Introduction
Macrophages in the immune response are considered to
be a phagocyte system, which may occasionally be dependent on the
state of the host immune reaction (the macrophage response may be
influenced for the synthesis of mediators by other immune cells and
interaction with other cells), as well as maintenance of the
antigen. Proinflammatory classical activation of macrophages is
characterized by the secretion of cytokines, such as interferon
(IFN)-γ and tumor necrosis factor (TNF)-α, from T helper cells
(Th1) and natural killer cells. This activation results in a
macrophage population that exhibits enhanced microbicidal
performance and increases the secretion of proinflammatory
cytokines, which enhance adaptive immunity (1–4).
Conversely, alternative activation is achieved by
the presence of interleukin (IL)-4 and −13, which polarize the Th2
response in the macrophages and are important in the immune
response in acute or chronic disease processes (5,6).
Currently, macrophages are designated as
macrophage-profile 1 (M1) and macrophage-profile 2 (M2), analogous
to the division of the Th1 and Th2 profiles. However, M2
macrophages are divided into three subtypes (M2a, M2b and M2c). M2a
are stimulated by IL-4 or −13, M2b are stimulated by immune
complexes on the Toll-like receptor and the production of
proinflammatory cytokines (IL-1β, TNF-α and IL-6), and M2c are
stimulated by IL-10 or transforming growth factor (TGF) -β when
subjected to anti-inflammatory glucocorticoid hormones (7,8).
Solid tumors recruit macrophages within the
microenvironment (tumor-associated macrophages; TAMs) that exhibit
a complex association with the neoplastic cells of the tumor. These
neoplastic cells perform various roles during tumor development,
which are occasionally antagonistic. It was hypothesized that TAM
cells presented an antitumor effect as, in a satisfactory
environment (when there are immune cells that produce cytokines and
other mediators with antitumor profiles), TAMs are capable of
causing tumor cell death. However, experimental and clinical
studies indicate that rather than promote the neutralization of the
tumor, TAMs facilitate angiogenesis, extracellular matrix breakdown
and remodeling, and promote tumor cell motility (9–12).
With regard to the role of macrophages in promoting
an antitumor response under the influence of physical activity, a
study comparing healthy animals versus animals with tumors,
identified that exercise resulted in a positive effect on the
function of macrophages, which was evidenced by reduced levels of
pulmonary metastasis (13).
Moreover, the exercise training resulted in greater cytolytic
activity in vitro (14).
However, few studies have investigated exercise, the
function of macrophages and the production of their cytokines in
the presence of tumors. In the present study, the immunological
profile of mice subjected to DMBA, with or without physical
activity, was evaluated.
Materials and methods
Experimental groups and tumor
induction
Female BALB/c virgin mice (age, eight weeks) were
obtained from the Institute for Research in Oncology, Federal
University of the Triângulo Mineiro (Uberaba, Brazil) and were
group-housed. The present study was approved by the Ethics
Committee of the Federal University of Triângulo Mineiro (Uberaba,
Brazil; registration no. 160).
The mice were divided into the following our groups
(n=14 per group): i) No tumor/non-trained; ii) no tumor/trained
(swim training five days/week for eight weeks); iii)
tumor/non-trained and iv) tumor/trained (following a matching
protocol to group ii). In the tumor groups, the tumors were induced
by oral administration of DMBA at a concentration of 1 mg/ml by
daily gavage, for six weeks.
Peritoneal macrophage culture
The mice were euthanized with an overdose of
anesthetics, ketamine (50 mg/kg) and xylazine (15 mg/kg) and
peritoneal lavage was performed to obtain the macrophages. Three
lavages were centrifuged at 290 × g for 10 min at 4°C using
RPMI-1640 (Sigma-Aldrich, St. Loius, MO, USA). The cells were
counted, resuspended in complete RPMI medium and distributed into
24-well plates at a concentration of 1×106 cells/well to
obtain the adherent cells in a 1.0-ml volume, which were
subsequently stimulated with 10 μg/ml lipopolysaccharide (LPS).
Following 24 h of incubation at 37°C in a 5% CO2
atmosphere, the supernatant samples were obtained and the cells
were stored at −80°C.
