Introduction
Gastric carcinoma is one of the most common causes
of cancer-related mortality worldwide, and the prolonged survival
rate of patients with unresectable or metastatic gastric carcinoma
remains low (1–3). Determining the expression profiles of
key molecules involved in the survival pathways holds a great deal
of promise in improving the rate of diagnosis and prognosis of
patients with gastric carcinoma. Epidemiological studies have
demonstrated that diffuse (poorly differentiated) gastric
adenocarcinomas present tumor aggressiveness, as well as a poor
prognosis and response to chemotherapeutic interventions (4,5).
Autophagy has recently attracted attention as a novel potential
target in cancer treatment (6–8).
Autophagy is a homeostatic cellular process that
recycles proteins and organelles using lysosomal machinery
(9,10). Depending on the stimulus and cell
type, autophagy is a double-edged sword, acting as a cell death
mechanism whilst also aiding the prolonged survival of cancer cells
in tumorigenesis (11,12). Several lines of evidence suggest
that autophagy and apoptosis can coexist or occur sequentially. In
contrast to the pro-survival role of autophagy, this pathway can
culminate with caspase-independent programmed cell death (13,14).
Several proteins are involved in the molecular mechanisms that
result in apoptosis or autophagy. For example, cathepsin B
activation is cell type-specific and plays a role in apoptosis via
BH3 interacting-domain death agonist cleavage, release of
cytochrome c and subsequent caspase activation (15). More recently, Chen et al
(16) reported that decreased
expression of Beclin 1 in gastric adenocarcinoma may be important
in the acquisition of a metastatic phenotype, suggesting that
decreased Beclin 1 expression is an independent biomarker for a
poor prognosis in patients with gastric adenocarcinoma. Thus,
inducing or impairing autophagy for therapeutic purposes requires
an in-depth molecular knowledge of this process in a number of
cancer cell types. However, the autophagy-lysosome process in
gastric adenocarcinoma has not yet been elucidated. Understanding
the context-specific role for autophagy in cancer and the
mechanisms involved may be important to guide autophagy-based
therapeutic intervention.
It is evident that gastric adenocarcinomas have
distinct subtypes but the significance of these subtypes remains
unclear. The present study aimed to investigate the changes in
autophagic pathways, as well as the intracellular link between
autophagic signaling and the apoptotic cascade in poorly
differentiated human gastric adenocarcinomas.
Materials and methods
Patients and tissue specimens
Tissue samples from 20 patients with gastric cancer
were obtained from the archives of the Second Affiliated Hospital,
Soochow University (Suzhou, China), between November 2009 and
December 2011. Tumor grades were defined in accordance with the
criteria of the World Health Organization (2000) (17). The tumor-node-metastasis (TNM) stage
of all gastric adenocarcinomas was assessed according to the
criteria of the sixth edition of the TNM classification of the
International Union Against Cancer (2002) (18). All 20 samples were solitary
intramucosal gastric cancers of poorly differentiated types (TNMII,
14 cases; TNMIII, 6 cases). In addition, matched adjacent gastric
mucosal tissues removed for radical gastrectomy were included as
controls. The Institute Research Medical Ethics Committee of the
Second Affiliated Hospital of Soochow University granted approval
for this study. The patients provided written informed consent for
their participation in this study.
Immunoblotting
The tissue samples were homogenized in homogenizing
buffer containing 50 mmol/l Tris-HCl (pH 7.4), 0.5% Triton X-100, 4
mmol/l ethylene glycol tetraacetic acid, 10 mmol/l EDTA, 30 mmol/l
sodium pyrophosphate, 1 mmol/l Na3VO4, 50
mmol/l NaF, 100 nmol/l calyculin A, 50 μg/ml leupeptin, 25 μg/ml
pepstatin A, 50 μg/ml trypsin inhibitor and 1 mmol/l
dithiothreitol. The homogenates were centrifuged for 20 min at
15,000 × g to pellet the cellular debris and the protein
concentration of the supernatant was determined using the Bradford
method (Bio-Rad, Hercules, CA, USA). Equal volumes of samples were
resolved by 10–12% SDS-PAGE and electrotransferred onto a
polyvinylidene difluoride transfer membrane. This was probed
overnight with the indicated primary antibody at 4°C, followed by
incubation with the relevant horseradish peroxidase-conjugated
secondary antibody for 1 h at room temperature. The primary
antibodies used for immunoblotting included light chain 3 (LC3)
rabbit polyclonal antibody (Medical and Biological Laboratories,
Ltd., Nagoya, Japan), Beclin 1 (Cell Signaling Technology,
Inc., Beverly, MA, USA), cathepsin B (mouse monoclonal antibody;
Abcam, Cambridge, UK), lysosome-associated membrane protein 2
(Lamp2), B-cell lymphoma 2 (Bcl-2; Santa Cruz Biotechnology, Inc.,
CA, USA), Bcl-2 and nineteen-kilodalton interacting protein-37
(BNIP3) and β-actin (mouse monoclonal antibody; Sigma, St. Louis,
MO, USA). Immunoreactive bands were detected by autoradiography
with enhanced chemiluminescence (Amersham Biosciences, Little
Chalfont, UK).
