|
Bourboulia D, Jensen-Taubman S, Rittler
MR, Han HY, Chatterjee T, Wei B and Stetler-Stevenson WG:
Endogenous angionesesis inhibitor blocks tumor growth via direct
and indirect effects on tumor microenvironment. Am J Path.
179:2589–2600. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
DeClerck YA, Perez N, Shimada H, Boone TC,
Langley KE and Taylor SM: Inhibition of invasion and metastasis in
cells transfected with an inhibitor of mettaloproteinases. Cancer
Res. 52:701–708. 1992.PubMed/NCBI
|
|
14
|
Ceolin L, Siqueira DR, Romitti M, Ferreira
CV and Maia AL: Molecular basis of medullary thyroid carcinoma: the
role of RET polymorphisms. Int J Mol Sci. 13:221–239. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Puñales MK, da Rocha AP, Meotti C, Gross
JL and Maia AL: Clinical and oncological features of children and
young adults with multiple endocrine neoplasia type 2A. Thyroid.
18:1261–1268. 2008.PubMed/NCBI
|
|
16
|
Kloos RT, Eng C, Evans DB, Francis GL,
Gagel RF, Gharib H, Moley JF, Pacini F, Ringel MD, Schlumberger M
and Wells SA Jr: Medullary thyroid cancer: management guidelines of
the American Thyroid Association. Thyroid. 19:565–612. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mulligan LM, Kwok JB, Healey CS, Elsdon
MJ, Eng C, Gardner E, Love DR, Mole SE, Moore JK, et al: Germ-line
mutations of the RET proto-oncogene in multiple endocrine neoplasia
type 2A. Nature. 363:458–460. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hundahl SA, Fleming ID, Fremgen AM and
Menck HR: A National Cancer Data Base report on 53,856 cases of
thyroid carcinoma treated in the U.S., 1985–1995. Cancer.
83:2638–2648. 1998.PubMed/NCBI
|
|
19
|
Mitchell JC and Parangi S: Angiogenesis in
benign and malignant thyroid disease. Thyroid. 15:494–510. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maeta H, Ohgi S and Terada T: Protein
expression of matrix metalloproteinases 2 and 9 and tissue
inhibitors of metalloproteinase 1 and 2 in papillary thyroid
carcinomas. Virchows Arch. 438:121–128. 2001. View Article : Google Scholar
|
|
21
|
Siqueira DR, Romitti M, da Rocha AP,
Ceolin L, Meotti C, Estivalet A, Puñales MK and Maia AL: The RET
polymorphic allele S836S is associated with early metastatic
disease in patients with hereditary or sporadic medullary thyroid
carcinoma. Endocr Relat Cancer. 17:953–963. 2010. View Article : Google Scholar
|
|
22
|
O’Sullivan B and Shah J: New TNM staging
criteria for head and neck tumors. Semin Surg Oncol. 21:30–42.
2003.
|
|
23
|
Nelson AR, Fingleton B, Rothenberg ML and
Matrisian LM: Matrix metalloproteinases: Biologic activity and
clinical implications. J Clin Oncol. 18:1135–1149. 2000.PubMed/NCBI
|
|
24
|
Kumamoto H, Yamauchi K, Yoshida M and Ooya
K: Immunohistochemical detection of matrix metalloproteinases
(MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in
ameloblastomas. J Oral Pathol Med. 32:114–120. 2003. View Article : Google Scholar
|
|
25
|
Lee SJ, Tsang PS, Daz TM, Wei BY and
Stetler-Stevenson WG: TIMP-2 modulates VEGFR-2 phosphorylation and
enhances phosphodiesterase activity in endothelial cells. Lab
Invest. 90:374–382. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cho Mar K, Eimoto T, Tateyama H, Arai Y,
Fujiyoshi Y and Hamaguchi M: Expression of matrix
metalloproteinases in benign and malignant follicular thyroid
lesions. Histopathology. 48:286–294. 2006.PubMed/NCBI
|
|
27
|
Korem S, Kraiem Z, Shiloni E, Yehezkel O,
Sadeh O and Resnick MB: Increased expression of matrix
metalloproteinase-2: a diagnostic marker but not prognostic marker
of papillary thyroid carcinoma. Isr Med Assoc J. 4:247–251.
2002.
|
|
28
|
Albini A, Melchiori A, Santi L, Liotta LA,
Brown PD and Stetler-Stevenson WG: Tumor cell invasion inhibited by
TIMP-2. J Natl Cancer Inst. 83:775–779. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ring P, Johansson K, Höyhtyä M, Rubin K
and Lindmark G: Expression of tissue inhibitor of
metalloproteinases TIMP-2 in human colorectal cancer - a predictor
of tumour stage. Br J Cancer. 76:805–811. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tomita T: Matrix metalloproteinases and
tissue inhibitors of metalloproteinases in thyroid C-cells and
medullary thyroid carcinomas. Histopathology. 31:150–156. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cavalheiro BG, Junqueira CR and Brandão
LG: Expression of matrix metalloproteinase 2 (MMP-2) and tissue
inhibitor of metalloproteinase 2 (TIMP-2) in medullary thyroid
carcinoma: prognostic implications. Thyroid. 8:865–871. 2008.
View Article : Google Scholar
|
|
32
|
Capp C, Wajner SM, Siqueira DM, Brasil BA,
Meurer L and Maia AL: Increased expression of vascular endothelial
growth factor and its rreceptors, VEGFR-1 and VEGFR-2 in medullary
thyroid carcinoma. Thyroid. 8:863–871. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bunone G, Vigneri P, Mariani L, Buto S,
Collini P, Pilotti S, Pierotti MA and Bongarzone I: Expression of
angiogenesis stimulators and inhibitors in human thyroid tumors and
correlation with clinical pathological features. Am J Pathol.
155:1967–1976. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
de la Torre NG, Buley I, Wass JA and
Turner HE: Angiogenesis and lymphangiogenesis in thyroid
proliferative lesions: relationship to type and tumour behaviour.
Endocr Relat Cancer. 13:931–944. 2006.PubMed/NCBI
|
|
35
|
Roy H, Bhardwaj S and Ylä-Herttuala S:
Biology of vascular endothelial growth factors. FEBS Lett.
580:2879–2887. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lamoreaux WJ, Fitzgerald ME, Reiner A,
Hasty KA and Charles ST: Vascular endothelial growth factor
increases release of gelatinase A and decreases release of tissue
inhibitor of metalloproteinases by microvascular endothelial cells
in vitro. Microvasc Res. 55:29–42. 1998. View Article : Google Scholar
|