Flow cytometry
Isolated peritoneal macrophages were placed in 1 ml
phosphate-buffered saline (PBS) supplemented with 2 μl protein
transport inhibitor (BD GolgiStop™; BD Biosciences,
Franklin Lakes, NJ, USA) per 3 ml peritoneal macrophage solution,
and incubated for ≥20 min at 4°C. The cells were washed with PBS by
centrifugation, using the method described above, to remove excess
proteins.
Following centrifugation, the cells were
resuspended, counted and subjected to extracellular immunolabeling
with fluorescent anti-cluster of differentiation (CD)-14 antibody
(BD Biosciences, San Diego, CA, USA). The cells were incubated with
each antibody for 30 min at 4°C in the dark and washed with PBS to
remove excess antibodies. Permeabilization and fixation were
performed using BD Cytofix/Cytoperm™ solution (BD
Biosciences) at 4°C for 20 min in the dark.
The cells were subjected to intracellular
immunolabeling using antibodies against IL-12 and TNF-α. Following
intracellular labeling, the cells were incubated at 4°C for 30 min
in the dark and washed in buffer solution (BD Perm/Wash™
Buffer; BD Biosciences) to remove excess labeling molecules. Cell
aliquots were resuspended in 500 μl PBS for flow cytometry analysis
in a FACSCalibur™ cytometer (BD Biosciences).
Cytokine levels
The presence of cytokines (IFN-γ, IL-4, IL-10,
IL-12, TNF-α and TGF-β) in the supernatant samples was measured by
an enzyme-linked immunosorbent assay using pairs of monoclonal
antibodies (BD OptEIA™; BD Biosciences, San Diego, CA,
USA). The procedure was performed according to the manufacturer’s
instructions.
Statistical analysis
Data are presented as the mean ± standard error of
the mean and the results were analyzed using the analysis of
variance test. Proportions were compared using the χ2
test and statistical analysis and graphing were performed with
GraphPad Prism version 5.0 (GraphPad Software, Inc., La Jolla, CA,
USA). P≤0.05 was considered to indicate a statistically significant
difference.
Results
Profile of immunocompetent cells and
expression of cytokines
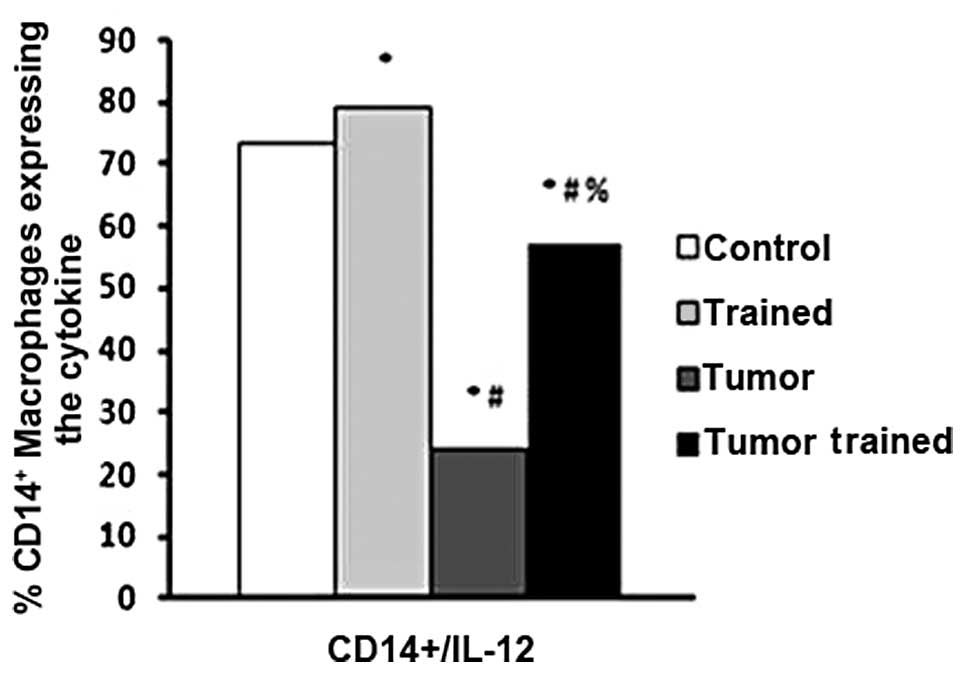
Ex vivo CD14/IL-12 double-labeling
experiments identified that physical activity increased the
quantity of CD14+/IL-12+ cells, compared with
that of the no tumor/non-trained control group (P<0.0001;
Fig. 1). Furthermore, mice in the
tumor/non-trained group exhibited a sharp reduction in
CD14+/IL-12+ cell frequency (P<0.0001),
when compared with the control group. However, subjecting the mice
with tumors to physical training attenuated the tumor-induced
reduction, as tumor/trained mice exhibited significantly greater
quantities of double-labeled cells than mice in the
tumor/non-trained group (P<0.0001; Fig. 1). There were no group differences
observed in CD14+/TNF-α+ double-labeled cells
(data not shown).