Confocal scanning immunofluorescence
microscopy
Immunolocalization and changes in LC3 and cathepsin
B in human gastric adenocarcinoma were examined by confocal
microscopy. Briefly, slices were prepared for fresh frozen coronal
sectioning (15 μm thick). For immunohistochemical staining, slices
were incubated with antibodies against LC3 (rabbit polyclonal
antibody; Cell Signaling Technology, Inc.) and cathepsin B, or
Beclin 1 (goat polyclonal antibody; Santa Cruz Biotechnology) and
Bcl-2, overnight at 4°C. This was followed by immunofluorescence
using a standard protocol from Perkin-Elmer (Waltham, MA, USA).
Immunofluorescence was visualized using a Zeiss LSM 510 confocal
microscope (Carl Zeiss AG, Oberkochen, Germany).
Statistical analysis
The statistical analysis of experimental data was
performed using a Student’s paired t-test (comparison of two
groups). P<0.05 was considered to indicate a statistically
significant difference. All data were expressed as the mean ±
standard deviation.
Results
Changes in autophagy signaling in human
gastric adenocarcinoma
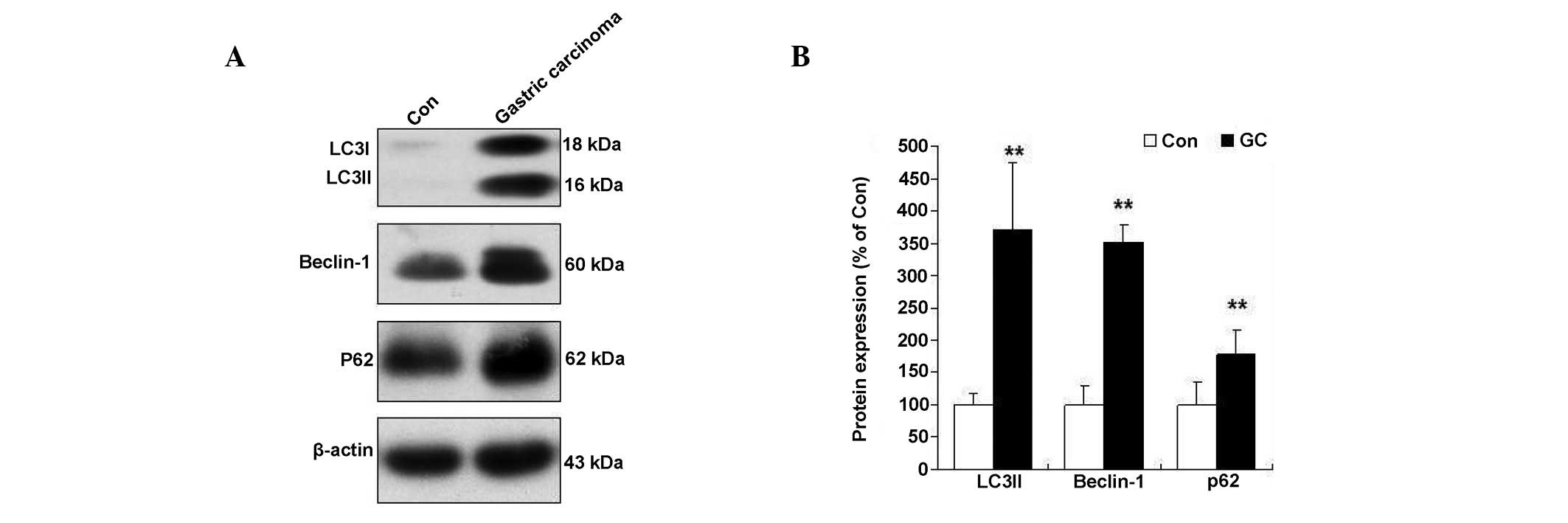
The protein changes in autophagy signaling were
monitored as a means of assessing autophagy progression in human
gastric adenocarcinoma. The induction of an autophagy event was
indicated by detection of membrane-bound LC3
phosphatidylethanolamine conjugate (LC3-II) by immunoblotting
(19). The data showed LC3-I/II
conversion in patients with low grade differentiated gastric
adenocarcinoma (Fig. 1). The
autophagy protein p62, a marker of autophagosome formation, was
also monitored (20). A significant
elevation of p62 was observed in patients with low grade
differentiated gastric adenocarcinoma (Fig. 1). In addition, Beclin 1 is a
critical component in the class III phosphatidylinositide 3 kinase
complex that induces the formation of autophagosomes in mammalian
systems (16). A significant
increase of Beclin 1 was observed in patients with low grade
differentiated gastric adenocarcinoma (Fig. 1).