Production of cytokines in the
supernatant of peritoneal macrophage cultures
Cultures of macrophages, from the mice in the groups
that were trained, exhibited greater concentrations of IFN-γ than
the cultures obtained from the sedentary groups (P=0.0119, no
tumor/trained vs. no tumor/non-trained and P=0.0112, tumor/trained
vs. tumor/non-trained; Fig. 2A).
The presence of the tumor alone was sufficient to increase the
synthesis of IFN-γ (P=0.0464, tumor/non-trained vs. no
tumor/non-trained). The combination of the tumor and training
markedly induced the expression of IFN-γ relative to the no
tumor/non-trained control group (P=0.0178).
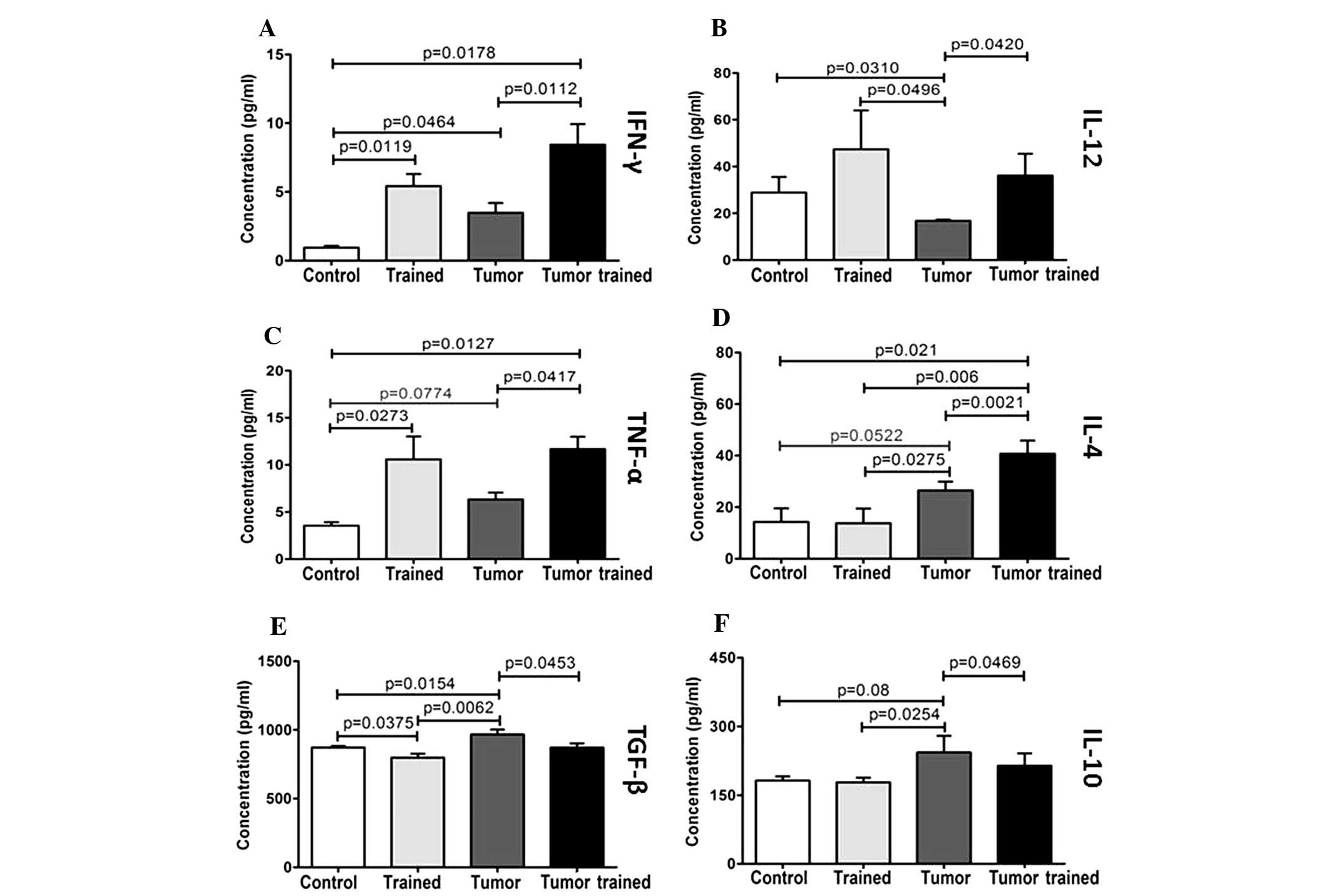 | Figure 2Concentrations of (A) IFN-γ, (B)
IL-12, (C) TNF-α, (D) IL-4, (E) TGF-β and (F) IL-10 per group,
obtained from 24-h cultures with peritoneal macrophages. IFN,
interferon; IL, interleukin; TNF, tumor necrosis factor; TGF,
transforming growth factor; control, no tumor/non trained; trained,
non tumor/trained; tumor, tumor/non trained; tumor trained,
tumor/trained. |
As with Th1 cytokines, after 24 h of culturing,
there was an increase in levels of IL-12 in the trained groups and
a decrease in the group with a tumor alone (Fig. 2B). Relative to the no
tumor/non-trained control group, no tumor/trained mice yielded
increased levels of IL-12 (P=0.0310) and tumor/non-trained mice
yielded reduced levels of IL-12 (P=0.0496). Conversely, physical
activity opposed this effect, as tumor/trained mice exhibited
greater levels of IL-12 than tumor/non-trained mice (P=0.0420).
TNF-α synthesis followed IL-12 production (Fig. 2C); either physical activity or the
presence of a tumor increased TNF-α expression compared with the no
tumor/non-trained control group (P=0.0273 and P=0.0127,