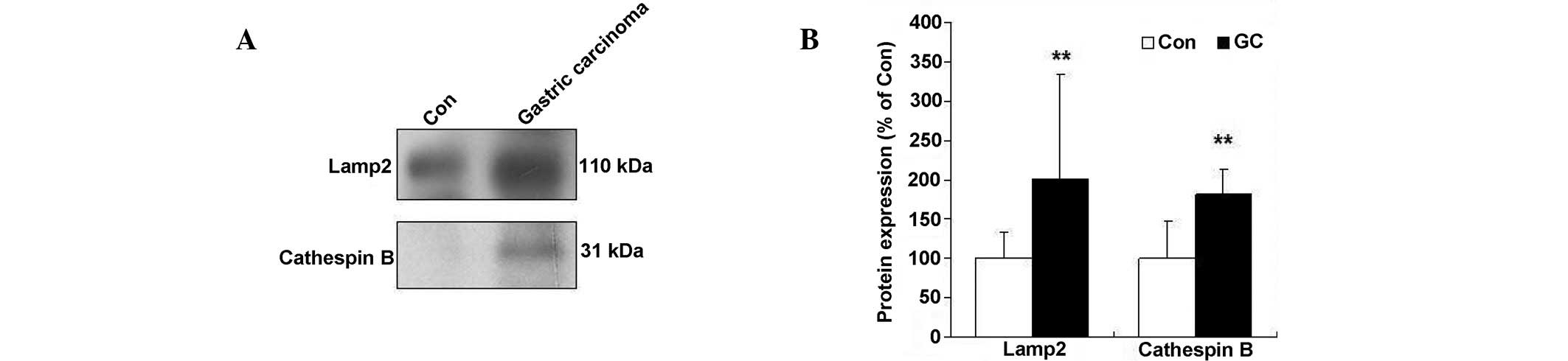
Expression of Lamp2 and cathepsin B
protein levels in human gastric adenocarcinoma
It is becoming increasingly clear that the
accumulation of autophagosomes alone cannot be used as an indicator
of increased autophagy. Thus, measurement of lysosomal protein
levels is essential for reflecting the function of the entire
autophagy pathway (10). In the
present study, the lysosomal protein expression was similar to the
patterns observed for LC3-II formation, including the induction of
Lamp2 and cathepsin B in patients with low grade differentiated
gastric adenocarcinoma (Fig. 2).
Lamp2 protein levels increased 2.0-fold in poorly differentiated
human gastric carcinomas, compared with normal tissues. A
significant decrease in the processing of the precursor forms of
cathepsin B to their lower molecular weight mature forms (31 kDa)
was observed in human gastric adenocarcinoma (Fig. 2). Thus, these data indicate that the
lysosome process is involved in low grade differentiated gastric
adenocarcinoma.