respectively). Exercise training in mice with tumors further
increased TNF-α expression beyond that which was observed in the
tumor/non-trained group (P=0.0417).
Training alone did not significantly affect IL-4
levels (Fig. 2D); by contrast, the
presence of a tumor tended to increase the IL-4 concentration. IL-4
levels in the tumor/non-trained group increased relative to the
control group (P=0.0552) and were significantly greater than the
levels observed for the no tumor/trained group (P=0.0275).
Practicing physical activity in combination with the presence of a
tumor produced IL-4 levels that were significantly higher than the
IL-4 levels observed in each of the three other groups (P=0.021 vs.
no tumor/non-trained; P=0.006 vs. no tumor/trained; P=0.0021 vs.
tumor/non-trained).
Conforming to the immunosuppressive cytokines,
relative to the no tumor/non-trained control group, TGF-β
concentration (Fig. 2E) was
increased after 24 h of culture in the isolated presence of a tumor
(P=0.0154); however, it was decreased in the no tumor/trained group
(P=0.0375). TGF-β concentration was also lower in the no
tumor/trained group than in the tumor/non-trained group (P=0.0062).
Furthermore, training resulted in a reduction of TGF-β
concentration in cultures from mice with tumors (P=0.0453,
tumor/trained vs. tumor/non-trained).
Over a 24 h time period, the IL-10 levels (Fig. 2F) in the no tumor/trained group were
analogous to IL-10 levels in the no tumor/non-trained control group
and significantly lower than IL-10 levels in the tumor/non-trained
group (P=0.0254). In the presence of the tumor, implementing
physical activity attenuated the expression of IL-10 (P=0.0469
tumor/trained vs. tumor/non-trained) towards control group levels
(P>0.05, tumor/trained vs. no tumor/non-trained; Fig. 2F).
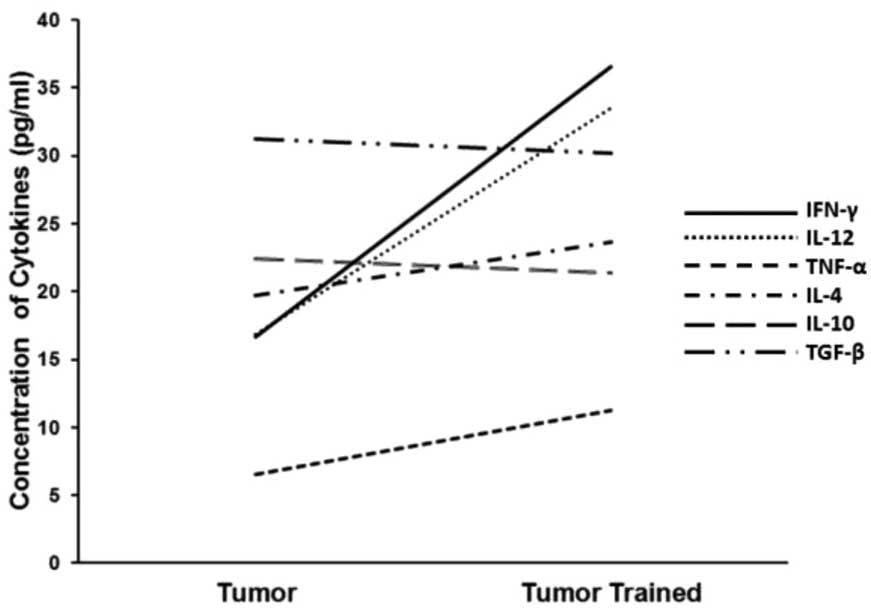
To observe the changes resulting from performing
physical activity, a tendency analysis to compare the M1 cytokines
(IFN-γ, TNF-α and IL-12) to the M2 cytokines (IL-4, IL-10 and
TGF-β) was conducted. The tumor/trained group exhibited greater
concentrations of the three M1 cytokines than the tumor/non-trained
group, whereas the trends of the M2 cytokines were less pronounced
(Fig. 3). Two M2 cytokines (IL-10
and TGF-β) were marginally greater in the tumor/non-trained group
than in the tumor/trained group and one cytokine (IL-4) was
marginally greater in the tumor/trained group than in the
tumor/non-trained group.
Discussion
The tumors may affect how the functionality of
macrophages change via modification of the microenvironment and
thus the immune response, in such a way that the immune system
itself is used as a tumor escape mechanism. Therefore,
understanding how tumor cells interfere with the action of
macrophages and investigating the potential application of this
information, may be via identification of the soluble or inhibitory
factors that permit the tumors to change the plasticity of the
macrophages (15).
To enable the macrophages to act efficiently, IL-12
expression is important and, in the presence of tumors, its
expression is correlated with increased survival rates (16). Thus, in the present study it was
identified that, in the ex vivo system and with the
production of IL-12 in the supernatant of the macrophage culture,
physical activity was capable of optimizing the expression of IL-12
in the macrophages. Trained mice exhibited greater percentages of
CD14+ cells expressing IL-12 than the non-trained
groups, particularly with the non-trained group that underwent
chemical carcinogenesis with DMBA. The results of the present
study, therefore, indicate that physical activity is a significant
factor that is capable of modulating the immune system and aiding
in the antitumor response. The activity of the Th1-pattern
cytokines analyzed in the present study, such as IFN-γ and TNF-α,
reinforced this hypothesis.
Kizaki et al (17) analyzed two groups of mice, which
were sedentary or moderately trained (50–75% VO2max; 30
min of stair climbing at 18 m/min, 5 days/week for three weeks). It
was observed that the trained group exhibited increased
concentrations of IFN-γ, TNF-α and nitric oxide, and a reduction of
IL-10. Furthermore, the synthesis of cytokines was not observed to
be correlated with the quantity of adrenergic β receptors. Thus,
Kizaki et al (17)
demonstrated that the adaptation of macrophages to moderate
exercise improved microbicidal activity and the capacity for a
Th1-type response. These data corroborate the observations of the
present study, in which mice in the no tumor/trained and
tumor/trained groups exhibited higher levels of IFN-γ and TNF-α, as
well as IL-12, and diminished levels of IL-10 and TGF-β. Thus, by
characterizing these macrophages as M1, it may be inferred that
these cells were capable of promoting a positive antitumor
response.