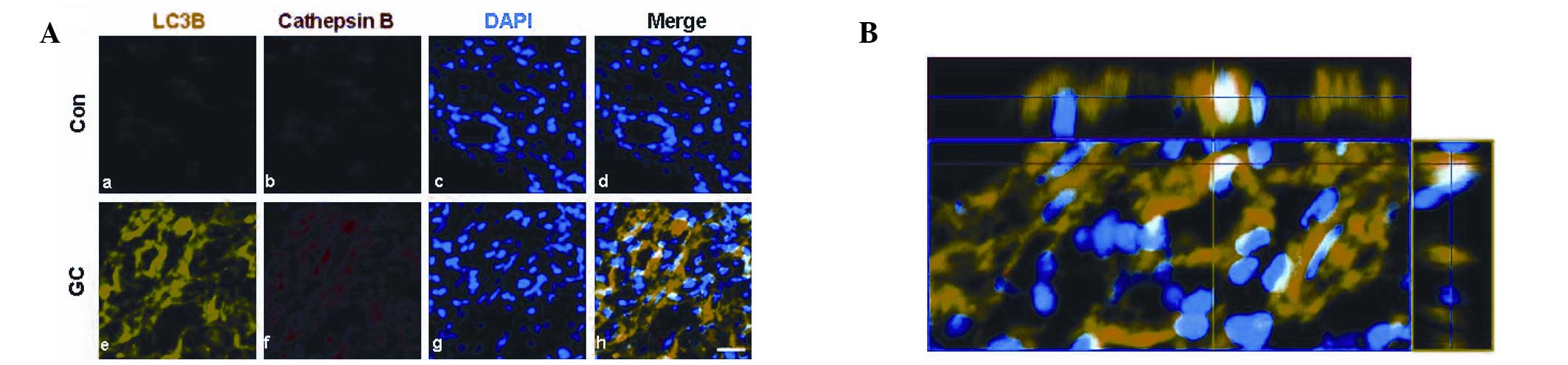
Immunofluroscence of autophagy-lysosome
signaling in human gastric adenocarcinoma
To further study autophagy-lysosome signaling in
human gastric adenocarcinoma, immunocytochemical studies were
performed using anti-LC3 and anti-cathepsin B antibodies. When
human gastric adenocarcinomas were dual-labeled with antibodies,
formation of LC3 and immunoreactivity against cathepsin B markedly
increased compared with normal tissues (Fig. 3). As shown in Fig. 3B, the LC3 puncta largely colocalized
with cathepsin B immunoreactivity in poorly differentiated human
gastric carcinomas, indicating that gastric adenocarcinomas undergo
both stages of the autophagy/lysosome process.
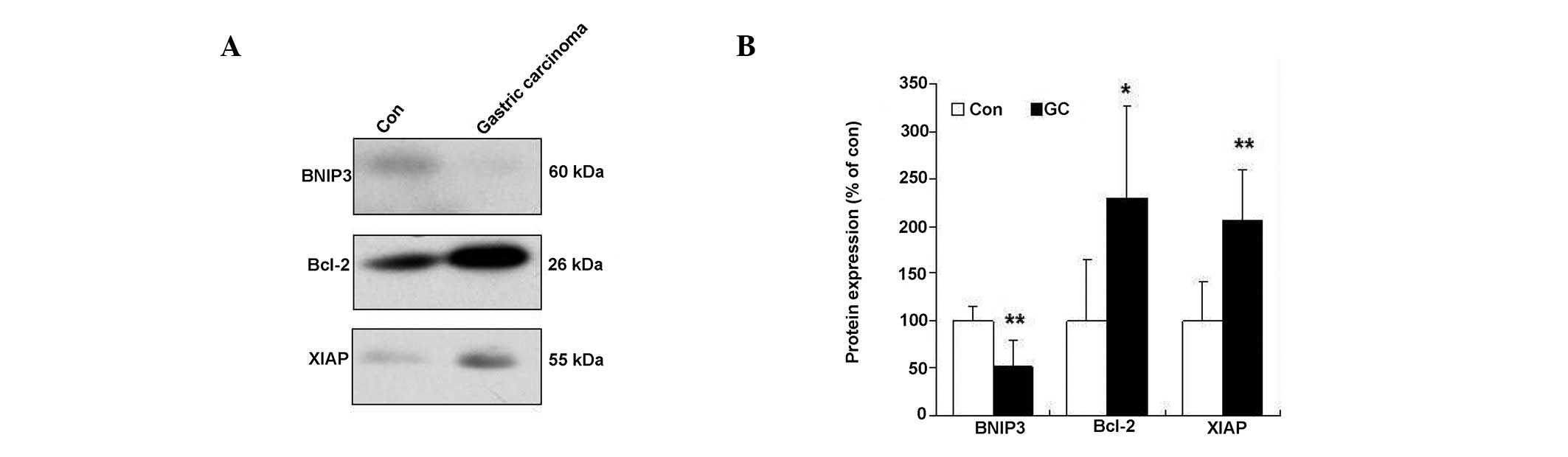
Changes in anti-apoptotic signaling in
human gastric adenocarcinoma
To gain further insight into the changes in
anti-apoptotic signaling in human gastric adenocarcinoma, the
changes in BNIP3, Bcl-2 and X-linked inhibitor of apoptosis (XIAP)
were determined by immunoblotting. The data demonstrated that BNIP3
levels significantly decreased in patients with low grade
differentiated gastric adenocarcinoma. Consistent with this
finding, similar results were obtained showing XIAP and Bcl-2
protein levels to increase in patients with low grade
differentiated gastric adenocarcinoma (Fig. 4).