Lu et al (18) verified that chronic physical
activity improved the antitumor activity of macrophages. When
macrophages were placed in a culture with IFN-γ and LPS, greater
quantities of cytolytic macrophages were observed in the trained
groups, independent of the age of the animals. Woods et al
(19) confirmed this outcome.
Bombarda et al (20) investigated the effect of a session
of exercise, performed below the anaerobic threshold, on the
function of neutrophils and circulating monocytes in Wistar rats.
The functional activity of circulating phagocytes was evaluated
using a Saccharomyces cerevisiae phagocytosis assay and a
nitro blue tetrazolium (NBT) test. No statistically significant
difference was identified between the groups with regard to the
total and differential number of leukocytes. However, the
neutrophils in the groups that underwent training phagocytosed an
increased number of S. cerevisiae and exhibited greater
efficiency in reducing NBT than the control group. Therefore,
exercise performed at an intensity below the anaerobic threshold
was sufficient to increase the phagocytic and microbicidal activity
of the neutrophil in an animal model.
Such findings indicate that macrophages may mediate
immune system defense against tumors. Early liberation of IFN-γ
contributes to the differentiation of T cells from Th1 cells. Thus,
the initial production of IFN-γ, IL-12 and TNF-α is significant in
generating innate immunity and M1 macrophages, as well as improving
adaptive defenses against infections. Conversely, IL-4 (an M2
cytokine) and the production of TGF-β promotes a change from Th1 to
Th2 or Tregs, suppressing antitumor resistance, thus indicating
that low production of IL-4, IL-10 and TGF-β, as seen in the
peritoneal macrophages of trained mice, may contribute to the
Th1-type immune response against tumors (21,22).
As identified in previous studies, where the profile
of T helper lymphocytes in animals with tumors subjected to
physical activity was evaluated and a greater expression of
Th1-pattern cytokines and a reduction in the expression of Th2
cytokines was demonstrated (23),
the data from the present study identified that trained mice and
mice with a tumor that practice physical activity exhibit an M1
profile. By contrast, mice with a tumor that remain sedentary have
an M2 profile. Thus, regular physical activity is advised for
patients with cancer, alongside conventional therapies (such as
chemotherapy, radiation treatment and surgery) and in conjunction
with novel therapies, such as immunotherapies. However, further
investigation is required to standardize training regimes and the
frequency, intensity and methods of delivering them in order to
verify the point at which physical activity becomes beneficial, or
to establish whether too much activity may damage the immune system
and favor the development and aggravation of tumors.
In conclusion, inducing tumors in sedentary mice
reduced the cytokine synthesis of M1 macrophages and increased the
presence of M2 macrophages. However, practicing physical activity
in the presence of a tumor promoted a reduction in tumor
development and polarized the immunological response in the
direction of the antitumor M1 profile.
Acknowledgments
The authors would like to thank the Studies and
Projects Funding Body (Financiadora de Estudos e Projetos, FINEP),
the Foundation for Research Assistance of the State of Minas Gerais
(Fundação de Amparo à Pesquisa do Estado de Minas Gerais, FAPEMIG),
the National Council for Scientific and Technical Development
(Conselho Nacional de Desenvolvimento Científico e Tecnológico,
CNPq) and the Uberaba Foundation for Teaching and Research
(Fundação de Ensino e Pesquisa de Uberaba, FUNEPU) for their
financial assistance.
References
|
1
|
van Furth R, Cohn ZA, Hirsch JG, Humphrey
JH, Spector WG and Langevoort HL: The mononuclear phagocyte system:
a new classification of macrophages, monocytes, and their precursor
cells. Bull World Health Organ. 46:845–852. 1972.PubMed/NCBI
|
|
2
|
Mackaness GB: The immunological basis of
acquired cellular resistance. J Exp Med. 120:105–120. 1964.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gordon S and Taylor PR: Monocyte and
macrophage heterogeneity. Nat Rev Immunol. 5:953–964. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gordon S: The macrophage: past, present
and future. Eur J Immunol. 37(Suppl 1): S9–S17. 2007. View Article : Google Scholar
|
|
5
|
Gordon S: Alternative activation of
macrophages. Nat Rev Immunol. 3:23–35. 2003. View Article : Google Scholar
|
|
6
|
Martinez FO, Helming L and Gordon S:
Alternative activation of macrophages: an immunologic functional
perspective. Annu Rev Immunol. 27:451–483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mills CD, Kincaid K, Alt JM, Heilman MJ
and Hill AM: M-1/M-2 macrophages and the Th1/Th2 paradigm. J
Immunol. 164:6166–6173. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lefèvre L, Galès A, Olagnier D, Bernad J,
Perez L, et al: PPARγ ligands switched high fat diet-induced
macrophage M2b polarization toward M2a thereby improving intestinal
Candida elimination. PLoS One. 5:e128282010.