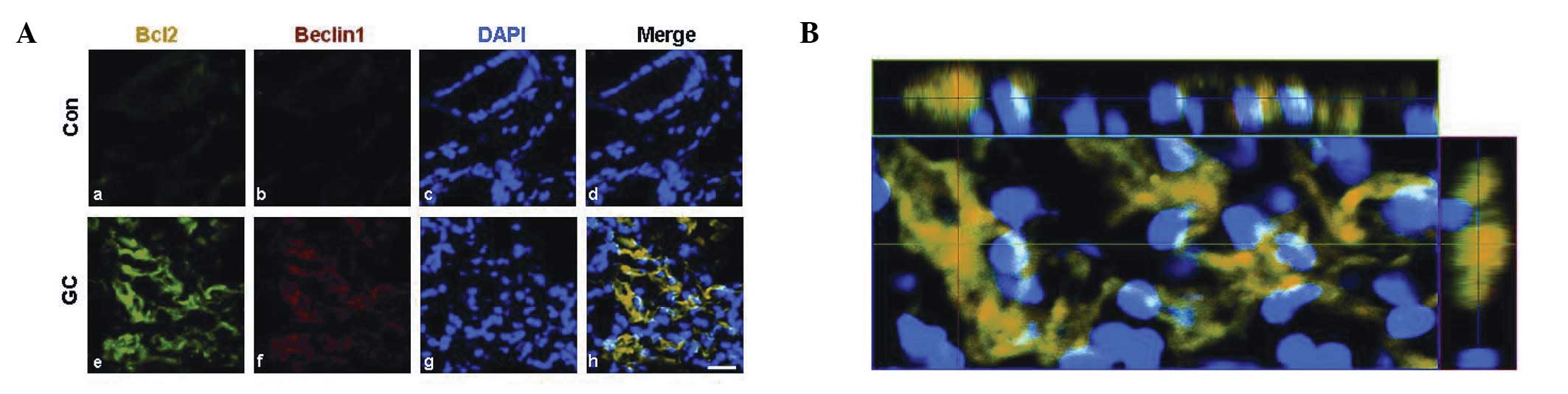
Disturbance of Bcl-2/Beclin 1 is
associated with cross-talk between the apoptotic cascade and
autophagic signaling
The balance of Bcl-2/Beclin 1 signaling also plays a
critical role during the cross-talk between the apoptotic cascade
and autophagic signaling (21–23).
It was reported that Beclin 1 expression was inversely correlated
with tumor differentiation, nodal and distant metastasis and tumor
relapse (24–26). To further study this result,
immunocytochemical studies were performed using anti-Bcl-2 and
Beclin 1 antibodies. When gastric adenocarcinoma cells were
dual-labeled with these two antibodies, Bcl-2 immunoreactivity
colocalized with Beclin 1 immunoreactivity in poorly differentiated
human gastric adenocarcinoma (Fig.
5). Immunoreactivities against Bcl-2 and Beclin 1 markedly
increased compared with those observed in normal tissue (Fig. 5).
Discussion
The role of autophagy in oncogenesis and tumor
survival is the subject of ongoing debate and remains unresolved
(19). Results of the present study
demonstrate that autophagic signaling participates in the
pathological process of low grade differentiated gastric
adenocarcinoma. In addition, Bcl-2/Beclin 1 signaling may be the
intracellular link between autophagic signaling and the apoptotic
cascade. Therefore, molecules involved in autophagic signaling in
gastric adenocarcinoma may represent potential prognostic and
therapeutic markers.
The biological significance and clinical impact of
the variation in expression of autophagic markers in cancer appear
to be associated with tumor types and tissue context (11,12).
Therefore, it follows that identification of the specific autophagy
profile in tissue specimens may offer important information for the
diagnosis and tailored treatment of this cancer. The majority of
gastric cancers are gland-forming adenocarcinomas (1). In the present study, the observed
alternations in autophagy signaling were based on measurements of
p62 and LC3 in human low grade differentiated gastric
adenocarcinoma. The biochemical hallmark of autophagic initiation
is the formation and subcellular redistribution of LC3-II (9,10).
Significant elevations of LC3-II/I and p62 were observed in human
gastric adenocarcinoma. Autophagy captures intracellular components
in autophagosomes and delivers them to lysosomes, where they are
degraded and recycled (9,10). In light of the key role of lysosome
signaling in autophagy and apoptosis, the alternations in
autophagy-lysosome progression were studied next, based on
measurements of Lamp2 and cathepsin B levels in human gastric
adenocarcinoma. Significant elevation of cathepsin B and Lamp2
levels in human gastric adenocarcinoma was found. Thus, induction
of autophagy-lysosome signaling participates in the pathological
processes of poorly differentiated human gastric
adenocarcinoma.
There remains much controversy over the precise
clinicopathological significance of autophagy signaling in gastric
cancer (27). Recent evidence
indicates that excessive autophagy may confer a tumor suppressive
function and trigger a subsequent caspase-dependent apoptotic
pathway (28). By contrast,
Wojtkowiak et al (12) have
shown that chronic autophagy occurs as a survival adaptation in
mouse tumors. In the present study, protein analysis of human
gastric adenocarcinoma revealed significant upregulation of
autophagy regulatory proteins, LC3, cathepsin B and Lamp2. However,
anti-apoptosis markers, particularly XIAP, were markedly increased
in low grade differentiated gastric adenocarcinoma cells.
Additionally, protein levels of the apoptosis inducer, BNIP3,
decreased in the low grade differentiated gastric adenocarcinoma
cells. Thus, data suggests that autophagy-lysosome signaling
participates in poorly differentiated gastric adenocarcinoma in
humans, and may be associated with poor prognosis in gastric
carcinoma.
These observations bring into question whether the
gastric adenocarcinoma-mediated apoptotic cascade is linked to
autophagic signaling, and participates in the progression of human
gastric adenocarcinoma. To evaluate the possible relationship
between the apoptotic cascade status and autophagic signaling,
immunoblotting was conducted to analyze changes in the autophagic
protein, Beclin 1, which has long been identified as a
Bcl-2-interacting partner (29).
Results demonstrated that Beclin 1 is highly expressed in low grade
differentiated gastric adenocarcinoma cells, compared with normal
mucosal tissues. The dimeric mitochondrial protein encoded by the
Beclin 1 gene is known to induce apoptosis (29). A significant elevation of survival
protein Bcl-2 was observed in the same context, indicating a
possible binding of Beclin 1 to the survival protein Bcl-2. Here,
the apoptotic events are characterized by the activation of
anti-apoptotic proteins, for example decreased BNIP3 and elevated
XIAP in human gastric adenocarcinoma, which interact with apoptotic
and/or anti-apoptotic molecules in human gastric
adenocarcinoma.
Although variable loss expression of Beclin 1 has
been observed in several types of human tumors (30), the relationship between Beclin 1
expression and gastric adenocarcinoma patient survival remains
unclear. At present, elevation of Beclin 1 protein levels in human
cancers (31) and decreases in
Beclin 1 expression have been reported (32). Bcl-2 antiapoptotic proteins inhibit
Beclin 1-dependent autophagy (21).
With regard to gastric adenocarcinoma, double immunostaining
analyses of the present study demonstrated colocalization of Beclin
1 and Bcl-2-positive immunoreactivity in human gastric
adenocarcinoma. Consistent with this, Ahn et al (33) analyzed expression of Beclin 1 in a
number of gastric adenocarcinomas. Weak or no immunoreactivity was
detected in surface mucosal and mucosal glandular cells. In the
context of the present study, we hypothesize that Bcl-2/Beclin 1
signaling may be a critical regulator of the biological effects of
gastric adenocarcinoma and may be a key signaling element for the
autophagic process, reflecting the prognostic result of patients.