|
|
9
|
Mantovani A, Bottazzi B, Colotta F,
Sozzani S and Ruco L: The origin and function of tumor-associated
macrophages. Immunol Today. 13:265–270. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mantovani A, Schioppa T, Porta C, Allavena
P and Sica A: Role of tumor-associated macrophages in tumor
progression and invasion. Cancer Metastasis Rev. 25:315–322. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sica A, Larghi P, Mancino A, Rubino L,
Porta C, Totaro MG, et al: Macrophage polarization in tumour
progression. Semin Cancer Biol. 18:349–355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dannenmann SR, Thielicke J, Stöckli M,
Matter C, von Boehmr L, Cecconi V, Hermanns T, Hefermehl L, Schraml
P, Moch H, et al: Tumor-associated macrophages subvert T-cell
function and correlate with reduced survival in clear cell renal
cell carcinoma. Oncoimmunology. 2:e235622013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Woods JA: Exercise and resistance to
neoplasia. Can J Physiol Pharmacol. 76:581–588. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pedersen BK and Hoffman-Goetz L: Exercise
and the immune system: regulation, integration, and adaptation.
Physiol Rev. 80:1055–1081. 2000.PubMed/NCBI
|
|
15
|
Cassetta L, Cassol E and Poli G:
Macrophage polarization in health and disease.
ScientificWorldJournal. 11:2391–2402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zijlmans HJ, Fleuren GJ, Baelde HJ, Eilers
PH, Kenter GG and Gorter A: Role of tumor-derived proinflammatory
cytokines GM-CSF, TNF-alpha, and IL-12 in the migration and
differentiation of antigen-presenting cells in cervical carcinoma.
Cancer. 109:556–565. 2007. View Article : Google Scholar
|
|
17
|
Kizaki T, Takemasa T, Sakurai T, Izawa T,
Hanawa T, Kamiya S, Haga S, Imaizumi K and Ohno H: Adaptation of
macrophages to exercise training improves innate immunity. Biochem
Biophys Res Commun. 372:152–156. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu Q, Ceddia MA, Price EA, Ye SM and Woods
JA: Chronic exercise increases macrophage-mediated tumor cytolysis
in young and old mice. Am J Physiol. 276:R482–R489. 1999.PubMed/NCBI
|
|
19
|
Woods J, Lu Q, Ceddia MA and Lowder T:
Special feature for the Olympics: effects of exercise on the immune
system: exercise-induced modulation of macrophage function. Immunol
Cell Biol. 78:545–553. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bombarda J, Melo JC, De Souza ER, Nóbrega
OT and Córdova C: Exercise below the anaerobic threshold increases
phagocytic and microbicide activities of neutrophils in Wistar
rats. J Bras Patol Med Lab. 45:9–15. 2009.(In Portuguese).
|
|
21
|
Schmieder A, Michel J, Schönhaar K, Goerdt
S and Schledzewski K: Differentiation and gene expression profile
of tumor-associated macrophages. Semin Cancer Biol. 22:289–297.
2012. View Article : Google Scholar
|
|
22
|
Shirabe K, Mano Y, Muto J, Matono R,
Motomura T, Toshima T, Takeishi K, Uchiyama H, Yoshizumi T,
Taketomi A, et al: Role of tumor-associated macrophages in the
progression of hepatocellular carcinoma. Surg Today. 42:1–7. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abdalla DR, Murta EF and Michelin MA: The
influence of physical activity on the profile of immune response
cells and cytokine synthesis in mice with experimental breast
tumors induced by 7,12-dimethylbenzanthracene. Eur J Cancer Prev.
22:251–258. 2013. View Article : Google Scholar
|