However, there are various other regulators of autophagic cell
death that are involved in autophagic cell death in gastric
carcinoma (34,35). Clearly, further studies are required
to fully understand the potential function of Beclin 1 in human
gastric carcinoma pathogenesis and to identify the signaling
pathway involved in the tumorigenesis.
In summary, results of the present study indicate
that autophagy-lysosome cascades may represent a low grade
differentiated gastric adenocarcinoma feature of carcinoma cells.
Furthermore, we hypothesize that increased Bcl-2/Beclin 1 signaling
in human gastric adenocarcinoma may contribute to the interplay of
apoptotic and autophagic events. The present study indicates that
increased expression of autophagy-lysosome signaling molecules
represents a new adverse independent prognostic factor in low grade
differentiated gastric carcinoma. This insight may lead to an
improved understanding of the role of autophagy in poorly
differentiated human gastric adenocarcinoma. In addition, it may
act as a valuable biomarker for the development of improved
aggressive postsurgical adjuvant anticancer therapies.
References
|
1
|
Milne AN, Carneiro F, O’Morain C and
Offerhaus GJ: Nature meets nurture: molecular genetics of gastric
cancer. Hum Genet. 126:615–628. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heatley MK: Immunohistochemical biomarkers
of value in distinguishing primary ovarian carcinoma from gastric
carcinoma: a systematic review with statistical meta-analysis.
Histopathology. 52:267–276. 2008. View Article : Google Scholar
|
|
3
|
Corso G, Marrelli D, Pascale V, Vindigni C
and Roviello F: Frequency of CDH1 germline mutations in gastric
carcinoma coming from high- and low-risk areas: metanalysis and
systematic review of the literature. BMC Cancer. 12:82012.
View Article : Google Scholar
|
|
4
|
Hass HG, Smith U, Jäger C, Schäffer M,
Wellhäuber U, Hehr T, Markmann HU, Nehls O and Denzlinger C: Signet
ring cell carcinoma of the stomach is significantly associated with
poor prognosis and diffuse gastric cancer (Lauren’s): single-center
experience of 160 cases. Onkologie. 34:682–686. 2011.
|
|
5
|
Shah MA, Khanin R, Tang L, et al:
Molecular classification of gastric cancer: a new paradigm. Clin
Cancer Res. 17:2693–2701. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bhutia SK, Das SK, Azab B, et al:
Autophagy switches to apoptosis in prostate cancer cells infected
with melanoma differentiation associated gene-7/interleukin-24
(mda-7/IL-24). Autophagy. 7:1076–1077. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Janku F, McConkey DJ, Hong DS and Kurzrock
R: Autophagy as a target for anticancer therapy. Nat Rev Clin
Oncol. 8:528–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Buchser WJ, Laskow TC, Pavlik PJ, Lin HM
and Lotze MT: Cell-mediated autophagy promotes cancer cell
survival. Cancer Res. 72:2970–2979. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han F, Chen YX, Lu YM, et al: Regulation
of the ischemia-induced autophagy-lysosome processes by nitrosative
stress in endothelial cells. J Pineal Res. 51:124–135. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Klionsky DJ, Abdalla FC, Abeliovich H, et
al: Guidelines for the use and interpretation of assays for
monitoring autophagy. Autophagy. 8:445–544. 2012. View Article : Google Scholar
|
|
11
|
White E: Deconvoluting the
context-dependent role for autophagy in cancer. Nat Rev Cancer.
12:401–410. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wojtkowiak JW, Rothberg JM, Kumar V, et
al: Chronic autophagy is a cellular adaptation to tumor acidic pH
microenvironments. Cancer Res. 72:3938–3947. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo Z, Yu G, Lee HW, et al: The
Nedd8-activating enzyme inhibitor MLN4924 induces autophagy and
apoptosis to suppress liver cancer cell growth. Cancer Res.
72:3360–3371. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaminskyy VO, Piskunova T, Zborovskaya IB,
Tchevkina EM and Zhivotovsky B: Suppression of basal autophagy
reduces lung cancer cell proliferation and enhances
caspase-dependent and -independent apoptosis by stimulating ROS
formation. Autophagy. 8:1032–1044. 2012. View Article : Google Scholar
|
|
15
|
Nagaraj NS, Vigneswaran N and Zacharias W:
Cathepsin B mediates TRAIL-induced apoptosis in oral cancer cells.
J Cancer Res Clin Oncol. 132:171–183. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen YB, Hou JH, Feng XY, et al: Decreased
expression of Beclin 1 correlates with a metastatic phenotypic
feature and adverse prognosis of gastric carcinomas. J Surg Oncol.
105:542–547. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fenoglio-Preiser C, Muñoz N, Carneiro F,
et al: Pathology and genetics of tumours of the digestive system.
World Health Organization Classification of Tumours. IARC Press;
Lyon: pp. 37–66. 2000
|
|
18
|
Sobin LH and Wittekind CH: International
union against cancer. TNM Classification of Malignant Tumours. 6th
ed. Wiley; New York, NY: 2002
|
|
19
|
Wen YD, Sheng R, Zhang LS, et al: Neuronal
injury in rat model of permanent focal cerebral ischemia is
associated with activation of autophagic and lysosomal pathways.
Autophagy. 4:762–769. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mathew R, Karp CM, Beaudoin B, et al:
Autophagy suppresses tumorigenesis through elimination of p62.
Cell. 137:1062–1075. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pattingre S, Tassa A, Qu X, et al: Bcl-2
antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell.
122:927–939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Erlich S, Mizrachy L, Segev O, et al:
Differential interactions between Beclin 1 and Bcl-2 family
members. Autophagy. 3:561–568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhong JT, Xu Y, Yi HW, et al: The BH3
mimetic S1 induces autophagy through ER stress and disruption of
Bcl-2/Beclin 1 interaction in human glioma U251 cells. Cancer Lett.
323:180–187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng HY, Zhang YN, Wu QL, Sun XM, Sun JR
and Huang X: Expression of beclin 1, an autophagy-related protein,
in human cervical carcinoma and its clinical significance. Eur J
Gynaecol Oncol. 33:15–20. 2012.PubMed/NCBI
|
|
25
|
Morselli E, Galluzzi L, Kepp O, et al:
Anti- and pro-tumor functions of autophagy. Biochim Biophys Acta.
1793:1524–1532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Elgendy M, Sheridan C, Brumatti G and
Martin SJ: Oncogenic Ras-induced expression of Noxa and Beclin-1
promotes autophagic cell death and limits clonogenic survival. Mol
Cell. 42:23–35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nicotra G, Castino R, Follo C, Peracchio
C, Valente G and Isidoro C: The dilemma: does tissue expression of
cathepsin D reflect tumor malignancy? The question: does the assay
truly mirror cathepsin D mis-function in the tumor? Cancer Biomark.
7:47–64. 2010.
|
|
28
|
Gozuacik D and Kimchi A: Autophagy as a
cell death and tumor suppressor mechanism. Oncogene. 23:2891–2906.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kang R, Zeh HJ, Lotze MT and Tang D: The
Beclin 1 network regulates autophagy and apoptosis. Cell Death
Differ. 18:571–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Priault M, Hue E, Marhuenda F, Pilet P,
Oliver L and Vallette FM: Differential dependence on Beclin 1 for
the regulation of pro-survival autophagy by Bcl-2 and Bcl-xL in
HCT116 colorectal cancer cells. PLoS One. 5:e87552010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qu X, Yu J, Bhagat G, et al: Promotion of
tumorigenesis by heterozygous disruption of the beclin 1 autophagy
gene. J Clin Invest. 112:1809–1820. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qian W, Liu J, Jin J, Ni W and Xu W:
Arsenic trioxide induces not only apoptosis but also autophagic
cell death in leukemia cell lines via up-regulation of Beclin-1.
Leuk Res. 31:329–339. 2007. View Article : Google Scholar
|
|
33
|
Ahn CH, Jeong EG, Lee JW, et al:
Expression of beclin-1, an autophagy-related protein, in gastric
and colorectal cancers. APMIS. 115:1344–1349. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Levine B, Sinha S and Kroemer G: Bcl-2
family members: dual regulators of apoptosis and autophagy.
Autophagy. 4:600–606. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Djavaheri-Mergny M, Maiuri MC and Kroemer
G: Cross talk between apoptosis and autophagy by caspase-mediated
cleavage of Beclin 1. Oncogene. 29:1717–1719. 2010. View Article : Google Scholar : PubMed/NCBI
|